Abstract
Background
We wished to evaluate the treatment methods for vertebral Langerhans cell histiocytosis (LCH) (a rare reticuloendothelial disorder) at a tertiary care pediatric centre and compare treatment and outcomes with those reported in the recent literature.
Methods
A total of 55 charts were retrieved between 1980 and 2003 for children with LCH. Only those children who were under 18 years of age, had a diagnosis of LCH, histiocytosis X or eosinophilic granuloma and had documented vertebral involvement were included. The data collected were compared with data in the literature with respect to epidemiologic features, symptoms, investigations and procedures done, treatment, outcome and follow-up.
Results
Of the 8 children who met the inclusion criteria for vertebral LCH, the most common presenting complaint was back or neck pain. The thoracic vertebrae were most commonly affected followed equally by cervical and lumbar spines. Most children underwent a complete diagnostic work-up. A single solitary lesion was found in only 1 child. Biopsies were attempted in all cases with 6 positive results. Treatment varied depending on the severity of the presenting complaint; however, none of the tumours was completely resected. Follow-up averaged 3.4 years, and only 1 child has had a recurrence.
Conclusion
A multidisciplinary investigation is recommended for children with suspected vertebral LCH. Treatment depends on the severity of the disease.
Abstract
Contexte
Nous voulions évaluer les méthodes de traitement de l'histiocytose vertébrale à cellules de Langerhans (HCL) (trouble rare du système réticuloendothélial) à un centre pédiatrique de soins tertiaires et comparer le traitement et les résultats à ceux que l'on signale dans des publications récentes.
Méthodes
On a extrait au total 55 dossiers d'enfants atteints d'HCL entre 1980 et 2003. On a inclus seulement les enfants de moins de 18 ans, chez lesquels on avait diagnostiqué une HCL, une histiocytose X ou un granulome à éosinophiles et une atteinte vertébrale documentée. On a comparé les données recueillies à celles des publications sur les plans des caractéristiques épidémiologique, des symptômes, des investigations et des interventions pratiquées, des traitements, des résultats et des suivis.
Résultats
Les huit enfants qui satisfaisaient aux critères d'inclusion se plaignaient le plus souvent de douleur au dos ou au cou. Les vertèbres thoraciques étaient les plus souvent atteintes, suivies également par les cervicales et les lombaires. La plupart des enfants ont subi des examens complets de diagnostic. On a trouvé une seule lésion isolée chez un enfant seulement. On a pratiqué une biopsie dans tous les cas et obtenu six résultats positifs. Le traitement a varié selon la gravité de la plainte, mais aucune des tumeurs n'a été réséquée complètement. Le suivi a duré en moyenne 3,4 ans et il y a eu récidive chez un enfant seulement.
Conclusion
On recommande une investigation multidisciplinaire dans le cas des enfants chez lesquels on soupçonne une HCL vertébrale. Le traitement dépend de la gravité de la maladie.
Langerhans cell histiocytosis (LCH) is a relatively uncommon disorder involving the reticuloendothelial system in children and young adults. Although it was discovered by Hand in 1893, the etiology remains unknown,1 but viruses, bacteria and genetic factors have been implicated. Familial occurrence is very rare.2
LCH is now used to designate various clinicopathologic conditions previously known as Hand–Schüller –Christian disease, Abt–Letterer–Siwe disease, Hashimoto–Pritzker disease, eosinophilic granuloma of bone and histiocytosis X. LCH was agreed upon by the Writing Group of the Histiocyte Society in 1987 in order to acknowledge the central role of the Langerhans cell in these diseases. Although the classification system is in continual flux, the most contemporary nomenclature divides the disorder into a malignant form (malignant disorders) or a nonmalignant form, which ranges from self-limited to lethal (disorders of varied biological behaviour). LCH is classified as a disorder of varied biological behaviour and is dendritic-cell-related. Furthermore, LCH has been stratified on clinical grounds as single-system involving a single solitary site, single system affecting multiple sites or multisystem disease.3
LCH is characterized by an abnormal proliferation of histiocytes with a variable granulomatous and inflammatory component. The histologic picture is one of reactive rather than neoplastic proliferation.4 Multi-system LCH generally presents with a triad of exophthalmos, diabetes insipidus and lytic bone lesions.5 The clinical spectrum of LCH is broad, depending on the number and location of the lesions. Bone involvement is almost always present with the lesions usually located in the skull or long bones (or both)5 as well as the viscera. Other common anatomical locations (and findings) of LCH involvement include skin (rash), lungs (dysfunction), liver (dysfunction), gastrointestinal tract, hematologic system (dysfunction), the pituitary gland (diabetes insipidus) and the nervous system. Sites such as the spleen (dysfunction), lymph nodes, subcutaneous tissues overlying a bony lesion (pain), urinary tract, eye (uveitits, exophthalmos) and ear (chronic infections or discharge) may also be involved. General symptoms like fever, weakness and failure to thrive may be present.6,7 Not all children have all of these involvements, and although no single prognostic indicator is accepted, factors predicting a poor prognosis appear to be young age (<1 yr), signs of organ dysfunction (liver, bone-marrow, lung or digestive involvement), or dysfunction of one of the vital organs (liver, lung or bone marrow) or involvement of more than 3 organs, or a combination of these.1,6,7
Solitary bone involvement in contrast to the multisystem form, has the best prognostic result. LCH involving bone at a single site (formerly eosinophilic granuloma) tends to be self-limiting. LCH accounts for less than 1% of bone tumours, and up to 80% of children will present before they reach the age of 10 years. Involvement of the spine has been reported to occur in 7%–10% of cases.8,9,10
Despite the relative infrequency of LHC, solitary LCH is the commonest form, accounting for 60%–80% of cases. Detection is important to manage solitary LCH properly.11 Acute awareness is required for diagnosis, and the Langerhans cell is essential for the diagnosis of LCH, particularly in its solitary form. The Langerhans cell is thought to be derived from the bone marrow and is related to the mononuclear phagocyte.9 Definitive diagnosis of LCH is made by histopathological evidence of Birbeck granules and immunohistochemical detection of S-100 and CD1a antigens in the tissue samples.3,11
In addition to a clinical examination, the investigations, usually dictated by the organ system(s) involved, consist of laboratory investigations (complete blood count, hepatic function testing, inflammatory markers, measurement of blood and urine osmolarities, and hormone levels such as antidiuretic hormone, thyroid stimulating hormone and growth hormone).6 To help confirm the diagnosis, multiple imaging modalities have been recommended, such as CT and MRI, a skeletal survey and tissue diagnosis.1,4 Myelography has also been used in the past.10 Technetium bone scintigraphy is not recommended as a screening tool because of its poor sensitivity.1
Treatment methods for vertebral involvement have been variable, yet all have had successful outcomes.12 The goals of treatment include spinal stability, preservation of neurologic function and eradication of the lesion. Some authors recommend conservative treatment with biopsy and immobilization and the use of steroids when there is no neurologic deficit.9,10,12 However, the treatment of children with neurologic deficit is more controversial. Some have advocated that immobilization and radiation are appropriate in children with mild neurologic deficit.12 Others believe that the neural elements should be decompressed and then fused, with the debatable addition of radiation or chemotherapy. Still others believe that radiation is unnecessary except when the disease continues to progress,1 and there are those who believe that chemotherapy is justified for multiple lesions or involved visceral organs, or when surgery and radiation have failed, but it is not recommended in cases of solitary LCH.1,10,12
Several studies of solitary osseous LCH have shown that resolution occurred at a rate unaffected by the mode of treatment and this reinforces the opinion that solitary LCH is a self-limiting process.1 Hence there is no clear evidence that treatment affects the natural history of this entity.4
In this study we compare the treatment regimen and outcome of children with vertebral LCH at a tertiary care pediatric centre and compare it to those reported in the literature.
Methods
With the use of the International Classification of Diseases, Ninth Revision diagnostic codes, we reviewed charts of patients admitted between 1980 and 2003 to the Children's Hospital of Eastern Ontario (a tertiary care pediatric centre) that were coded with the following: eosinophilic granuloma (EG), histiocytosis X, vertebra, spine and vertebra plana. Fifty-five charts were retrieved, but in only 8 cases were the patients under 18 years of age with the diagnosis of histiocytosis X, LCH or EG with vertebral involvement. All other charts were excluded.
Information collected from the charts included demographic data, date of birth, age, sex, medical history, symptoms, neurologic and radiologic findings, visceral involvement, additional imaging modalities such as skeletal survey, CT, MRI and bone scanning (Table 1). Procedures such as biopsies, repeat biopsies, biopsy results, resection and fusion were noted. In addition, laboratory investigations such as leukocyte counts and erythrocyte sedimentation rates were recorded. The working diagnosis and the histologic findings were noted. Treatment was identified as requiring a brace, steroids, radiation and or chemotherapy. Outcome was evaluated on follow-up visits and classified as improved, resolved or worse. Follow-up interval was also noted. Data were collected when available in the chart, and not all data collected are present in Table 1. In addition to the chart review, the imaging was reviewed in all 8 cases by both a pediatric radiologist and a pediatric orthopedic surgeon.
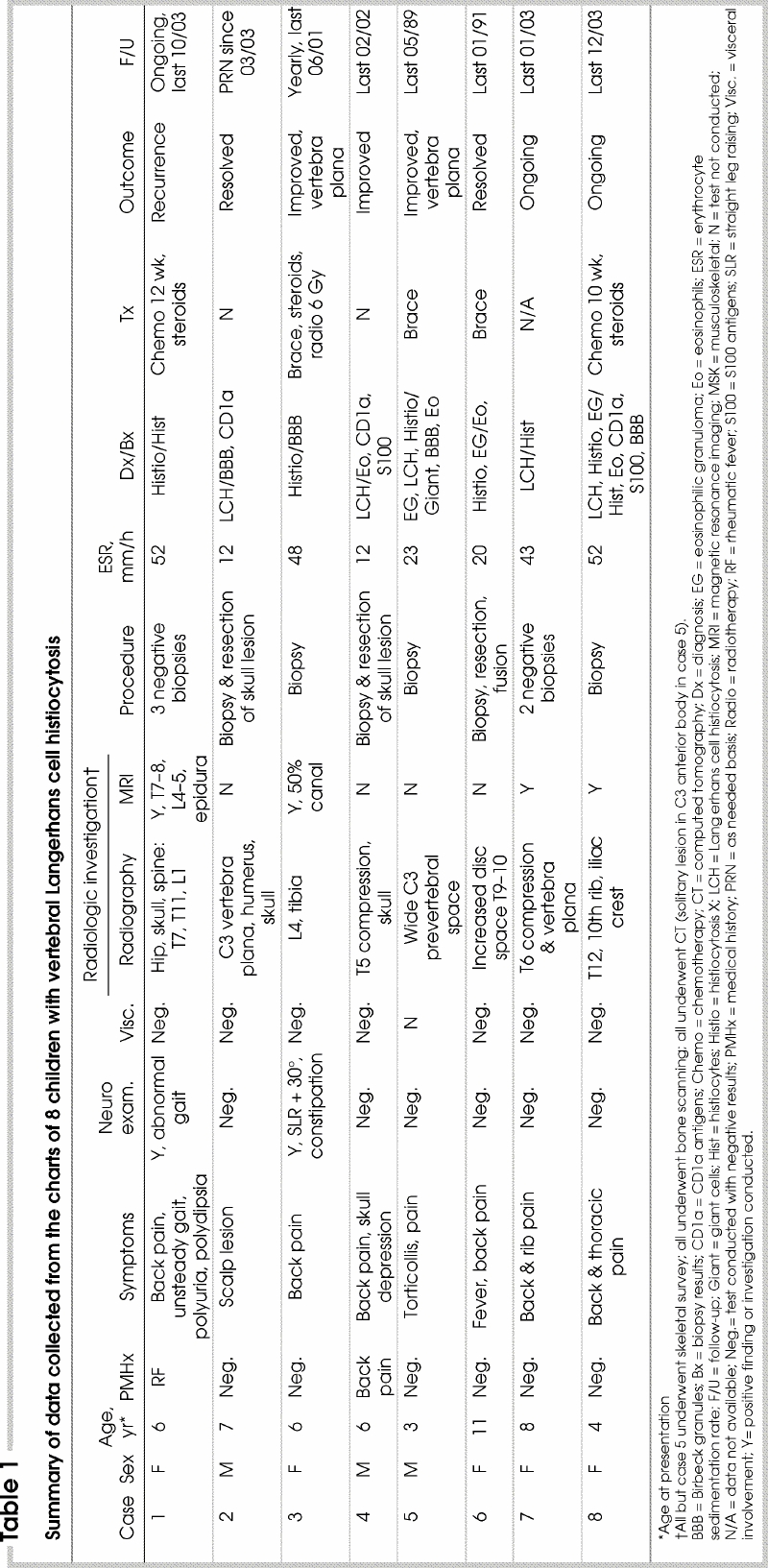
Table 1
A comprehensive literature search was conducted using Ovid MEDLINE, and all data were compared with available recent literature (Table 2 4,5,9,10,12,13,14).
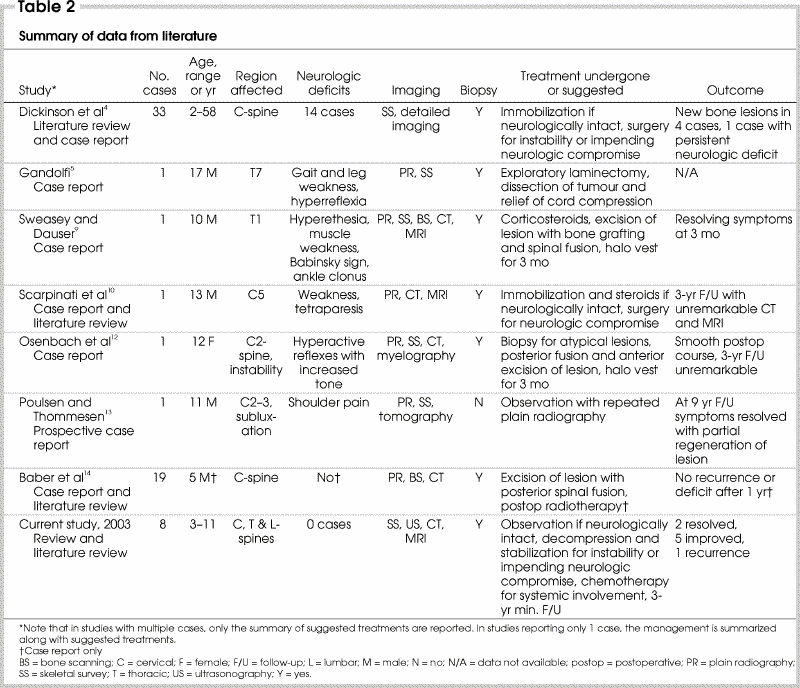
Table 2
Results
The 8 patients (3 boys, 5 girls) ranged in age from 3 to 11 years (mean 6.4 yr). The most common presenting symptom was back or neck pain (7 children). The remaining child had only a lesion on the scalp, found incidentally. Findings suggestive of neurologic compromise were noted in 2 children on physical examination: 1 child had an abnormal gait and polyuria, the other had a positive straight-leg-raising test at 30° and constipation.
Radiologic investigations consisted of plain radiography, bone scanning and CT in all cases. Skeletal survey and ultrasonography to determine visceral involvement was performed in 7 children and MRI was performed in 4 (Fig. 1, Fig. 2). Multiple bony and systemic lesions were found in 7 children, and 1 had a single solitary lesion located at C3. A total of 11 thoracic vertebrae were involved, ranging from T5 to T11. Two cervical vertebrae were involved, affecting C3 in both cases, and 2 lumbar vertebrae were involved, affecting L1 and L4. Vertebra plana was noted in 4 children, involving the vertebral body of L4, T6 and the vertebral body of C3 (2). In both children who presented with possible neurologic symptoms, investigations confirmed that the epidura was involved in 1, and 50% canal compromise was found in the other (Fig. 1).
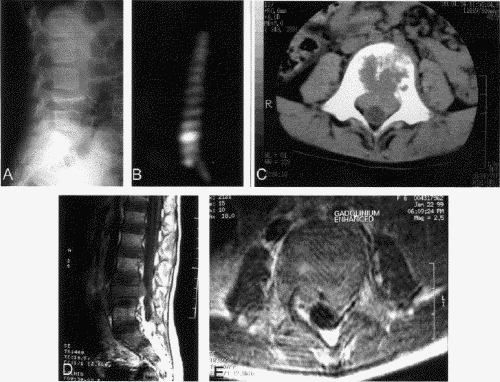
FIG. 1. Case 3. There is 50% canal compromise at the L4 vertebra. The lateral radiograph (A) demonstrates the classic vertebra plana of the anterior vertebral body of L4. The bone scan (B) shows uptake of contrast at L4. CT (C) and MRI (D,E) images further demonstrate the degree of canal compromise at this level.
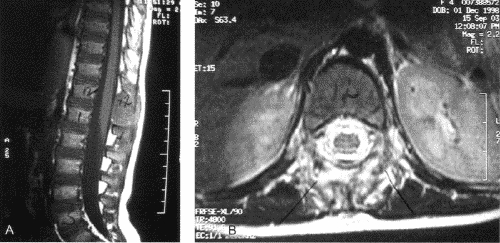
FIG. 2. Case 8. Sagittal (A) and transverse (B) MR images demonstrate the solitary lesion of Langerhans cell histiocytosis involving the spinous process of T12 as well as the paraspinal soft tissues.
Procedures consisted of a biopsy attempt in all cases; however, positive results were found in only 6 children; multiple attempts were made in the other 2, and all gave negative results. Laboratory investigations at the time of presentation were variable. The erythrocyte sedimentation rate (ESR) was measured routinely and found to range from 12 to 52 mm/h in the 8 children.
Diagnosis was based on positive biopsy results when available, and in the 2 children with negative results was correlated with the clinical presentation. In 6 children the diagnosis was LCH exhibiting multiple lesions and in 2 as LCH with solitary lesions. Tissue biopsies demonstrated histiocytes, Langerhans cells, eosinophils, giant cells and Birbeck granules (Fig. 3).
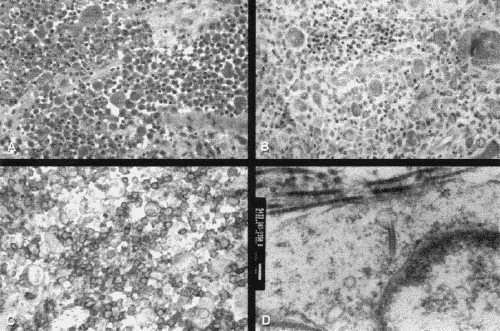
FIG. 3. Case 8. Histologic sections show an atypical histiocytic infiltrate (A; hematoxylin-phloxine-saffron stain, original magnification х25), eosinophils in the background (B; Giemsa stain, original magnification х25), positive CD1a staining (C; original magnification х25) and Birbeck granules on electron microscopy (D; original magnification х55 000).
Bracing was used in 3 cases and consisted of either a body cast followed by a thoracolumbar sacral orthosis, a soft-tissue collar or a Jewett brace. The duration of bracing varied according to resolution of symptoms. Surgery for vertebral fusion was performed in 1 child with a thoracotomy and débridement of the lesion followed by anterior strut grafting. Adjuvant therapy consisted of the use of dexamethasone in 1 child and prednisone in 2 children. One child had radiotherapy (6 Gy). Two children received chemotherapy with vinblastine. One of them required a repeat 12-week course 6 years after the first.
Follow-up varied from 3 to 5 years (mean 3.4 yr). Three children are still undergoing scheduled follow-up, and 1 has a recurrence. Three children improved such that they had follow-up on a yearly or as-needed basis, and 2 had resolution of their lesions with follow-up as needed (Table 1).
Discussion
Our review suggests that treatment of LCH in the spine in the absence of multisystem disease or instability is treated with immobilization and observation. Surgery is reserved for patients with instability or neurologic deficit,1,4,8,10 and chemotherapy is reserved for systemic involvement.
The difficulty seems to be in making the diagnosis, given that the pathognomonic clinical picture for LCH has been questioned as a result of the presentation of unusual cases.12,13 Although some maintain that radiographically typical lesions do not require biopsy and can safely be followed with serial radiography, most advocate a tissue diagnosis.12 In our study, all of the children underwent biopsy, but the findings in 2 were inconclusive. In such cases, we believe that other “menacing” entities should be eliminated and the diagnosis then based on the clinical picture. In both children LCH was diagnosed; the treatment regimen was chemotherapy for systemic involvement in 1 child and observation in the other. Both are still being followed-up.
LCH involving the vertebra has no particular predilection for race, age or sex.2 However, the results of our review are in keeping with the current literature, which states that 80% of cases occur in children younger than 10 years of age.8 Our study revealed a female preponderance, which contradicts the sex distribution of other studies.1 Two cases of LCH involving the C3 vertebra were identified, whereas C2 was most often involved in a study of 39 cervical vertebrae by Dickinson and Farhat.4
The most common presenting complaint (in 7 children) in this series was back or neck pain,8,14 and despite several studies reporting a history of trauma before presentation,8,9 only 2 children presented after minor trauma. This is in keeping with the findings in other studies that trauma is usually absent but that minor trauma may induce pain by causing a pathological fracture.12
One study has shown that the ESR is elevated, findings that are similar to ours.13 However, in the study of Bertram and associates1 the ESR was not elevated, a finding that is in keeping with the findings in the majority of studies reporting inconclusive laboratory results.
In our study the thoracic spine was most often involved, in agreement with reports in the literature.1,8,12 In 1995 there were only 35 documented cases of vertebral LCH, and only 3 of solitary LCH were found to have caused spinal cord compression.10 The cord was not involved in any of our patients. However, the epidura was involved in 1 child and there was 50% canal compromise in another.
With respect to imaging, all of our children underwent CT, and MRI was performed in 4 children when the degree of involvement was questionable or when there was clinical evidence of spinal cord compression. A recent study advocates MRI as the procedure of choice for staging multifocal LCH in the skull and central nervous system, as well as for monitoring response to therapy.15
Three children with systemic involvement in our study received chemotherapy, and this regimen is advocated by others.1,10,12 Similarly, only 1 child underwent radiotherapy. The use of radiation has fallen out of favour despite having been reported to be as effective as immobilization or surgery. Radiotherapy has been shown to promote healing and lead to a more rapid reduction in pain than other modes of treatment. Whether radiation adversely affects the enchondral ossification centres and limits the regrowth potential of the vertebral body continues to be debated.12 However, recent literature supports the notion that radiotherapy can destroy the vertebral end plates and pose a risk for secondary malignancy or radiation myelitis.1 Although our findings do not adequately reflect the use of radiotherapy, it should not be use for all cases of LCH but may be of value for cases in which the disease continues to progress.1
Only 1 child required surgery, through a right thoracotomy with débridement of the body of T9 followed by anterior strut grafting. Although surgery for instability is recommended by others,1,4,9,12 it is not clear what criteria they used for instability.
Follow-up investigations will depend on the nature and location of the presenting lesion. It is recommended that the imaging investigation that demonstrates the abnormal finding at the time of diagnosis be repeated at routine follow-up visits. However, for the majority of our patients, radiography has been the method of choice, and MRI has been advocated for assessing the response to treatment.15
Outcome results of our study demonstrate that over an average 3-year follow-up, 2 patients have had resolution of their lesions, 5 have improved, and 1 patient has had a recurrence. Similar studies suggest that follow-up of at least 4 years is required and advise long-term follow-up of these patients.16 The 2 children who presented with clinical findings suggestive of neurologic involvement did not have any evidence of spinal cord or nerve involvement on imaging.
Potential problems with this study are those attributed to retrospective reviews and the small case sample (owing to the rarity of LCH involving the vertebra). Also, our study population had predominantly disseminated LCH. Some may argue that this confounds the results; however, there is no clear evidence to indicate that these may be 2 distinct entities. It may also indicate that our observations are of a different population from those of the other studies. Further, most of the data were collected prior to the nomenclature adopted in 1997, so the diagnosis reported could possibly be different.
Summary and conclusions
Our results support the concept of solitary osseous LCH as a benign self-limiting disorder when systemic disease is absent and suggest therapeutic conservatism as recommended by others.9,10,12,17 Based on our results and review of the literature (Table 2), we recommend that any child with suspected solitary LCH of the vertebra undergo a full diagnostic investigation consisting of a skeletal survey and abdominal ultrasonography to rule out multiple lesions. Although bone scanning is not sensitive for LCH,1 it is also recommended because it is important to rule out other diagnoses. CT is warranted for the vertebral lesion followed by MRI if there is any question of neural involvement. A biopsy is highly recommended for a diagnosis at the time of presentation and should be attempted in any suspicious lesion that is easily accessible using the least invasive technique such as fine-needle aspiration. However, in the case of solitary LCH involving a remote region such as the anterior vertebral body, a CT-guided biopsy or an open biopsy would be more appropriate. If there is any neural involvement, then decompression and stabilization are warranted. There is no role for routine radiotherapy. However, there may be a role for radiotherapy when the disease continues to progress. Chemotherapy is reserved for multiple systemic lesions. Follow-up should be a minimum of 3 years or until symptoms or the lesion has resolved. Follow-up investigations should be individualized but should consist of radiography and MRI. Bracing and the use of steroids were not consistently used in our review, and so their value in the treatment regimen cannot be evaluated.
Competing interests: None declared.
Correspondence to: Dr. Christopher W. Brown, Division of Orthopaedic Surgery, Children's Hospital of Eastern Ontario, 401 Smyth Rd., Ottawa ON K1H 8L1; fax 613 737-8837; cbrownone@hotmail.com
Accepted for publication July 23, 2004
References
- 1.Bertram C, Madert J, Eggers C. Eosinophilic granuloma of the cervical spine [review]. Spine 2002;27:1408-13. [DOI] [PubMed]
- 2.Hanapiah F, Yaacob H, Ghani KS, Hussin AS. Histiocytosis X: evidence for a genetic etiology. J Nihon Univ Sch Dent 1993;35: 171-4. [DOI] [PubMed]
- 3.Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, et al. Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society [review]. Med Pediatr Oncol 1997;29:157-66. [DOI] [PubMed]
- 4.Dickinson LD, Farhat SM. Eosinophilic granuloma of the cervical spine. A case report and review of the literature. Surg Neurol 1991;35:57-63. [DOI] [PubMed]
- 5.Gandolfi A. Vertebral histiocytosis-X causing spinal cord compression. Surg Neurol 1983;19:369-72. [DOI] [PubMed]
- 6.Donadieu J. Langerhans cell histiocytosis. Orphanet Encyclopedia May 2003. Available: www.orpha.net/data/patho/GB/uk-LCH.pdf (accessed 2005 Apr 27).
- 7.Egeler RM, Nesbit ME. Langerhans cell histiocytosis and other disorders of monocyte-histiocyte lineage. Crit Rev Oncol Hematol 1995;18:9-35. [DOI] [PubMed]
- 8.Bilge T, Barut S, Yaymaci Y, Alatli C. Solitary eosinophilic granuloma of the lumbar spine in an adult. Case report. Paraplegia 1995;33:485-7. [DOI] [PubMed]
- 9.Sweasey TA, Dauser RC. Eosinophilic granuloma of the cervicothoracic junction. Case report. J Neurosurg 1989;71:942-4. [DOI] [PubMed]
- 10.Scarpinati M, Artico M, Artizzu S. Spinal cord compression by eosinophilic granuloma of the cervical spine. Case report and review of the literature. Neurosurg Rev 1995;18:209-12. [DOI] [PubMed]
- 11.Puzzilli F, Mastronardi L, Farah JO, Ruggeri A, Lunardi P. Solitary eosinophilic granuloma of the calvaria. Tumori 1998;84:712-6. [DOI] [PubMed]
- 12.Osenbach RK, Youngblood LA, Menezes AH. Atlanto-axial instability secondary to solitary eosinophilic granuloma of C2 in a 12-year-old girl. J Spinal Disord 1990;3:408-12. [PubMed]
- 13.Poulsen JO, Thommesen P. An unusual case of histiocytosis X in the spine. Acta Orthop Scand 1976;47:59-62. [DOI] [PubMed]
- 14.Baber WW, Numaguchi Y, Nadell JM, Culicchia F, Robinson AE. Eosinophilic granuloma of the cervical spine without vertebrae plana. J Comput Tomogr 1987;11:346-9. [DOI] [PubMed]
- 15.Moore JB, Kulkarni R, Crutcher DC, Bhimani S. MRI in multifocal eosinophilic granuloma: staging disease and monitoring response to therapy. Am J Pediatr Hematol Oncol 1989;11:174-7. [PubMed]
- 16.Sessa S, Sommelet D, Lascombes P, Prevot J. Treatment of Langerhans-cell histiocytosis in children. Experience at the Children's Hospital of Nancy. J Bone Joint Surg Am 1994;76:1513-25. [DOI] [PubMed]
- 17.Sartoris DJ, Parker BR. Histiocytosis X: rate and pattern of resolution of osseous lesions. Radiology 1984;152:679-84. [DOI] [PubMed]


