Abstract
Purpose
To determine how long patients in Ontario waited for major breast, colorectal, lung or prostate cancer surgery in the years 1993–2000.
Methods
“Surgical waiting time” was defined as the interval from date of preoperative surgeon consult to date of hospital admission for surgery. We created patient cohorts by linking appropriate diagnosis and procedure codes from Canadian Institutes of Health Information data. Scrambled unique surgeon identifiers were obtained from Ontario Health Insurance Plan data. Changes in median surgical waiting times were assessed with univariate time-trend analyses and multilevel models. Models were controlled for year of surgery and other patient (age, gender, comorbid conditions, income level, area of residence) and hospital level characteristics (teaching status, procedure volume status).
Results
Compared with 1993, median surgical waiting times in the year 2000 increased 36% for patients with breast cancer (to 19 d), 46% with colorectal (to 19 d), 36% with lung (to 34 d) and 4% with prostate cancer (to 83 d). Multilevel models confirmed significant increases in waiting times for all procedures. There were no concerning or consistent differences in waiting times among the categories of hospitals and patients examined.
Discussion
There were significant increases in surgical waiting times among patients undergoing breast, colorectal, lung or prostate cancer surgery in Ontario over years 1993–2000. Administrative databases can be used to efficiently measure such waits.
Abstract
Objet
Déterminer combien de temps des patients de l'Ontario ont attendu avant de subir une chirurgie majeure contre un cancer du sein, du poumon, de la prostate ou un cancer colorectal entre 1993 et 2000.
Méthodes
Le temps d'attente avant la chirurgie a été défini comme étant l'intervalle entre la consultation préopératoire avec le chirurgien et l'admission à l'hôpital pour la chirurgie. Nous avons créé les cohortes de patients en reliant les codes de diagnostic et d'intervention appropriés tirés des données de l'Institut canadien d'information sur la santé. Des identificateurs codés uniques ont été obtenus pour les chirurgiens à partir de données sur le Régime d'assurance-maladie de l'Ontario. Les changements au chapitre du temps d'attente médian avant la chirurgie ont été évalués au moyen d'analyses à une variable des tendances temporelles et de modèles multiniveaux. Les modèles tenaient compte de l'année au cours de laquelle la chirurgie a été pratiquée et des caractéristiques des patients (âge, sexe, comorbidité, revenu, lieu de résidence) et des hôpitaux (hôpital d'enseignement, volume d'interventions).
Résultats
En 2000, les temps d'attente médians avant chirurgie chez les patients ayant subi une intervention contre un cancer du sein (19 j), un cancer colorectal (19 j), un cancer du poumon (34 j) ou un cancer de la prostate (83 j) étaient considérablement plus longs que ceux enregistrés en 1993, qu'ils dépassaient de 36 %, 46 %, 36 % et 4 %, respectivement. L'utilisation des modèles multiniveaux a démontré que ces constatations étaient significatives pour toutes les interventions. L'examen des groupes d'hôpitaux et de patients n'a pas révélé de différences préoccupantes ou régulières au chapitre du temps d'attente.
Conclusion
Nos constatations sur l'accroissement significatif des temps d'attente avant une chirurgie contre le cancer du sein, du poumon, de la prostate ou le cancer colorectal en Ontario au cours de la période de 1993 à 2000 indiquent que les bases de données administratives peuvent servir à mesurer efficacement ces périodes d'attente.
Lengthy waits for cancer services may harm patients by causing psychological distress1,2,3 or lessening the effectiveness of available treatments.4,5,6,7 Waiting times for surgery are of particular relevance, since for most patients with cancer the cornerstone of curative therapy is surgical extirpation of the offending lesion.8 Moreover, in countries such as Canada, where cancer is a leading cause of death and disability, problems with access to cancer treatments likely run parallel to problems with general access to medical care.
There is a paucity of information on actual waiting times for cancer surgery in most jurisdictions. Currently in a number of Canadian provinces patient waiting lists are being created for cancer and most other major surgeries.9,10 Such efforts are important, since it is probable that policy-makers and administrators will only respond to perceptions of worrisome delays when perceptions are corroborated by data. But surgical waiting lists require considerable resources to maintain, and are subject to gaming or poor management by physicians; implicit assumptions that patients will welcome the chance to be referred to regions with shorter waits or even better outcomes are not necessarily true.11,12,13,14,15,16
Administrative databases at the provincial level may provide a more efficient method of assessing waiting times. Such databases contain comprehensive information on all patients treated within a given geographic area.17 In our study we used such Ontario hospital discharge data and physician billing data to determine how long patients in Ontario waited for breast, colorectal, lung or prostate cancer surgery in years 1993 to 2000. We also determined how hospital and patient characteristics influenced waits for cancer surgery.
Methods
We defined surgical waiting time as the interval between 2 key events for patients undergoing major cancer surgery (for breast, colorectal, lung or prostate cancer): the date of the preoperative consult by the surgeon who does the surgery, and the date of admission to hospital for the operative procedure. It is during this interval that a surgeon will request test results and consults from other specialists to assist in treatment decisions, as well as operating-room time.
As sources of data, we used databases of the Canadian Institute for Health Information (CIHI) and the Ontario Health Insurance Plan (OHIP) from fiscal years 1991 through 2000. The CIHI database contains information on all patients discharged from Ontario hospitals. The OHIP database contains billing information from individual clinicians across the province. OHIP officials routinely assess billing patterns and audit individual clinicians to ensure billing accuracy. Researchers have reported18,19,20,21 that these databases contain accurate and comprehensive information for many data fields, such as major diagnoses, major procedures, admission date, length of stay and discharge status of patients.
Deyo and colleagues' validated modification22 of a comorbidity index for the ICD-9-CM database was used to define comorbidity. Postal Code Conversion File Plus (Ottawa: Statistics Canada; 2001) incorporates Canadian census data from 1996; we used PCCF+ to link postal codes for all patients to household income (adjusted for household size) and rural versus urban place of residence. Patient cohorts were divided into 5 income levels — high, high-medium, medium, low-medium and low — each of which contained approximately equal numbers of patients. Calculations for hospital procedure volume considered the administrative merging of institutes over time. Teaching hospitals were affiliated directly with a medical school.
Linking relevant CIHI diagnosis and procedure codes allowed the identification of patients with breast, colorectal, lung or prostate cancer who were treated with major extirpative surgery in 1993 through 2000. Patients were assigned to the calendar year in which they were admitted to hospital for surgery. For each diagnosis and for each patient only the first admission for major cancer surgery was used, thereby decreasing the likelihood of measuring waits for recurrent cancer surgery. Patients from the CIHI cohorts were then linked to OHIP data by means of unique patient identifiers: encrypted health card numbers. An OHIP procedure bill was accepted as the definitive cancer surgery bill if the billing date was during, 7 days prior to, or 7 days after the CIHI date of admission. Finally, preoperative consult bills were linked to surgical procedure bills via encrypted unique physician identifiers, which are attached to all OHIP bills.
We limited our time window for pre-surgery consult to 4 months for patients with breast, colorectal or lung cancer, to avoid including pre-surgery consults for other unrelated surgical issues. For patients with prostate cancer we extended this to 12 months, since many consults occurred more than 4 months before surgery. For patients with 2 or more consults in the relevant time window we selected the consult closest to surgery as our anchor point, though we also assessed how selecting the first consult would affect the results.
Patients were excluded from the study if their consult occurred the day prior to or during the hospital admission; we speculated that such a waiting time was for emergency surgery. For all cancer sites but the breast, if the length of hospital stay was ≤ 1 day and the patient was discharged alive, the admission was excluded since we judged that recovery from major cancer surgery could not have occurred in such a short time. Patients treated at Southeastern Academic Medical Organization hospitals were also removed; with the initiation of an alternate funding plan for physicians in 1994 the accuracy and comprehensiveness of OHIP billing is likely incomplete.23 Patients with cancer in any site(s) except prostate were excluded if they received chemotherapy or a consultation with a radiation oncologist within 4 months before surgery.
We used descriptive, univariate and multivariate analyses of data. All tests of hypothesis were 2-sided and significant if p ≤ 0.05. Univariate regression was used to examine linear time trends in numerous patient and outcome measures, including median surgical waiting times. Multivariate multilevel linear models were designed to consider clustering of the patient data at the hospital level.24 Year of surgery and log-transformed surgical waiting time were independent and dependent variables, respectively. Other covariates of interest included patient demographics (age, gender, income level and area of residence) and comorbid conditions, and hospital teaching status and procedure volume levels. Residual analyses were performed to assess normality. When a propensity was noted for hospitals with high versus low procedure volumes to be teaching centres, we measured for interactions among the hospital teaching and procedure-volume groups. All analyses were done with Stata (version 6.0, Stata Corporation, College Station, Tex.) and SAS (version 8.2, SAS Institute Inc., Cary, NC).
The study received ethics approval from the Sunnybrook and Women's College Health Sciences Centre Research Ethics Board.
Results
We linked 95% of eligible patients in the CIHI database to an OHIP procedure bill; the range for the 4 cancer sites was 93%–97%. We linked 86% of eligible patients in the CIHI database to both an OHIP procedure and a consult bill; range for the 4 sites was 86%– 88%. There was more than 1 consult bill from the operating surgeon in the relevant time window for 1% of patients undergoing breast, 2% colorectal, 1% lung and 3% prostate cancer surgery, respectively. Using the first rather than the most proximate consult as the anchor point of the surgical waiting time for these patients did not change the magnitude or significance of our results. The consult of interest was dated the day before or during hospital admission for 22% of patients with colorectal cancer; this percentage declined through time. Consultations dated in this interval rarely occurred for other sites of cancer.
Numbers of patients and their median and 75th-percentile surgical waiting times for cancer surgery are shown in Table 1. In the year 2000 there were 14% more patients undergoing surgery for breast cancer than in 1993; 33% more for colorectal; 2% more for lung; and 96% more for prostate cancer surgery than in 1993. Median surgical waiting times increased for all sites of cancer: by 36% for breast, 46% for colorectal, 36% for lung and 4% for prostate cancer surgery. These increases were significant for all but prostate cancer.
Table 1
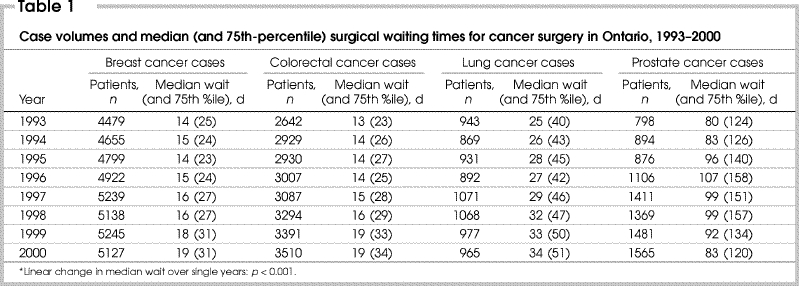
Multilevel regression models revealed significant proportional increases in median surgical waiting times from 1993–1994 to 1999–2000. Patients undergoing breast cancer surgery had a proportional increase in waiting period of 25%; colorectal, 34%; lung, 36%; and prostate cancer surgery, 5%. Other factors associated with longer delays in waiting time included elderliness of patients (for all sites but prostate) and presence of 1 or more comorbidities (all sites; Table 2). Patients in the low- versus high-income group had longer waits for breast (estimate 1.04, 95% confidence interval [CI] 1.02– 1.06, p ≤ 0.001) and colorectal surgery (estimate 1.05, CI 1.02– 1.08, p = 0.001). The median wait in teaching centres was shorter for patients with prostate cancer (estimate 0.85, CI 0.73– 0.99, p = 0.042) than in non-teaching hospitals, but longer for those with breast cancer (estimate 1.16, CI 1.01–1.32, p = 0.032). Hospital procedure volume had no significant effect on surgical waiting times; neither were there significant hospital level interactions for any of the 4 sites.
Table 2

Discussion
Queues and accompanying periods of delay for a medical service are often viewed as the reasonable price paid by Canadians for a health system that promises universal, comprehensive and accessible care.25,26,27 As well, some pre-surgical delay should be expected to allow patients and physicians to optimize treatment decisions. Recently, however, some have questioned if the implicit rationing of care in Canada through organized or informal queues has become excessive.26,27,28 There is a surprisingly paucity of data on how long patients do wait for most medical services, a prerequisite for further discussion and decision-making. Our study found that increases in surgical waiting times in Ontario for breast, colorectal, lung and prostate cancer operations over years 1993 to 2000 were significant. Such information is especially important in the Canadian health care debate since cancer in this country is a major source of morbidity and mortality.
Our results add to results from 2 studies29,30 that also used administrative databases to measure waiting times for surgery. Mayo and associates29 showed that for patients from Quebec, the median interval from first diagnostic procedure to breast cancer surgery had increased 45% from 1991 to 1998. (The interval from surgeon consult to surgery was not reported in their results.) Decoster and colleagues30 found that surgical waiting times in the province of Manitoba had not increased significantly from 1991 to 1995. Of the 10 procedures examined, only major breast surgery was cancer-related. It is of interest that in our investigation the increases in surgical waiting times for breast cancer were most pronounced after 1995. These 2 reports and our own demonstrate that in Canada cancer surgery waits can be efficiently measured by use of administrative databases. Such measuring, and comparisons among jurisdictions, can encourage the focusing of attention on access to surgical care for patients diagnosed with cancer.
There were no concerning or consistent differences in surgical waiting times observed among the hospital and patient groups examined. The longer waiting times seen for elderly patients and those with comorbidities are understandable; such patients often require relatively more preoperative tests, consults or preparation for the operating room. The lengthier median waits for breast (4% longer) and colorectal surgery (5% longer) for patients in low- versus high- income groups were probably of little clinical importance. Hospital teaching status influenced waiting times inconsistently: patients treated in teaching hospitals experienced longer waits for breast surgery, shorter waits for prostate surgery, and similar waits for colorectal and lung surgery than those in non-teaching hospitals. Hospital procedure volumes appear to be unrelated to changes in waiting times.
A key question is whether the waiting times observed in this study were excessive or harmful to patients. There is little evidence that such delays (in 2000, a median of 19 d for breast cancer surgery, 19 d for colorectal, 34 d for lung and 83 d for prostate) have a negative impact on clinical outcomes such as operative mortality or long-term survival. That such evidence will be forthcoming is unlikely, given that cancer tumours typically grow over months and years prior to clinical presentation.8 Nevertheless, increased waits are known to increase psychological distress for patients and their families.1,2,3 We suggest that it is ultimately society that must decide, through interested stakeholders, what constitutes an acceptable wait. For example, was it acceptable in the year 2000 that 25% of patients awaited lung cancer surgery for at least 51 days?
There are limitations in our study. Although some may question the use of retrospective administrative data to measure surgical waiting times, the accuracy and comprehensiveness of the databases used here have, as mentioned, been assessed previously.18,19,20,21 Waits in our study are also comparable to those from study data collected prospectively on 547 patients in the year 2000 by surgeons affiliated with an Ontario regional cancer centre,31 which furnished median waits from surgeon consult to admission of 24 days for breast cancer, 22 days for colorectal, 29 days for thoracic, and 43.5 days for prostate cancer operations [M. Simunovic, unpublished data, 2001]. Our current study also lacks cancer staging information. Yet our main interest was to measure waiting times for all of Ontario, across time, and by various patient and hospital characteristics. Since it is unlikely there were major changes in disease stage over the years 1993 to 2000, we do not believe a lack of staging data biased our results.
In conclusion, over years 1993 to 2000 patients from Ontario undergoing breast, colorectal, lung or prostate cancer surgery experienced significant increases in surgical waiting times. There were no concerning or consistent differences in waiting times among the examined hospital and patient groups. Administrative databases can be used to efficiently measure waiting times for cancer surgery.
Reprints will not be available from the authors.
Competing interests: None declared.
Correspondence to: Dr. Marko Simunovic, Hamilton Regional Cancer Centre, 699 Concession St., Hamilton ON L8V 5C2; fax 905 575-6326; marko.simunovic@hrcc.on.ca
Accepted for publication Apr. 26, 2004
References
- 1.Risberg T, Sorbye S, Norum J, Wist E. Diagnostic delay causes more psychological distress in female than in male breast cancer patients. Anticancer Res 1996;16(2): 995-9. [PubMed]
- 2.Benedict S, Williams RD, Baron PL. Recalled anxiety: from discovery to diagnosis of a benign breast mass. Oncol Nurs Forum 1994;21(10):1723-7. [PubMed]
- 3.Gray R, Fitch M, Phillips C, Labrecquie M, Klotz L. Presurgery experiences of prostate cancer patients and their spouses. Cancer Pract 1999;7(3):130-5. [DOI] [PubMed]
- 4.Christensen ED, Harvald T, Jendresen M, Aggestrup S, Petterson G. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardiothorac Surg 1997;12(6):880-4. [DOI] [PubMed]
- 5.Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer 1999;79(5-6):858-64. [DOI] [PMC free article] [PubMed]
- 6.Roncoroni L, Pietra N, Violi V, Sarli L. Delay in the diagnosis and outcome of colorectal cancer: a prospective study. Eur J Surg Oncol 1999;25(2):173-8. [DOI] [PubMed]
- 7.Huang J, Barbera, L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol 2003;21:555-63. [DOI] [PubMed]
- 8.Tannock I, Hill RP, editors. The basic science of oncology. 3rd ed. New York: McGraw Hill; 1998.
- 9.Pilot project to track surgery wait times. Cancer Care Ontario 2003;1(4). Available (accessed 2005 Mar 9): www.cancercare.on.ca/OntarioCancerNewsArchives/200306/index_0603story2.html.
- 10.Saskatchewan Surgical Care Network. Frequently asked questions. Available: www.sasksurgery.ca/faq.html (a Web site of the government of Saskatchewan, accessed 2005 Mar 16).
- 11.Hanning M, Spangberg UW. Maximum waiting time — a threat to clinical freedom? Implementation of a policy to reduce waiting times. Health Policy 2000;52(1):15-32. [DOI] [PubMed]
- 12.Sheldon T. Dutch waiting lists increase despite £36m [US$54 000 000] campaign [news]. BMJ 2000;321:530. [PMC free article] [PubMed]
- 13.Newton JN, Henderson J, Goldacre MJ. Waiting list dynamics and the impact of earmarked funding. BMJ 1995;311:783-5. [DOI] [PMC free article] [PubMed]
- 14.Surgical waiting lists in Victorian hospitals. The Standards Sub-Committee of the Victorian State Committee of the Royal Australasian College of Surgeons. Med J Aust 1991;154(5):326-8. [PubMed]
- 15.Brouwer W, van Exel J, Hermans B, Stoop A. Should I stay or should I go? Waiting lists and cross-border care in the Netherlands. Health Policy 2003;63(3):289-98. [DOI] [PubMed]
- 16.Finlayson SRG, Birkmeyer JD, Tosteson ANA, Nease RF. Patient preferences for location of care: implications for regionalization. Med Care 1999;37(2):204-9. [DOI] [PubMed]
- 17.Hong D, Tandan V, Goldsmith CH, Simunovic M. User's guide to the surgical literature: how to use an article reporting population-based volume–outcome relationships in surgery. Can J Surg 2002;45(2): 109-15. [PMC free article] [PubMed]
- 18.Williams J, Young W. A summary of studies on the quality of health care administrative databases in Canada. In: Goel V, Williams J, Anderson G, Blackstien-Hirsch P, Fooks C, Naylor CD, editors. Patterns of health care in Ontario: the ICES practice atlas. 2nd ed. Ottawa: Canadian Medical Association; 1996.
- 19.Simunovic M, To T, Langer B. The more the better? [letter]. CMAJ 1999;160(13):1820. Comment on Wexler MJ. More procedures, better quality of care? Is there a case for regionalization of pancreatic resection for neoplasm? CMAJ 1999;160(5):671-3, which comments on Simunovic M, To T, Theriault M, Langer B. Relation between hospital surgical volume and outcome for pancreatic resection for neoplasm in a publicly funded health care system. CMAJ 1999;160(5):643-8. [PMC free article] [PubMed]
- 20.Chan B, Anderson G, Dales R. Spirometry utilization in Ontario: practice patterns and policy implications. CMAJ 1997;156(2):169-76. [PMC free article] [PubMed]
- 21.Pinfold SP, Goel V, Sawka C. Quality of hospital discharge and physician data for type of breast cancer surgery. Med Care 2000;38(1):99-107. [DOI] [PubMed]
- 22.Deyo R, Cherkin D, Ciol M. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45(6):613-9. [DOI] [PubMed]
- 23.Southeastern Ontario Academic Medical Organization Backgrounder. In: SEAMO FAQ. Available: www.seamo.ca/Faq/faq.html (accessed 2005 Mar 16).
- 24.Goldstein H. Multilevel statistical models. 2nd ed. London: Edward Arnold; 1995.
- 25.Naylor CD. A different view of queues in Ontario. Health Aff 1991;10(3):110-28. [DOI] [PubMed]
- 26.Standing Senate Committee on Social Affairs, Science and Technology. The health of Canadians — the federal role. Volume six: Recommendations for reform. Ottawa: the Committee; 2002.
- 27.Romanow RJ. Building on values: the future of health care in Canada: final report. Saskatoon: Commission on the Future of Health Care in Canada; 2002. Available: www.hc-sc.gc.ca/english/care/romanow/hcc0023.html (accessed 2003 Aug 14).
- 28.Davies RF. Waiting lists for health care: a necessary evil? [editorial]. CMAJ 1999;160(10):1469-70. [PMC free article] [PubMed]
- 29.Mayo N, Scott S, Shen N, Hanley J. Waiting time for breast cancer surgery in Quebec. CMAJ 2001;164(8):1133-8. [PMC free article] [PubMed]
- 30.DeCoster C, Carriere K, Peterson S, Walld R, MacWilliam L. Waiting times for surgical procedures. Med Care 1999;37(6 Suppl):JS187-205. [DOI] [PubMed]
- 31.Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ 2001;165(4):421-5. [PMC free article] [PubMed]


