Abstract
Objective
We carried out a retrospective cohort study at the Ottawa Hospital–Civic Campus to determine the proportions of patients referred for and provided adjuvant therapy for colorectal cancer (CRC) among those eligible according to published clinical practice guidelines.
Method
Patients with stage III colon or stage II or stage III rectal cancer who had had potentially curative surgical resection for CRC and were seen at the Ottawa Hospital during 1999 and 2000 were eligible. We noted the number of medical or radiation oncology consultations, or both, and the subsequent receipt of adjuvant chemotherapy or radiotherapy, or both.
Results
Of 158 eligible patients, 135 (85%) had medical or radiation oncology consultations, or both. Of the total, 104 were less than 75 years of age and of these 99 (95%) were referred; of the 54 patients 75 years of age or older, 36 (67%) were referred. Of the 158 patients, 113 (72%) received adjuvant therapy, 90 (87%) eligible patients aged less than 75 years and 23 (43%) older patients. Increasing age and the presence of comorbidity were independent predictors of nonreferral and nontreatment. Gender and cancer site (colon or rectum) were not significant predictors of referral for, or receipt of, adjuvant therapy in general.
Conclusions
The observed rates of referral for and receipt of adjuvant therapy for CRC are greater than generally published and appear reasonably concordant with current clinical practice guidelines, but optimal rates are undefined. Older patients and those with comorbidity were less likely to be referred and treated. However, our knowledge of the factors important to the process of clinical decision-making about adjuvant therapy for CRC is incomplete, and there may be patients, especially older ones, for whom adjuvant therapy would be appropriate but who are not being referred or treated.
Abstract
Objectif
Nous avons effectué une étude de cohorte rétrospective au Campus civique de l'Hôpital d'Ottawa afin de déterminer quelle est la proportion des patients admissibles, selon les guides de pratique publiés, à un traitement adjuvant contre le cancer colorectal (CCR), qui ont été référés pour recevoir ce traitement et qui l'ont bel et bien reçu.
Méthode
Étaient admissibles les patients atteints d'un cancer du côlon de stade III ou d'un cancer du rectum de stade II ou III qui avaient subi une exérèse possiblement curative contre le CCR et ont été suivis à l'Hôpital d'Ottawa en 1999 et en 2000. Nous avons pris note du nombre de consultations médicales, de consultations en radio-oncologie, ou des deux, ainsi que de l'administration subséquente d'une chimiothérapie ou d'une radiothérapie adjuvante, ou des deux.
Résultats
Au nombre des 158 patients admissibles, 135 (85 %) s'étaient présentés à des consultations médicales ou à des consultations en radio-oncologie, ou aux deux. Au total, 104 avaient âgés de moins de 75 ans, dont 99 (95 %) ont été référés pour le traitement. Parmi les 54 patients âgés de 75 ans ou plus, 36 (67 %) ont été référés. Des 158 patients, 113 (72 %) ont reçu un traitement adjuvant, soit 90 (87 %) des patients admissibles âgés de moins de 75 ans et 23 (43 %) des patients plus âgés. L'âge croissant et la comorbidité étaient des prédicteurs indépendants de la non-référence et du non-traitement. En général, le sexe et le site du cancer (côlon ou rectum) n'étaient pas des prédicteurs significatifs de la référence pour un traitement adjuvant ou de l'administration du traitement.
Conclusions
Les taux observés de référence et d'administration du traitement adjuvant contre le CCR, sont supérieurs à ceux qui sont généralement publiés et semblent cadrer relativement bien avec les recommandations des guides de pratique en vigueur, encore que les taux optimaux ne soient pas définis. Les patients plus âgés et ceux présentant des troubles concomitants étaient moins susceptibles d'être référés, puis traités. Ceci dit, nos connaissances sur les facteurs qui revêtent de l'importance dans le processus de décision clinique au sujet du traitement adjuvant contre le CCR sont incomplètes, et il pourrait y avoir des patients, surtout parmi les plus âgés, chez qui le traitement adjuvant serait approprié, mais qui ne sont pas référés ou traités en conséquence.
Colorectal cancer (CRC) is the most common malignant lesion of the gastrointestinal tract and the second leading cause of cancer death in North America. Surgical resection is the primary therapy, and in patients at moderate or high risk of recurrence adjuvant therapy improves survival. The conclusions of a National Institutes of Health Consensus Conference about the role of adjuvant therapy after resection of CRC were published in 1990.1 In 1997 and 1998 Cancer Care Ontario (CCO), the provincial cancer agency for Ontario, published clinical practice guidelines (CPGs) recommending that adjuvant therapy be considered in patients with resected stage III colon cancer (chemotherapy) and stage II or stage III rectal cancer (combined chemoradiotherapy).2,3 Despite the considerable human and financial resources expended on their development and dissemination, the impact of these CPGs on clinical practice has not been evaluated, and we do not know how closely current clinical practice reflects them. A number of reports suggest that in the past adjuvant therapy has been underused, particularly in specific demographic groups such as older patients.4,5,6,7,8 The primary aim of this study was to evaluate the concordance of recent clinical practice with these CPGs by determining the proportion of patients eligible for adjuvant therapy who were referred for oncology consultation after surgical resection for CRC, and the proportion who actually received adjuvant therapy. Our secondary aim was to examine possible predictors of referral and treatment, including age, comorbidity and sex.
Methods
We conducted a retrospective cohort study at The Ottawa Hospital and the Ottawa Regional Cancer Centre (ORCC). The Ottawa Hospital was created in a recent merger of 2 adult teaching hospitals and 2 community hospitals, and accounts for the majority of CRC resections in Eastern Ontario. The ORCC is the tertiary cancer treatment facility that provides virtually all adjuvant therapy in Eastern Ontario. To identify all patients who had potentially curative resection for CRC at The Ottawa Hospital during 1999 and 2000, the following records were reviewed: inpatient health, pathology and operating room. For all potentially eligible patients identified, surgical pathology reports were reviewed to determine who had tumour and regional lymph-node status consistent with stage III colon cancer or stage II or stage III rectal cancer. The Ottawa Hospital and ORCC records for these patients were reviewed for information about distant metastases to complete TNM staging. For the eligible patients, we abstracted the following from Ottawa Hospital and ORCC records: age, birthdate, sex, residence postal code, comorbidity (Charlson Comorbidity Index),9,10 cancer site, surgical procedure, hospital admission and discharge dates, survival to discharge and date of surgery. The Charlson Comorbidity Index is a validated measure of comorbidity, originally developed as an index of operative risk. The presence or absence of a series of defined comorbidities is evaluated, and the weights assigned to each comorbidity are added to yield a summary score. We determined from ORCC records whether patients who were eligible for adjuvant therapy by cancer stage and were discharged alive from hospital had had medical or radiation oncology consultation, or both, and whether they subsequently received adjuvant chemotherapy or radiotherapy, or both.
We selected a 2-year period for review based on the number of cases required for adequate statistical modelling of covariates and the expectation that 250 patients eligible for adjuvant therapy would be identified, with 170 referred and 80 not referred. Proportions were compared by χ2 testing (Systat version 10; SPSS, Chicago). Age and comorbidity were evaluated as dichotomous variables using an arbitrary age cutoff point of 75 years and comorbidity coded as present (Charlson score ≥1) or absent. Age and comorbidity were also evaluated as continuous variables in ordinal logistic regression models where the dependent variable was defined to have 3 ordinal levels (not referred, referred but not treated, referred and treated), and age was evaluated as a nonlinear function using a smoothing spline (R version 1.7.1; The R Foundation for Statistical Computing, Vienna).
Results
During 1999 and 2000 at The Ottawa Hospital, 565 patients had surgical resection for CRC. Of these, 158 had stage III colon (n = 104) or stage II or stage III rectal cancer (n = 54) and survived to discharge (Table 1).
Table 1
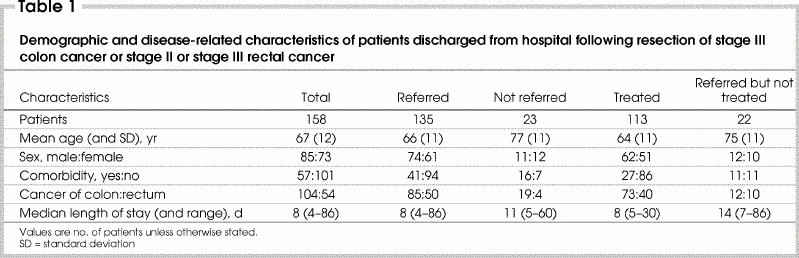
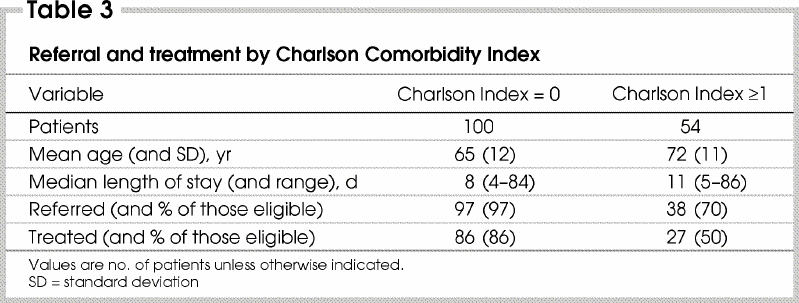
Medical oncology consultations were obtained for 130 patients (82% of those eligible for adjuvant therapy). The proportions were very similar for colon and rectal cancer. Forty-four patients with rectal cancer (81%) had radiation oncology consultations. Overall, 135 patients (85%) (95% confidence interval [CI] 78%–91%) had medical or radiation oncology consultation, or both: colon cancer 85 (82%) and rectal cancer 50 (92%). Thirty-nine (72%) patients with rectal cancer had both medical and radiation oncology consultations. On univariate analysis, patients not referred for medical oncology consultation were older (p < 0.001) and had greater comorbidity (p = 0.01) than those who were referred (Table 2 and Table 3). The pattern of referral for radiotherapy consultation was similar. Age and comorbidity were independent predictors of referral for medical or radiation oncology consultation on multivariate analysis. Among the 104 patients less than 75 years of age, 99 (95%) were referred for either medical or radiation oncology consultation, compared with 67% (36 of 54) of patients aged 75 years or older (p < 0.001).
Table 2
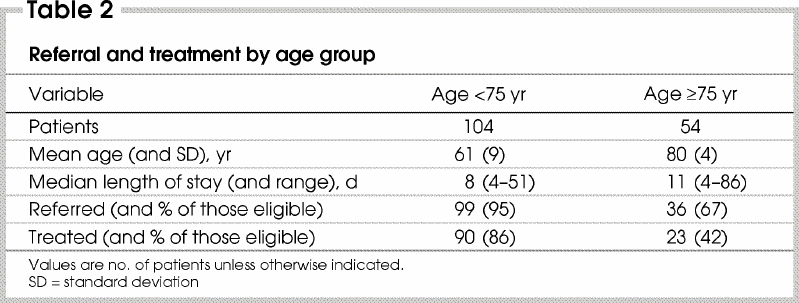
Table 3
Of the 158 patients eligible for adjuvant therapy, 107 (68%) received adjuvant chemotherapy: 73 (70%) with colon cancer and 34 (63%) with rectal cancer. They were younger (p < 0.001), had less comorbidity (p < 0.001) and shorter postoperative hospital stay (p < 0.01) on univariate analysis than patients who did not receive adjuvant chemotherapy. Thirty-seven patients with rectal cancer received adjuvant radiotherapy (68% of those eligible). Patients who received adjuvant radiotherapy were younger (p < 0.05) than those who did not but did not differ with respect to comorbidity. Patients who received adjuvant therapy (113 [72%] of the 158 eligible patients) (95% CI 62%–79%) were younger (p < 0.001) and had less comorbidity (p < 0.001) than patients who did not receive adjuvant therapy. As with referral, age and comorbidity were independent predictors on multivariate analysis. Of the 104 patients less than 75 years of age, 90 (86%) received adjuvant therapy (91% of those referred), compared with 23 (43%) of the 54 patients 75 years of age or older (64% of referred, p < 0.001).
The influence of age on referral and treatment was not linear (Fig. 1, Fig. 2). Its effects were minor until about age 70 years, after which there were progressive declines in the rates of referral and treatment that were not accounted for by comorbidity. Sex was not a significant predictor of referral or treatment. (The coefficients and p values of the variables included in the ordinal logistic regression are available from the authors.)
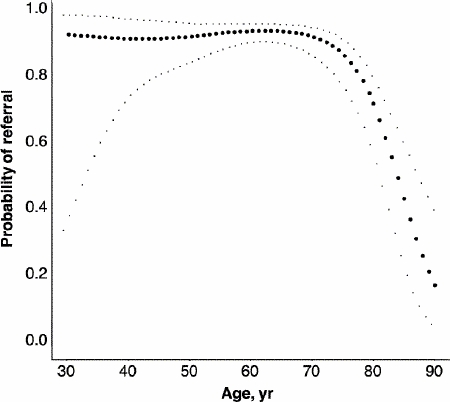
FIG. 1. The probability of referral for adjuvant therapy as a function of patient age (filled circles) with 95% confidence limits (light dots) derived in a generalized additive model. The curve is qualitatively similar when other covariates are included in the model.
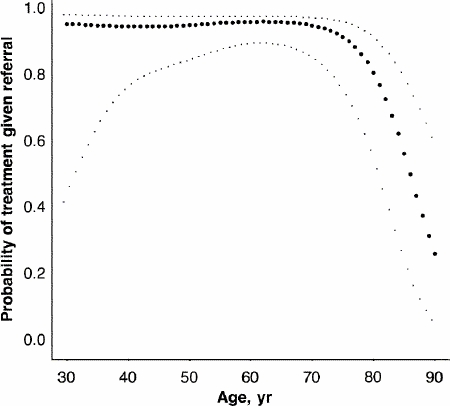
FIG. 2. The probability of adjuvant treatment (given referral) as a function of patient age (filled circles) with 95% confidence limits (light dots) derived in a generalized additive model. The curve is qualitatively similar when other covariates are included in the model.
Discussion
Colorectal cancer is a common cancer and one for which the benefits of advances in therapy may not be fully realized.11,12,13,14 Following the establishment of a scientific consensus about the survival benefit of adjuvant therapy for CRC, CPGs were developed by CCO and disseminated to surgeons and oncologists in Ontario in 1997 and 1998 as part of an ongoing program of evidence-based clinical care. However, there is little information about how closely clinical practice may have come to reflect the best available evidence as represented by these CPGs. Several reports examining clinical practice in other jurisdictions up to 1997 indicate that adjuvant therapy for CRC was underutilized, particularly in the elderly.4,5,6,7,8 There is also recent information to suggest that surgeons are not fully conversant with the evidence base for the care of patients with CRC.15 We conducted a retrospective cohort study in a single institution, identifying eligible patients by review of separately maintained inpatient health records, pathology records and operating room records. Population-based data would be desirable, and our findings may not be generalizable, but the 2 adult sites of the Ottawa Hospital represent the major provider of secondary and tertiary surgical care in Eastern Ontario, with a staff of 17 general surgeons with both community and academic backgrounds. Moreover, Ontario Cancer Registry data demonstrate that fewer than 1% of patients with CRC who reside in Eastern Ontario register at cancer centres elsewhere in the province. Thus, we believe that we have identified essentially all eligible patients and all patients who have had perioperative oncology consultations or adjuvant therapy, or both.
CPGs are systematically developed statements designed to assist and facilitate practitioner and patient decisions in specific clinical circumstances.16,17,18 We observed a referral rate of 85% for oncology consultation in this study. This observation is consistent with a high level of use of the CPGs in clinical practice. However, it is not possible to evaluate retrospectively how decisions about referral and adjuvant treatment were reached, what factors were considered and how they were weighed. For this and other reasons, caution must be used in applying CPGs as performance measures or standards.19 The rates of adjuvant treatment we observed in this study (chemotherapy 68%, radiotherapy 69% are greater than those in reports from other jurisdictions based on data acquired by a variety of methodologies, none describing treatment provided more recently than 1997.4,5,6,7 The rates of treatment we observed are quite similar to the rates identified in a recent, population-based study (chemotherapy 67%, radiotherapy 64%).8
The treatment rate we observed was modestly lower than the referral rate (i.e., not all referred patients were treated). This may reflect either oncologists' judgement or patient preference. Although the observed rates of referral and treatment appear reasonable, it is not possible with this study design to draw any causal inference about the impact of the publication of the CPGs by the CCO.
Several recent reports of aggregated clinical trial and population-based data confirm that the survival benefit for patients 65 years and older is equivalent to that in younger patients.20,21,22,23 Moreover, in a recent meta-analysis, neither survival benefit nor toxicity differed significantly in patients more than 70 years of age compared with younger patients.20 Since the incidence of CRC increases with age, it has been proposed that large numbers of older patients who have had surgical resection could derive worthwhile survival benefit from adjuvant therapy.23 Despite these observations or perhaps because of their publication only relatively recently, older patients in the cohort we studied were significantly less likely to be referred for and receive adjuvant therapy. The potential for greater use of adjuvant therapy in elderly patients should be explored by acquiring a better understanding of age-related risk factors for toxicity and adverse functional effects, the impact of comorbidity and competing risks on the survival benefit, and the basis on which elderly patients and their clinicians decide about adjuvant therapy.24,25
In summary, in a retrospective cohort of patients, rates of referral and adjuvant treatment following resection of colorectal cancer were 85% and 72%, respectively. Older age and the presence of comorbidity were consistent predictors of nonreferral and nontreatment. These observations are concordant with current CPGs. However, our knowledge of the factors important to, and the process of, clinical decision-making about adjuvant therapy for CRC is incomplete. There may be patients, especially older patients, for whom adjuvant therapy would be appropriate but who are not being referred or treated.
Sharon Ong is a resident in the Program in General Surgery, University of Ottawa.
Presented as a poster at the 2003 Canadian Surgery Forum, Vancouver, BC, Sept. 18–21, 2003
Acknowlegements: We acknowledge the contributions of Susan M. Kirkpatrick, RN, BScN, and James Jaffey, MSc. The work was supported by a grant from the Ottawa Regional Cancer Centre Foundation.
Competing interests: None declared.
Correspondence to: Dr. James M. Watters, Ottawa Hospital — Civic Campus, 737 Parkdale Ave., CPC 208, Ottawa ON K1Y 1J8; jwatters@ohri.ca
Accepted for publication June 14, 2004
References
- 1.NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264:1444-50. [PubMed]
- 2.Figueredo A, Fine S, Maroun J, Walker-Dilks C, Wong S, and the Gastrointestinal Cancer Disease Site Group. Adjuvant therapy for stage III colon cancer following complete resection. Cancer Prev Control 1997;1:304-19. [PubMed]
- 3.Figueredo A, Germond C, Taylor B, Maroun J, Agboola O, Wong R, et al. Postoperative adjuvant radiotherapy and/or chemotherapy for resected stage II or III rectal cancer. Available: www.cancercare.on.ca/access_1105.htm (accessed 2005 Apr 5).
- 4.Beart RW, Steele GD Jr, Menck HR, Chmiel JS, Ocwieja KE, Winchester DP. Management and survival of patients with adenocarcinoma of the colon and rectum: a national survey of the Commission on Cancer. J Am Coll Surg 1995;181:225-36. [PubMed]
- 5.Tropman SE, Hatzell T, Paskett E, Ricketts T, Cooper MR, Aldrich T. Colon cancer treatment in rural North and South Carolina. Cancer Detect Prev 1999;23:428-34. [DOI] [PubMed]
- 6.Jessup JM, McGinnis LS, Steele GD Jr, Menck HR, Winchester DP. The National Cancer Data Base Report on Colon Cancer. Cancer 1996;78:18-26. [DOI] [PubMed]
- 7.Roetzheim R, Pal N, Gonzalez E, Ferrante J, Van Durme D, Krischer J. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health 2000;90:1746-54. [DOI] [PMC free article] [PubMed]
- 8.Ayanian JZ, Zaslavsky AM, Fuchs CS, Guadagnoli E, Creech CM, Cress RD, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol 2003;21:1293-300. [DOI] [PubMed]
- 9.Charlson ME, Ales KA, Pompei P, Mackenzie CR. A new method of classification of prognostic comorbidity for longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [DOI] [PubMed]
- 10.Charlson ME, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47: 1245-51. [DOI] [PubMed]
- 11.Cancer Guidance Sub-Group of the Clinical Outcomes Group. Improving outcomes in colorectal cancer: the research evidence. London: NHS Executive, Department of Health; 1997.
- 12.Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst 2001;93:501-15. [DOI] [PubMed]
- 13.Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol 2002;20:1192-202. [DOI] [PubMed]
- 14.Midgley R, Kerr D. ABC of colorectal cancer: adjuvant therapy. BMJ 2000;321:1208-11. [DOI] [PMC free article] [PubMed]
- 15.Ward JE, Gattellari M, Soloman MJ. Management of patients with colorectal cancer. Do Australian surgeons know the scientific evidence? Arch Surg 2002;137:1389-94. [DOI] [PubMed]
- 16.Woolf S, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ 1999;318:527-30. [DOI] [PMC free article] [PubMed]
- 17.Grimshaw J, Russell I. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 1993;342:1317-22. [DOI] [PubMed]
- 18.Smith TJ, Hillner BE. Ensuring quality cancer care by the use of clinical practice guidelines and critical pathways. J Clin Oncol 2001;19:2886-97. [DOI] [PubMed]
- 19.Walter LC, Davidowitz NP, Heineken PA, Covinsky KE. Pitfalls of converting practice guidelines into quality measures: lessons learned from a VA performance measure. JAMA 2004;291:2466-70. [DOI] [PubMed]
- 20.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091-7. [DOI] [PubMed]
- 21.Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med 2002;136:349-57. [DOI] [PubMed]
- 22.Neugut AI, Fleischauer AT, Sundararajan V, Mitra N, Heitjan DF, Jacobson JS, et al. Use of adjuvant chemotherapy and radiation therapy for rectal cancer among the elderly: a population-based study. J Clin Oncol 2002;20:2643-50. [DOI] [PubMed]
- 23.Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol 2002;20:3992-8. [DOI] [PubMed]
- 24.Extermann M, Balducci L, Lyman GH. What threshold for adjuvant therapy in older breast cancer patients? J Clin Oncol 2000;18:1709-17. [DOI] [PubMed]
- 25.Watters JM, McClaran JC. Perioperative management of the elderly patient. In: Wilmore DW, Cheung LY, Harken AH, Holcroft JW, Meakins JL, editors. ACS surgery: principles and practice. New York: WebMD; 2002.


