Abstract
Fine-needle aspiration biopsy (FNAB) is considered a safe, reliable and cost-effective means of selecting thyroid nodules with risk for malignancy. However, there are limitations of this method including false positive/negative and “nondiagnostic” results that may be reduced by repeating FNAB.
Objective
To evaluate accuracy, sensitivity, specificity and costs of sequential FNAB in the management of thyroid nodular disease.
Methods
Charts of all patients who underwent thyroidectomy at a university teaching hospital in Toronto from 1998 to 2000 were reviewed. FNAB reports of “suspicious for malignancy,” “follicular lesion” and “cellular atypia” were considered to be positive. Data were analyzed with χ2 and z tests.
Results
There were 268 patients (225 women and 43 men; age range 18–89 yr; mean age 47 yr) who underwent a total of 449 FNABs (mean 1.7 FNABs/patient) within a year before thyroidectomy. Accuracy (63.8%), sensitivity (73.8%) and specificity (69%) were determined for single FNABs. Sequential FNAB increased the accuracy of method by 22.6%, sensitivity by 13.8% and specificity by 6.2%, with reduction of false positive/ negative results by 14.2% and “nondiagnostic” results by 100%. However, the costs of sequential cytology per patient were 70% higher than single FNAB.
Conclusions
Multiple FNABs are unpleasant for patients, but useful in the selection for treatment of patients with thyroid nodular diseases. Although sequential FNAB increases the costs of method, the improvement of precision of FNAB may imply a reduction in overall health-care costs.
Abstract
La biopsie par aspiration à l'aiguille fine (BAAF) est un moyen réputé sûr, fiable et rentable de sélection de nodules thyroïdiens présentant un risque de malignité. Toutefois, il y a des limitations à cette méthode, notamment des résultats faux positifs ou faux négatifs et «non diagnostiques», qui peuvent être réduites par la répétition de la BAAF.
Objectif
Évaluer l'exactitude, la sensibilité, la spécificité et les coûts de BAAF séquentielles dans la gestion des maladies à nodules thyroïdiens.
Méthodes
On a passé en revue le dossier de tous les patients ayant subi une thyroïdectomie à un hôpital universitaire de Toronto entre 1998 et 2000. Les rapports de BAAF présentant «une suspicion de malignité», «des lésions folliculaires» et «une atypie cellulaire» ont été considérés comme positifs. Les données ont été analysées à l'aide de tests χ2 et z.
Résultats
Au total, 268 patients (225 femmes et 43 hommes de 18 à 89 ans; moyenne d'âge de 47 ans) ont subi 449 BAAF (moyenne de 1,7 BAAF par patient) un an avant une thyroïdectomie. On a déterminé l'exactitude (63,8 %), la sensibilité (73,8 %) et la spécificité (69 %) des BAAF uniques. Les BAAF séquentielles ont amélioré l'exactitude de la méthode de 22,6 %, la sensibilité de 13,8 % et la spécificité de 6,2 %, tout en faisant diminuer les résultats faux positifs ou faux négatifs de 14,2 % et les résultats «non diagnostiques» de 100 %. Toutefois, les coûts de cytologies séquentielles par patient ont été 70 % plus élevés que pour une seule BAAF.
Conclusions
Les BAAF multiples sont désagréables pour les patients, mais utiles dans le choix du traitement des patients présentant des nodules thyroïdiens. Même si des BAAF séquentielles augmentent les coûts de la méthode, l'amélioration de la précision des BAAF peut amener une diminution des coûts globaux des soins de santé.
Martin and Ellis1 pioneered the puncture biopsy and aspiration of a thyroid nodule in 1930. Fine-needle aspiration biopsy (FNAB) of the thyroid was first reported in 1948. Since the 1970s, FNAB has been developed mainly by Scandinavian researchers at several European centres.2 Over the past 2 decades, FNAB has become widely acceptable as a diagnostic method for nodular thyroid disease.2 Currently, FNAB is considered a simple, safe, reliable and cost-effective means of screening thyroid nodules with risk for malignancy.3,4
Recent studies have demonstrated advantages of FNAB over open surgical and core-needle biopsy techniques. FNAB is not only more economical and has less morbidity than open surgical biopsy, but is also simpler, safer and better tolerated by patients than core needle or open surgical biopsies.5,6,7 FNAB nevertheless has limitations in the management of thyroid nodules, including results that are falsely positive / negative or “nondiagnostic.”3,8 Repeating FNAB may increase its accuracy when nondiagnostic results are obtained.9,10,11,12,13
This study was undertaken to evaluate and compare the accuracy, sensitivity, specificity and costs of sequential versus single FNAB in the management of thyroid nodular disease.
Methods
This retrospective study reviewed 268 consecutive patients who underwent thyroidectomy for nodular thyroid disease after undergoing FNAB for cytological examination. All patients were evaluated and underwent surgery from January 1998 through December 2000 at a teaching hospital in Toronto. Thyroid surgery was indicated when FNAB results showed cancer or were suspicious for malignancy. Furthermore, individuals with a benign diagnosis or a nondiagnostic FNAB result underwent thyroidectomy if they had rapid nodule growth, local compression symptoms or an associated risk factor based on gender, age and exposure to ionizing radiation.
All fine-needle aspirations were performed in outpatient clinics at the Mount Sinai Hospital or other centres by a trained surgeon (I.B.R.), cytologist or radiologist using sterile technique and no anesthesia. The standard technique includes the use of a 21- or 23-gauge hypodermic needle attached to a 20-mL disposable plastic syringe. Each nodule was aspirated at least twice. The aspirated material was smeared onto at least 3 glass slides per nodule, fixed immediately with 95% ethanol and then stained by means of the Papanicolaou method.
FNAB specimens were considered adequate if there was a minimum of 6 separate groups of at least 10 well-preserved follicular epithelial cells.14 Specimens with either no diagnostic cellular material or insufficient cells for a diagnosis were classified as nondiagnostic. Biopsy reports of “suspicious for malignancy,” “follicular lesion” and “cellular atypia” were considered to be positive, since they usually resulted in thyroid surgery.
To define false and true FNAB results in each case, cytological reports were compared with the corresponding histopathological examinations. An FNAB result diagnosed as benign that upon histopathologic examination revealed a carcinoma was considered a false-negative case. Conversely, a cytologic diagnosis of malignancy, revealed after surgical resection and histopathological examination to be a benign lesion, was defined as a false-positive.15
Sequential FNAB was defined as at least 2 cytological aspirations from the same patient on different dates during a follow-up period of up to 12 months, collected in the management of a thyroid nodular disease. FNAB was repeated during clinical follow-up for nodular thyroid disease whenever the previous FNAB was nondiagnostic and there was no other indication for thyroid surgery.
In many cases of sequential FNAB, aspirations were carried out by different physicians on different occasions. All cases were divided into 2 groups: patients who underwent 2 or more FNABs (the sequential-FNAB group, n = 110), and those who had a single FNAB (n = 268). Note that the latter group includes the first cytological evaluation from individuals who later underwent multiple (sequential) FNABs.
The accuracy, sensitivity and specificity of FNAB were estimated in both groups. Within the sequential-FNAB group, the result that most contributed to preoperative decision-making was chosen for analysis of its accuracy, sensitivity and specificity. Rates of false and true results, along with nondiagnostic reports, were also estimated in both groups.
Accuracy of FNAB was defined as the ratio between the number of true results and number of patients undergoing aspiration. Sensitivity was defined as the ratio between the number of patients with a positive cytology result and cancer at histology (true-positive results), and the number of patients with carcinoma at definitive histopathological report (true-positive plus false-negative results). Specificity was defined as the ratio between the number of patients with a negative cytological diagnosis as well as no tumour at histology (true-negative results) and the number of patients with no carcinoma at histology (true-negative plus false-positive results).
Accuracy, sensitivity and specificity of single and sequential FNAB were also calculated after exclusion of all incidental microcarcinomas (MC), defined as primary cancer of 10 mm or smaller (World Health Organization), that were found after thyroidectomy and study of the entire gland. These cases were further divided into subgroups with uninodular and multinodular disease, according to ultrasonographic evaluation of the thyroid.
Data were analyzed with χ2 and z tests (SigmaStat for Windows Version 2.03, SSPS Inc., Chicago, Ill.). Significance was assumed if p ≤ 0.05.
Results
The patients, 225 women and 43 men, were 18–89 years old (mean 47 yr). In this series, benign thyroid disease (47.8%) was about as frequent as thyroid cancer (52.2%; p = 0.351). The most common benign nodular disease was nodular hyperplasia (72 of 128 cases), followed by adenomas (45) and miscellaneous (11 cases). Thyroid malignancies were dominated by papillary thyroid carcinoma (118 of 140 cases) and its oxyphilic variant (18), excepting 3 cases of Hürthle cell and 1 case of follicular thyroid carcinoma. No other thyroid cancer was observed. Mean size of primary thyroid cancer was 25.4 mm (range 2–70 mm). The incidence of thyroid MC was 12.3% in our series (33 of 268 cases).
A total of 449 FNABs were taken from the 268 patients during a 12-month period before thyroidectomy, for a mean of 1.7 FNABs per patient (range 1–6). Considering that 29.5% of the patients in this series had ultrasound-guided FNAB, and using figures from the OHIP (Ontario Health Insurance Plan) fee schedule of the Ministry of Health and Long-Term Care,16 we calculated that sequential FNAB increased the cost of the method by 70%, from $84.24 to $143.20 (Table 1).
Table 1
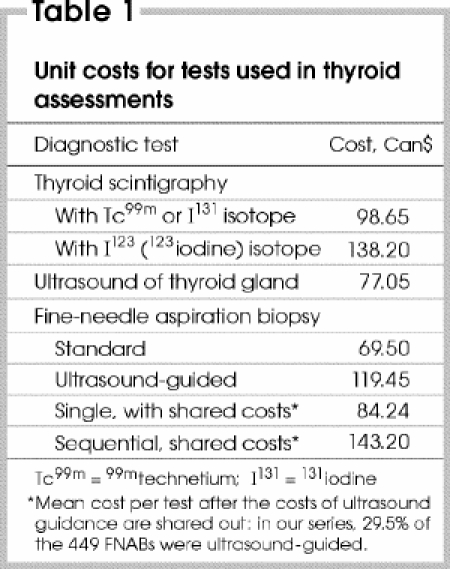
Sequential FNAB was carried out in 110 of 268 patients (41%). Ultrasound guidance was more frequently indicated in cases of microscopic (≤ 10 mm, 44.2%) than gross (> 10 mm) thyroid nodules (p = 0.034).
False results (both false-negative and false-positive) in the single-FNAB group (25.4%) were reduced by 14.2% with sequential FNAB (21.8%), although this was not statistically significant (p = 0.543). However, the difference in rates of nondiagnostic results (10.8% of single-FNAB cases v. 0 sequential) was significant at p < 0.001. In our centre, the accuracy of single FNAB was estimated to be 63.8%; sensitivity, 73.8%; and specificity, 69%. Sequential FNAB increased accuracy by 22.6% (p = 0.009) and sensitivity by 13.8% (p = 0.046) over that of the single-FNAB group. As well, the specificity of the method in the sequential group was 6.2% higher than in the single-FNAB group, although this difference was not significant (p = 0.48; Fig. 1, graph A).
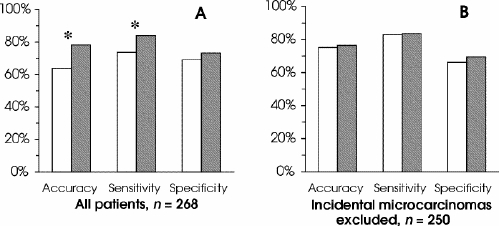
FIG. 1. Comparisons of means from groups of single versus sequential fine-needle aspiration biopsies (FNABs) for accuracy, sensitivity and specificity. White columns are single FNABs; dark columns, sequential FNABs. Asterisks indicate statistically significant differences. Graph A shows the entire series, and graph B, the new series after exclusion of cases of incidental microcarcinoma.
After the 18 cases of incidental MC were excluded, the 250 remaining cases had a similar distribution of malignant (48.8%) and benign disease (51.2%; p = 0.655). In this new series, 250 patients underwent a single FNAB, and 150 individuals, sequential FNAB. The mean number of aspirations per patient was 1.7 (range 1–6) in the sequential-FNAB group. There were no significant differences between the single and sequential groups for the accuracy (p = 0.898), sensitivity (p = 0.965) and specificity (p = 0.594) of FNABs after exclusion of cases of incidental MC (Fig. 1, graph B). Multinodular disease (175 of 250 patients) was more frequent than uninodular disease (75 cases) in this new series. Mean number of aspirations in the subgroup with multinodular disease (1.7 aspirations/patient, range 1–6) was similar to that of uninodular disease (1.6 per patient, same range).
There was a higher incidence of malignant disease in the group with uninodular disease (58.7% v. 44.6%), with the difference approaching statistical significance (p = 0.057; Fig. 2A). Although the increases in accuracy (by 6.5%; p = 0.439) and sensitivity (by 3.8%; p = 0.648) from the single-FNAB group to the sequential were not significant, in the group with a single nodule proven by ultrasound there was a significant increase in specificity (by 20.7%; p = 0.036). The estimated accuracy (p = 0.975), sensitivity (p = 0.94) and specificity (p = 0.991) among single-FNAB patients with multinodular thyroid disease were comparable to patients who had sequential FNAB (Fig. 2B).
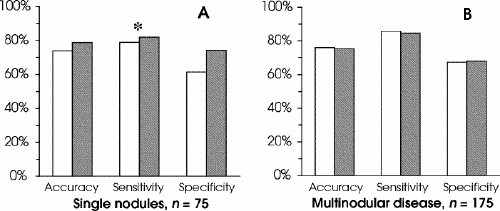
FIG. 2. Comparison of means between FNAB groups (legend as described for Fig. 1). Data is shown for cases of (A) single nodules confirmed by ultrasound ( n = 75) and (B) multinodular thyroid disease ( n = 175).
Discussion
Nodular thyroid disease is common and frequently has multinodular benign pathology, so that clinicians have been encouraged to use FNAB in preoperative selection of thyroid nodules.3 Economical criteria and test reliability have supported the frequent use of FNAB as the initial investigation for nodular thyroid disease over other diagnostic methods such as thyroid scintigraphy and ultrasound.17 Routine use of FNAB has decreased the need for thyroidectomy by at least 25% and the cost of care by 25%.18 These are impressive outcomes for cost-effectiveness, even though such studies to date have addressed single FNAB only.17,18,19
In our centre, the estimated accuracy, sensitivity and specificity of single FNAB were 63.8%, 73.8% and 69%, respectively. When sequential FNAB was used, the accuracy rose to 78.2%, sensitivity to 84% and specificity to 73.3%. These increases represent a significant improvement in the precision of FNAB, which fell in statistical significance after exclusion of all cases of incidental MC. This suggests that multiple FNAB may have increased odds of aspirating small nodules, reinforcing the importance of sequential FNAB. In addition, our results showed that sequential FNAB increases the specificity of the method in patients with uninodular thyroid disease, as these individuals have a higher incidence of cancer.
Our results are comparable to previous studies that reported accuracies for FNAB of 53%–98%, sensitivities of 53%–98% and specificities of 45%–99%.20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39 These parameters can vary considerably among different studies depending on the methods of data analysis.2 At least 5 major methodological issues have been identified that affect estimations of the precision of FNAB: exclusion criteria; features of the population and sample analyzed; the necessity of confirmatory histology for diagnosis; the rate of nondiagnostic results; and discrepancies between cytology and histology.
Exclusion criteria in different studies may differ considerably, causing confusion in the comparison of outcomes. For instance, some authors20,28,40 considered MC of the thyroid gland to be an incidental finding at surgery and therefore a negative result for the purpose of evaluating FNAB efficacy. Papillary and follicular thyroid MC occurred in 12.3% of our cases; since they may behave similarly to gross well-differentiated thyroid cancers and ultrasound-guided FNAB currently enhances their diagnosis,41,42,43,44,45,46 these were included in our study as positive results. Other studies2 have excluded “nondiagnostic” and “suspicious” samples before estimation of the accuracy of FNAB. In our centre, nondiagnostic results occurred in 10.8% of single FNAB and were included in the estimation of accuracy of FNAB since they were an important component for cost analysis.
The characteristics of the population and sample analyzed may affect the precision of FNAB considerably. For example, Corrias and colleagues47 demonstrated that FNAB is more accurate in detecting malignancy in adults than in children. The patients in our series were all mature, whereas others36,47,48 have included both children and adults. Furthermore, it is well known that the age at diagnosis influences the distribution of histological types of thyroid cancer, with increased incidences of differentiated thyroid carcinomas in childhood, and medullary and anaplastic thyroid cancers in the elderly.46 Our study sample comprised well- differentiated thyroid cancers only, with a predominance of papillary thyroid cancer; but other samples2,22,23,24,25,26,27,28,29,30,33,35 have included different histological types, such as medullary and anaplastic thyroid cancer, primary thyroid lymphoma and metastatic thyroid malignancies. Previous studies2,29 have shown that the sensitivity of FNAB may differ significantly in the diagnosis of thyroid cancer depending on the histological type of the lesion.
The necessity of confirmatory histology for diagnosis results in more selective samples that interfere in estimations of the precision of FNAB. Malignancy has been reported in 1.5%–37% of individuals with thyroid nodules, based on clinical and cytological assessments, but confirmed in 8.5%–67% of patients treated surgically.2,4,8,9,10,11,12,21,22,23,24,25,27,28,33,34,35,36,37,38,39,40,49,50 Selective surgery based on FNAB has led, in several studies,51,52,53 to an increase in the incidence of cancer in resected specimens, from 9.6% to 40%, 31% to 50%, and 10% to 69%. The elevated frequency of thyroid cancer in our series (52.2%) indicates that patients in this hospital-based surgical unit were highly selected for thyroidectomy, which is comparable to previous investigations.13,49,50
Nondiagnostic results reduce the estimation of FNAB precision in most studies. Nondiagnostic FNABs, which included inadequate smears and “inconclusive” or “insufficient” test results, occurred in 10.8% of our single-FNAB group, whereas no “insufficient” results were returned in our sequential FNAB series. Nondiagnostic FNABs have been reported10,24,54 at incidences of 10%–30% of smears. Although rates of samples inadequate for FNAB of 1.6%–32.4% have been documented,6,10,28 Oertel and Oertel7 have suggested that the proportion of unsatisfactory specimens should not exceed 5%.
Use of ultrasound-guided FNAB may improve the diagnostic yield in selected patients with nondiagnostic FNAB and reduce the number of false results.2,45,55 Leenhardt and associates56 found that adequacy of cytological material significantly increased with nodule size, outlining the limits of ultrasound-guided FNAB in small nodules. Alexander and co-authors57 recommended that repeat aspiration should be the standard approach after nondiagnostic ultrasound-guided FNAB, since there remains a significant risk of initial nondiagnostic cytology for malignancy. Despite its limitations, ultrasound-guided FNAB has shown advantages over standard FNAB in selected cases. Previous investigations28,54,58 have reported widely differing frequencies of use of ultrasound-guided FNAB, from 12% to 100%, depending on the experience and routines of health care services. At our centre, 29.5% of our patients had an ultrasound-guided aspiration, which was used significantly more often in the management of microscopic nodules (44.2%) than gross nodules (26.7%).
In addition, cytological smears may show some discrepancies when compared with postoperative histology, even though FNAB has been considered the most effective screening test for investigating thyroid nodules. Such inconsistencies were mostly attributed to cytological misinterpretation due to cytodiagnostic error, sampling error or technically suboptimal material.29
There is great concern about false diagnoses with FNAB, which represent the most clinically significant discrepancy between cytology and histology, since they may interfere in the management of nodular thyroid diseases and result in unnecessary surgery (in cases of false-positive results) or delayed resection (false negatives). Gharib3 reviewed the literature and found false-negative rates from 1% to over 11%, and false-positive rates from 0 to 10%. Caruso and colleagues59 noted that rates of false-negative diagnoses in FNAB have been reported from 1.5% to 15%, and rates of false-positive cytology from 0% to 12%. Sidawy and co-workers29 reported a false-negative rate of 16% and a false-positive rate of 6%. In our centre, false-negative and false-positive results occurred in 25.4% of single-FNAB cases and 21.8% of sequential, comparable to rates in the literature.29,57
Because sequential FNAB often reduces the discordance between cytology and histology, it has also been recommended as routine practice after nondiagnostic FNAB results by several authors.2,3,7,10,11,12,13,50,54,56,60,61 Our results support that suggestion, since there was an increase in the accuracy and sensitivity of FNAB and a reduction in nondiagnostic cytology with repetition of the aspirations.
Although the sequential FNAB was more efficient than a single cytological biopsy, sequential FNAB increased the cost of the diagnosis by 70% compared with single FNAB. In our centre, the cost of a sequential FNAB associated with ultrasound guidance (29.5% of aspirations) was similar to the cost of scintigraphy with I123, but 45% more expensive than scintigraphy with Tc99m or I131 and 86% more expensive than thyroid ultrasound. FNAB still offers better accuracy, sensitivity and specificity in detecting malignancy than less invasive approaches such as ultrasound and scintigraphy.12,56,59,60,61,62,63 Therefore, health care planners ought to consider a rate of 1.7 to quantify the total cost of the sequential FNAB method for the management of thyroid nodular disease.
Conclusions
Our results confirmed that fine-needle aspiration cytology is an important and highly reliable preoperative screening for nodular thyroid disease. Sequential FNAB was particularly valuable for elucidation of “insufficient” results, increasing significantly the accuracy and sensitivity of the method. Although sequential aspirations increased the cost of FNAB by 70%, its unit cost is considerably lower, and the precision of the method is significantly improved, in that its use can result in better selection of patients for thyroidectomy and a likely overall reduction of health care costs. Nonetheless, prospective randomized studies of cost-effectiveness are necessary to validate this suggestion.
Acknowledgments
The authors would like to thank Margaret Allen for her assistance in the preparation of the manuscript. This study was supported by a grant from The Head and Neck Cancer Foundation.
The data from this study was presented in the 2002 Annual Meeting of the American Society of Head and Neck Surgery (Boca Raton, Fla., May 11–13, 2002), the 2003 Canadian Surgery Forum (Vancouver, BC, Sept. 19, 2003), the 2003 Annual Meeting of the American Thyroid Association (Palm Beach, Fla., Sept. 17, 2003), and the 2003 Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Professional Conference and Annual Meetings (Ottawa, Ont., Oct. 16–17, 2003).
Competing interests: None declared.
Correspondence to: Dr. Julio C. Furlan, Toronto Western Hospital, 399 Bathurst St., Rm. 12-407, Toronto ON M5T 2S8; fax 416 603-5745; jfurlan@uhnres.utoronto.ca
Accepted for publication Nov. 10, 2003
References
- 1.Martin HE, Ellis EB. Biopsy by needle puncture and aspiration. Ann Surg 1930;92:169-81. [DOI] [PMC free article] [PubMed]
- 2.Belfiore A, La Rosa GL. Fine-needle aspiration biopsy of the thyroid. Endocrinol Metab Clin North Am 2001;30:361-400. [DOI] [PubMed]
- 3.Gharib H. Fine-needle aspiration biopsy of thyroid nodules: advantages, limitations, and effect. Mayo Clin Proc 1994;69:44-9. [DOI] [PubMed]
- 4.Giovagnoli MR, Pisani T, Drusco A, Scardella L, Antonaci A, Vecchione A. Fine needle aspiration biopsy in the preoperative management of patients with thyroid nodules. Anticancer Res 1998:18:3741-6. [PubMed]
- 5.Rimm DL, Stastny JF, Rimm EB, Ayer S, Frable WJ. Comparison of the costs of fine-needle aspiration and open surgical biopsy as methods for obtaining a pathologic diagnosis. Cancer 1997;81:51-6. [DOI] [PubMed]
- 6.Pisani T, Bononi M, Nagar C, Angelini M, Bezzi M, Vecchione A. Fine needle aspiration and core needle biopsy techniques in the diagnosis of nodular thyroid pathologies. Anticancer Res 2000;20:3843-7. [PubMed]
- 7.Oertel YC, Oertel JE. Thyroid cytology and histology. Baillieres Best Pract Res Clin Endocrinol Metabol 2000;14:541-57. [DOI] [PubMed]
- 8.Shaha AR. Controversies in the management of thyroid nodule. Laryngoscope 2000;110:183-93. [DOI] [PubMed]
- 9.Rosen IB, Wallace C, Strawbridge HG, Walfish PG. Reevaluation of needle aspiration cytology in detection of thyroid cancer. Surgery 1981;90:747-56. [PubMed]
- 10.Hamburger JI. Consistency of sequential needle biopsy findings for thyroid nodules. Arch Intern Med 1987;147:97-9. [PubMed]
- 11.Hamburger JI, Hisain M, Nishiyama R, Nunez C, Solomon D. Increasing the accuracy of fine-needle biopsy for thyroid nodules. Arch Pathol Lab Med 1989;113:1035-41. [PubMed]
- 12.Dwarakanathan AA, Staren ED, D'Amore MJ, Kluskens LF, Martirano M, Economou SG. Importance of repeat fine-needle aspiration biopsy in the management of thyroid nodules. Am J Surg 1993;166:350-2. [DOI] [PubMed]
- 13.Borman KR, Hume AT. Credibility and clinical utility of thyroid fine-needle aspiration biopsy in a teaching hospital. Am J Surg 1995;170:638-42. [DOI] [PubMed]
- 14.McHenry CR, Walfish PG, Rosen IB. Non-diagnostic fine-needle aspiration biopsy: a dilemma in management of nodular disease. Am Surg 1993;59:415-9. [PubMed]
- 15.The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules. Mod Pathol 1996;9:710-5. [PubMed]
- 16.Ministry of Health and Long-Term Care. Ontario Health Insurance Plan (OHIP) schedule of benefits and fees. Available: www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html (accessed Jan 17, 2005).
- 17.Caplan RH, Wester SM, Lambert PJ, Rooney BL. Efficient evaluation of thyroid nodules by primary care providers and thyroid specialists. Am J Manag Care 2000;6:1134-40. [PubMed]
- 18.Gharib H. Changing concepts in the diagnosis and management of thyroid nodules. Endocrinol Metab Clin North Am 1997;26:777-800. [DOI] [PubMed]
- 19.Mason J, Freemantle N, Nazareth I, Eccles M, Haines A, Drummond M. When is it cost-effective to change the behavior of health professionals? JAMA 2001;286: 2988-92. [DOI] [PubMed]
- 20.Åkerman M, Tennvall J, Biörklund A, Mårtensson H, Möller T. Sensitivity and specificity of fine needle aspiration cytology in the diagnosis of tumors of the thyroid gland. Acta Cytol 1985;29:850-5. [PubMed]
- 21.Hall TL, Layfield LJ, Philippe A, Rosenthal DL. Sources of diagnostic error in fine needle aspiration of the thyroid. Cancer 1989;63:718-25. [DOI] [PubMed]
- 22.Altavilla G, Pascale M, Nenci I. Fine needle aspiration cytology of the thyroid gland diseases. Acta Cytol 1990;34:251-6. [PubMed]
- 23.La Rosa GL, Belfiore A, Giuffrida D, Sicurella C, Ippolito O, Russo G, et al. Evaluation of the fine needle aspiration biopsy in the preoperative selection of cold thyroid nodules. Cancer 1991;67:2137-41. [DOI] [PubMed]
- 24.RodrÍguez JM, Parrilla P, Sola J, Bas A, Aguilar J, Moreno A, et al. Comparison between preoperative cytology and intraoperative frozen-section in the diagnosis of thyroid nodules. Br J Surg 1994;81:1151-4. [DOI] [PubMed]
- 25.Mandreker SR, Nadkarni NS, Pinto RG, Menezes S. Role of fine needle aspration cytology as initial modality in the investigation of thyroid lesions. Acta Cytol 1995;39:898-904. [PubMed]
- 26.Holleman F, Hoekstra JB, Ruitenberg HM. Evaluation of fine needle aspiration (FNA) cytology in the diagnosis of thyroid nodules. Cytopathology 1995;6:168-75. [DOI] [PubMed]
- 27.Giovagnoli MR, Pisani T, Drusco A, Scardella L, Antonaci A, Vecchione A. Fine needle aspiration biopsy in the preoperative management of patients with thyroid nodules. Anticancer Res 1998;18:3741-6. [PubMed]
- 28.Cáp J, RyŠka A, Rehorková P, Hovorková E, Kerekes Z, Pohnetalová D. Sensitivity and specificity of the needle aspiration biopsy of the thyroid: clinical point of view. Clin Endocrinol (Oxf) 1999;51:509-15. [DOI] [PubMed]
- 29.Sidaway MK, Del Vecchio D, Knoll SM. Fine-needle aspiration of thyroid nodules: correlation between cytology and histology and evaluation of discrepant cases. Cancer 1997;81:253-9. [DOI] [PubMed]
- 30.Yeoh GPS, Chan KW. The diagnostic value of fine-needle aspiration cytology in the assessment of thyroid nodules: a retrospective 5-year analysis. Hong Kong Med J 1999;5:140-4. [PubMed]
- 31.Ravetto C, Colombo L, Dottorini ME. Usefulness of fine-needle aspiration in the diagnosis of thyroid carcinoma: a retrospective study in 37,895 patients. Cancer 2000;90:357-63. [PubMed]
- 32.Bi J, Lu B. Advances in the diagnosis and management of thyroid neoplasms. Curr Opin Oncol 2000;12:54-9. [DOI] [PubMed]
- 33.Bakhos R, Selvaggi SM, DeJong S, Gordon DL, Pitale SU, Hermann M, et al. Fine-needle aspiration of the thyroid: rate and causes of cytohistopathologic discordance. Diagn Cytopathotol 2000;23:233-7. [DOI] [PubMed]
- 34.Carrillo JF, Frias-Mendivil M, Ochoa-Carrillo FJ, Ibarra M. Accuracy of fine-needle aspiration biopsy of the thyroid combined with an evaluation of clinical and radiologic factors. Otolaryngol Head Neck Surg 2000;122:917-21. [DOI] [PubMed]
- 35.Mandel DL, Genden EM, Mechanick JI, Bergman DA, Biller HF, Urken ML. Diagnostic accuracy of fine-needle aspiration and frozen section in nodular thyroid disease. Otolaryngol Head Neck Surg 2001;124:531-6. [DOI] [PubMed]
- 36.de Vos tot Nederveen Cappel RJ, Bouvy ND, Bonjer HJ, Van Muiswinkel JM, Chadha S. Fine needle aspiration cytology of thyroid nodules: how accurate is it and what are the causes of discrepant cases? Cytopathology 2001;12:399-405. [DOI] [PubMed]
- 37.Duek SD, Goldenberg D, Linn S, Krausz MM, Hershko DD. The role of fine-needle aspiration and frozen section in the surgical management of solitary thyroid nodules. Surg Today 2002;32:857-61. [DOI] [PubMed]
- 38.Caraci P, Aversa S, Mussa A, Pancani G, Ondolo C, Conticello S. Role of fine-needle aspiration biopsy and frozen-section evaluation in the surgical management of thyroid nodules. Br J Surg 2002;89:797-801. [DOI] [PubMed]
- 39.Abboud B, Allam S, Chacra LA, Ingea H, Tohme C, Farah P. Use of fine-needle aspiration cytology and frozen section in the management of nodular goiters. Head Neck 2002;25:32-6. [DOI] [PubMed]
- 40.Gershengorn MC, McClung MR, Chu EW, Hanson TAS, Weintraub BD, Robbins J. Fine-needle aspiration cytology in the preoperative diagnosis of thyroid nodules. Ann Intern Med 1977;87:265-9. [DOI] [PubMed]
- 41.Furlan JC, Bedard Y, Rosen IB. Biological basis for the treatment of microscopic, occult well-differentiated thyroid carcinoma. Surgery 2001;130:1050-4. [DOI] [PubMed]
- 42.Yokozawa T, Fukata S, Kuma K, Matsuzuca F, Kobayashi A, Hirai K, et al. Thyroid cancer detected by ultrasound-guided fine-needle aspiration biopsy. World J Surg 1996;20:848-53. [DOI] [PubMed]
- 43.Mikosch P, Wartner U, Kresnik E, Gallowitsch HJ, Heinisch M, Dinges HP, et al. Results of preoperative ultrasound guided fine needle aspiration biopsy of solitary thyroid nodules as compared with the histology: a retrospective analysis of 538 patients. Nuklearmedizin 2001;40:148-54. [PubMed]
- 44.Yang GC, LiVolsi VA, Baloch ZW. Thyroid microcarcinoma: fine-needle aspiration diagnosis and histologic follow-up. Int J Surg Pathol 2002;10:133-9. [DOI] [PubMed]
- 45.Solymosi T, Toth GL, Bodo M. Diagnostic accuracy of fine needle aspiration cytology of the thyroid: impact of ultrasonography and ultrasonographically guided aspiration. Acta Cytol 2001;45:669-74. [DOI] [PubMed]
- 46.Hundahl SA, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. An American College of Surgeons Commission on cancer patient care evaluation study. Cancer 2000;89:202-17. [DOI] [PubMed]
- 47.Corrias A, Einaudi S, Chiorboli E, Weber G, Crinò A, Andreo M, et al. Accuracy of fine needle aspiration biopsy of thyroid nodules in detecting malignancy in childhood: comparison with conventional clinical, laboratory, and imaging approaches. J Clin Endocrinol Metabol 2001;86:4644-8. [DOI] [PubMed]
- 48.Brooks AD, Shaha AR, DuMornay W, Huvos AG, Zakowski M, Brennan MF, et al. Role of fine-needle aspiration biopsy and frozen section analysis in the surgical management of thyroid tumors. Ann Surg Oncol 2001;8:92-100. [DOI] [PubMed]
- 49.Baskin HJ, Guarda LA. Influence of needle biopsy on management of thyroid nodules: reasons to expand its use. South Med J 1987;80:702-5. [DOI] [PubMed]
- 50.Ogawa Y, Kato Y, Ikeda K, Aya M, Ogisawa K, Kitani K, et al. The value of ultrasound-guided fine-needle aspiration cytology for thyroid nodules: an assessment of its diagnostic potential and pitfalls. Surg Today 2001;31:97-101. [DOI] [PubMed]
- 51.Al-Sayer HM, Krukowski ZH, Williams VMM, Matheson NA. Fine needle aspiration cytology in isolated thyroid swellings: a retrospective two-year evaluation. BMJ 1985;290:1490-2. [DOI] [PMC free article] [PubMed]
- 52.Franklyn JA, Fitzgerald MG, Oates GD, Sheppard MC. Fine needle aspiration cytology in the management of euthyroid goitre. Q J Med 1987;248:997-1003. [PubMed]
- 53.Kendall CH. Fine needle aspiration of thyroid nodules: three years' experience. J Clin Pathol 1989;42:23-7. [DOI] [PMC free article] [PubMed]
- 54.Gharib H, Goellner JR. Fine-needle aspiration biopsy of thyroid: an appraisal. Ann Intern Med 1993;118:282-9. [DOI] [PubMed]
- 55.Mittendorf EA, Tamarkin SW, McHenry CR. The results of ultrasound-guided fine-needle aspiration biopsy for evaluation of nodular thyroid disease. Surgery 2002;132:648-54. [DOI] [PubMed]
- 56.Leenhardt L, Hejblum G, Franc B, Fediaevsky LD, Delbot T, Le Guillouzic D, et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab 1999;84:24-8. [DOI] [PubMed]
- 57.Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, et al. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab 2002;87:4924-7. [DOI] [PubMed]
- 58.Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, et al. Usefulness of ultrassonography in the management of nodular thyroid disease. Ann Intern Med 2000;133:696-700. [DOI] [PubMed]
- 59.Caruso D, Mazzaferri EL. Fine needle aspiration biopsy in the management of thyroid nodules. Endocrinologist 1991;1:194-202.
- 60.Mazzaferri E. Management of a solitary thyroid nodule. N Engl J Med 1993;328: 553-8. [DOI] [PubMed]
- 61.Woeber KA. Cost-effective evaluation of the patient with a thyroid nodule. Surg Clin North Am 1995;75:357-63. [DOI] [PubMed]
- 62.Burch H, Burman K, Reed L, Buckner L, Raber T, Ownbey J. Fine needle aspiration of thyroid nodules. Acta Cytol 1996;40:1176-83. [DOI] [PubMed]
- 63.Gutman P, Henry M. Fine needle aspiration cytology of the thyroid. Clin Lab Med 1998;18:461-82. [PubMed]


