Abstract
Introduction
The use of the biofragmentable anastomotic ring (BAR) has been reported in the literature with good results. Our purpose in this review was to document the clinical outcomes after gastrointestinal anastomoses performed with use of the BAR.
Methods
Data were gathered systematically through chart review with the help of data collection forms from 159 patients who underwent 173 intestinal anastomoses performed with use of the BAR between 1992 and 1999. Of the 165 patients who had anastomoses (6 had 2 anastomoses constructed on separate occasions and were considered separately), 23 (13.9%) had surgery with anastomosis under emergency conditions, and 44 (26.7%) were steroid-dependent patients. The indications for surgery were malignant disease in 63 (38.2%) patients, inflammatory bowel disease in 54 (32.7%) patients, diverticular disease in 13 (7.9%) patients and other conditions in 35 (21.2%) patients.
Results
A clinical anastomotic leak developed in the first 2 weeks after surgery in 7 (4.2%) patients, 6 of whom required reoperation. All recovered well, with no deaths related to use of the BAR. Early small-bowel obstruction developed in 13 patients (7.9%), none of whom required reoperation. The average postoperative length of hospital stay was 9.0 days, the average time to pass the first flatus was 3.2 days, and the average time to begin oral fluid intake was 3.3 days. The rate of leakage at the anastomosis in our series was comparable to that found in randomized trials with the BAR (2.0%–4.4%) and as reported with hand-sewn and stapled anastomoses (1.9%–8.2%).
Conclusions
Our data indicate that use of the BAR is safe and effective in both elective and emergent surgery. The rate of leakage is comparable to that reported in the literature when a BAR is used.
Abstract
Introduction
On a signalé dans la littérature scientifique que l'utilisation de l'anneau d'anastomose biofragmentable (AAB) donne de bons résultats. Cette étude visait à documenter les résultats cliniques après des anastomoses gastro-intestinales effectuées au moyen de l'AAB.
Méthodes
On a réuni systématiquement des données en analysant des dossiers à l'aide de formulaires de collecte de données recueillis auprès de 159 patients ayant subi 173 anastomoses intestinales effectuées au moyen de l'AAB entre 1992 et 1999. Des 165 patients ayant subi une anastomose (six en ont subi deux au cours d'interventions distinctes, que l'on a comptées séparément), 23 (13,9 %) ont subi une intervention chirurgicale et l'anastomose d'urgence et 44 (26,7 %) étaient des patients asservis aux stéroïdes. La présence d'une maladie maligne chez 63 (38,2 %) des patients, d'une affection intestinale inflammatoire chez 54 (32,7 %), d'une diverticulite chez 13 (7,9 %) et d'autres problèmes chez 35 (21,2 %) d'entre eux a constitué l'indication pour l'intervention chirurgicale.
Résultats
Une fuite à l'anastomose clinique a fait son apparition dans les deux semaines qui ont suivi l'intervention chirurgicale chez sept (4,2 %) des patients, dont six ont dû subir une nouvelle intervention. Tous se sont bien rétablis et l'on n'a signalé aucun décès relié à l'utilisation de l'AAB. Une occlusion de l'intestin grêle s'est produite peu après l'intervention chez 13 patients (7,9 %) dont aucun n'a dû subir une nouvelle intervention. La durée moyenne de l'hospitalisation après l'intervention s'est établie à 9,0 jours et il a fallu en moyenne aux patients 3,2 jours pour passer la première flatulence et 3,3 jours pour commencer à prendre des liquides par la bouche. Le taux de fuite au niveau de l'anastomose chez notre série de patients se comparait à celui qu'ont révélé les essais cliniques randomisés dans le cas de l'AAB (2,0 %-4,4 %) et qu'on a signalé dans le cas des anastomoses refermées par des points cousus à la main et par des agrafes (1,9 %-8,2 %).
Conclusions
Nos données indiquent que l'AAB est sécuritaire et efficace à la fois pour les interventions chirurgicales électives et dans les cas d'urgence. Le taux de fuite se compare à celui qu'on rapporte dans la littérature scientifique pour un AAB.
The concept of a sutureless anastomosis was introduced in 1826 by Denans,1 as an intraluminal device. Mechanical devices were described later by others, including Bonnier in 18862 and Murphy3 in 1892. The principle was the same: a circular segment of intestinal tissue was trapped between the 2 rings of the anastomotic device. Necrosis and sloughing of the trapped bowel wall released the device, which then passed into the feces. Surgeons were reluctant to use this device because of anastomotic stenosis (resulting from ischemia secondary to the tight device) and of obstruction of the fecal flow (when the device was in situ).
In 1985, Hardy and associates4 introduced a biofragmentable anastomotic device made of absorbable polyglycolic acid and 12.5% barium sulfate, which renders it radiopaque (Valtrac Biofragmentable Anastomosis Ring [BAR]).5,6 It is made of 2 identical segments attached together on a central frame. Their scalloped rims prevent tissue ischemia; there is a 6-mm gap in the open position, and a 1.5-, 2.0- or 2.5-mm gap in the closed position. A variety of external diameter sizes are available: 25, 28, 31 and 34 mm, each 14 mm larger than the internal lumen.7 The bowel anastomosis is created by tying the pursestring suture inserted into each end of the bowel around each component of the BAR. The device is then snapped shut. Between 11 and 16 days after surgery the fragmented BAR is passed into the stool. An inverted serosa-to-serosa anastomosis is thus created, which can withstand an intraluminal pressure of 70 mm Hg, far above that encountered in intestinal obstruction in humans.8
There have been several randomized prospective trials with the BAR,9,10,11,12,13,14,15 demonstrating that it is a safe alternative to the hand-sewn and stapled techniques of bowel anastomosis. The aim of this chart review was to document outcomes after use of the BAR at our institution in consecutive unselected patients presenting for colon resection.
Methods
Using a registry of cases, we reviewed the charts of 159 patients who underwent elective or emergent small- and large-bowel resection and anastomosis with use of the BAR. The data gathered included the following: patient demographics, the indication for surgery, the date and nature of the operation, the type and location of the anastomosis, size of the BAR used, preoperative state (immunosuppression with steroids and presence of localized sepsis) of the patient, intraoperative and postoperative complications (both related and unrelated to the intestinal procedure), the time to recovery (time to first oral intake and passage of flatus, postoperative length of hospital stay), the need for reoperation and readmission, and the death rate. Early postoperative bowel obstruction was recorded in the first month postoperatively when the patient was unable to tolerate oral intake after documented return of bowel function.
The BAR was used routinely when an anastomosis could be safely created after mobilization of a 2–3-cm segment of bowel proximal and distal to the area of resection, except when extreme size discrepancy precluded its use, and when creating rectal anastomoses within 10 cm from the anal verge. The use of steroids was not considered a contraindication to use of the BAR.
Preoperatively the bowel was mechanically cleansed with oral administration of a sodium phosphate solution, and 3 doses of neomycin (1 g) and metronidazole (750 mg) given by mouth the day before surgery. This was accompanied by a diet restricted to clear fluids and was administered at home to allow same-day admission to hospital (after February 1995). In addition, patients received 1 g cefazolin intravenously 1 hour before incision. In emergency cases, bowel preparation was omitted, and patients received broad-spectrum antibiotics intravenously before operation. The technical details for insertion of the BAR were as described by Hardy and associates.4,5,6 A special metal clamp was used to facilitate insertion of the pursestring suture, which was a 3-0 MAXON suture on a straight needle. The luminal size was determined by inserting a graduated cone. The size that could be accommodated snugly but not to the point of rupture was selected. The gap size was 2.0 mm in general for colon, 1.5 mm for small bowel and 2.5 mm for thicker tissue. Drains were not used, and wounds were closed primarily. Postoperatively, the patients were kept fasting until signs of return of bowel function appeared, at which time their diets were gradually advanced. When steroids were used preoperatively, these were tapered rapidly after surgery.
Unless otherwise specified, the denominator for the percentages calculated was the number of patients who had anastomoses performed (counting patients with concurrent anastomoses only once). This number was 165. Student's t-test was used to compare average time to passage of flatus, oral intake and postoperative length of hospital stay among groups of patients according to an emergent indication for surgery, an immunocompromised state, and the presence of intra-abdominal sepsis. A probability value of less than 0.05 was considered significant.
Results
Between 1992 and 1999, 1 surgeon (P.B.) constructed 173 anastomoses with the BAR using standard technique.4,5,6 Six patients had 2 simultaneous anastomoses, 1 patient had 3 simultaneous anastomoses, and 6 patients had 2 anastomoses constructed on separate occasions. There were 84 women and 75 men, with a mean age of 53.9 years (range from 18–86 yr).
The indications for surgery are shown in Table 1, the types of anastomoses performed are shown in Table 2. All 4 sizes of the BAR were used, with the 28-mm (2-mm gap) most frequently inserted (Table 3).
Table 1

Table 2
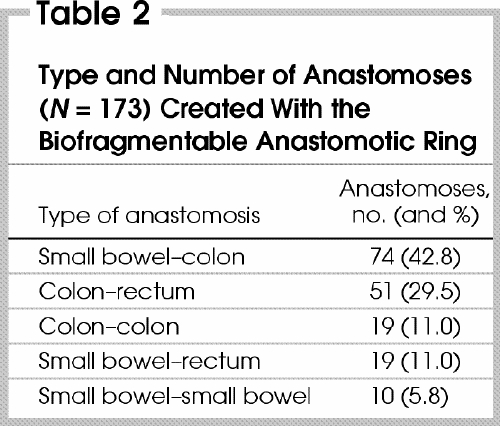
Table 3
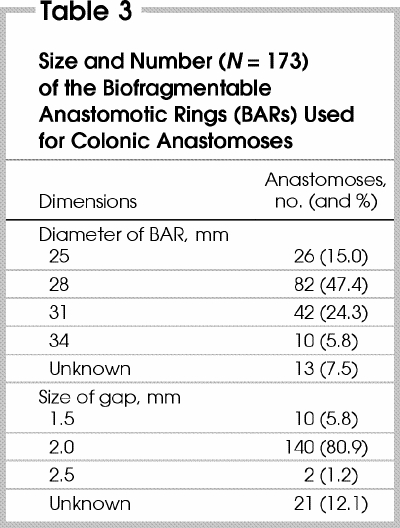
In 23 (13.9%) of the patients, anastomosis was performed under emergent conditions (mainly for bowel obstruction or diverticulitis). In 44 patients (26.7%) the patient was immunosuppressed (most were taking steroids for inflammatory bowel disease). Thirty (18.2%) patients were found to have localized intra-abdominal sepsis at the time of operation. Sixteen anastomoses were constructed in patients with abscess or fistula secondary to Crohn's disease, 12 in patients with diverticular abscess or fistula, 3 in a patient with a perforated tumour with abscess, and 2 in a patient with a postoperative enterocutaneous fistula. The BAR was never used in the presence of generalized peritonitis.
Complications
There were 7 instances intraoperatively where use of the BAR proved difficult: in 3 patients the anastomosis required suture reinforcement, in 2 instances the pursestring needed to be constructed by hand due to technical difficulties with the pursestring device, in 1 patient the entire anastomosis was reconstructed by hand, and in 1 patient there was difficulty inserting the BAR for a distal rectal anastomosis. In no case was use of the BAR abandoned completely for technical reasons once it was considered to meet the criteria of size accommodation.
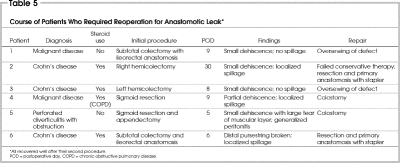
Postoperatively, 7 (4.2%) patients had clinically determined anastomotic leaks, which occurred in the first 2 weeks after the procedure (Table 4). Four of these 7 patients were immunosuppressed (taking steroids). These 7 patients had undergone a variety of procedures. Three with inflammatory bowel disease underwent subtotal colectomy with ileorectal anastomosis, right hemicolectomy and left hemicolectomy. A fourth with malignant disease, who was taking prednisone for chronic obstructive pulmonary disease (COPD), had sigmoid resection. A fifth patient underwent emergent sigmoid resection and appendectomy for acute diverticulitis with fistula to the appendix and large-bowel obstruction. Spasm of the distal bowel was seen, and the anastomosis was reinforced with sutures. The sixth patient had a subtotal colectomy with ileorectal anastomosis for malignant disease. The last patient underwent a second stage Hartmann procedure (first stage performed emergently for ischemic colitis). The first 6 patients required repeat laparotomy with either primary repair if the leak was diagnosed within 6 hours and with minimal contamination, or stoma formation with repair when advanced reaction was found (Table 5). The seventh patient was managed conservatively. All 7 patients responded to treatment.
Table 4

Table 5
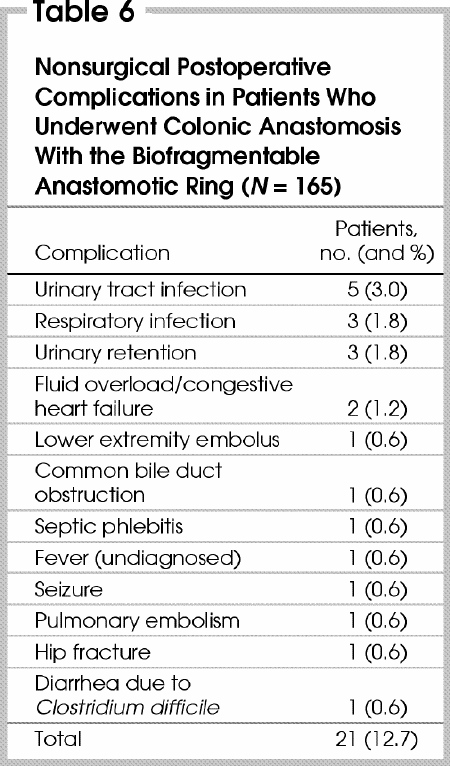
Small-bowel obstruction developed in 13 patients (7.9%) (Table 4); 3 (23%) of them required readmission for bowel obstruction. All 13 episodes resolved without operative intervention. We examined the role of device size and rate of obstruction and could not find a correlation. Three (1.8%) patients had a late stricture, all within 1 year of operation (Table 4). Two (colorectal and colocolonic anastomoses) were successfully treated with endoscopic dilatation on 2 or fewer occasions. The third patient (colorectal anastomosis), who had had an anastomotic leak treated conservatively, required endoscopic dilatation 8 months postoperatively, and later had an episode of small-bowel obstruction, treated conservatively. Subsequently, he had 2 episodes of documented Ogilvie's syndrome. There were no deaths directly related to use of the BAR. The surgical postoperative complications are shown in Table 4, and the nonsurgical complications in Table 6.
Table 6
Recovery
The average hospital stay postoperatively was 9.0 days (range from 3–112 d). Prolonged stay was seen in patients with multiple medical problems, advanced malignant disease and complex postoperative courses. The average time to passage of first flatus was 3.2 days (range from 1–8 d). Patients were allowed a clear fluid diet an average of 3.3 days postoperatively (range from 1–10 d). All patients were tolerating a solid diet at the time of discharge.
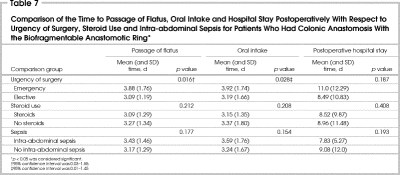
The effects of emergent indication for surgery, immunosuppression with steroids and presence of intra-abdominal sepsis on the average time to passage of flatus and to oral intake, and on postoperative hospital stay were assessed (Table 7). It is interesting to note that there was a marginally significant difference in the time to passage of flatus (3.9 v. 3.1 d) and oral intake (3.9 v. 3.2 d) between the emergent and elective surgery groups, with shorter times in the elective group.
Table 7
Discussion
Three criteria must be fulfilled for any intestinal anastomosis: maintenance of an adequate blood supply, serosal apposition without tension and an uncompromised lumen with a watertight seal. In experimental studies on dogs and pigs,4 the BAR has been shown to cause the least amount of tissue necrosis when compared with stapled and hand-sewn techniques. Its edges are scalloped to prevent tissue strangulation, and in the closed position, the gap is 1.5, 2.0 or 2.5 mm wide. This allows the surgeon to account for bowel-wall thickness and postoperative swelling by using the appropriate-sized device.
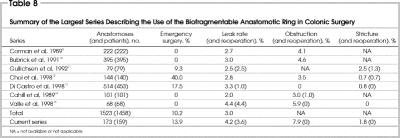
The BAR is designed to create and maintain serosa-to-serosa apposition with a watertight seal. It was shown to withstand an intraluminal pressure of 70 mm Hg immediately after its construction and to have the highest burst pressure on day 0 and equal pressures on days 7 and 16 compared with hand-sewn and stapled anastomoses.4 In the largest series using the BAR,9,10,11,12,13,14,15 the aggregate clinical leak rate was 3.0% (Table 8). This represents 46 leaks in 1458 anastomoses. In those studies that were randomized controlled trials, the leak rates obtained with the BAR (2.0%–3.0%)9,10,11,12,14 were comparable to those obtained with stapled anastomoses (1.9%–6.2%) and hand-sewn anastomoses (2.5%–8.2%).9,10,11,12,14 Our study showed a leak rate of 4.2% (7 leaks in 165 patients), comparable to the rates obtained in the largest series (Table 8 9,10,11,12,13,14,15). Conclusions from such comparisons are clearly limited by the fact that ours was not a randomized controlled trial.
Table 8
It is interesting that 4 of the 7 leaks in the current study occurred in the group of 44 patients taking corticosteroids at the time of surgery. The numbers in the series are too small to permit valid statistical analysis; however in a retrospective study of 764 patients, Golub and associates16 found a leak rate of 3.4% among anastomoses performed with the hand-sewing and stapling techniques. In their univariate, and then multivariate analysis, use of corticosteroids was found to be an independent risk factor for the occurrence of an anastomotic leak. The other variables that independently predicted anastomotic leaks were the presence of COPD, bowel obstruction, peritonitis, intraoperative transfusion of more than 2 units, and a serum albumin level less than 3.0 g/L. In our series, one patient who had an anastomotic leak had COPD and another had a large-bowel obstruction. The role these variables played in our leak rate is unclear due to our small number of patients and leaks.
One question that remains unanswered is the safety of the BAR in high-risk colonic anastomoses. The largest series9,10,11,12,13,14,15 to date using the BAR included a mix of high- and average-risk anastomoses, so more detailed comparisons cannot be made. In their 1997 randomized study of 100 patients, Pahlman and colleagues17 reported similar complication rates in high-risk and average-risk colonic anastomoses, including leak rates. Two studies18,19 of anastomoses in irradiated bowel in dogs showed that the BAR method was as safe as the hand-sewn and stapled techniques, with a suggestion in one study of a lower leak rate with use of the BAR and the indication that a 2.0-mm gap is better than a 1.5-mm gap. In a study done by Maney and colleagues,20 mean burst/leak pressures were similar in anastomoses done with the BAR, sutures and staples in dogs immunosuppressed perioperatively with steroids. Our series includes immunosuppressed patients (26.7%), emergent operations (13.9%) and the presence of intra- abdominal sepsis (18.2%) in a significant number of anastomoses. Although not limited to outcomes in high-risk cases, we have shown good results in this heterogeneous group of patients.
The minimal internal diameter of the BAR is 14 mm, and studies have shown similar rates of early bowel obstruction when comparing the BAR to conventional methods of anastomosis.9,10,11,14 In our study, the rate of postoperative small-bowel obstruction was comparable to that of other series (Table 8). In 1993, Gullichsen and associates21 published the results of long-term follow-up in 30 patients with a BAR anastomosis (mean time elapsed from operation was 24.5 mo). Only 1 patient required reoperation for stricture, and none required endoscopic treatment. In our study, 3 patients had anastomotic strictures, all within 1 year of surgery, and all strictures were successfully treated with endoscopic dilatation.
A study by Dyess and associates22 has shown that anastomosis with the BAR is rapid. The time to complete an intestinal anastomosis was 22 minutes with the BAR (range from 5–43 min), 37 minutes with sutures (range from 15–90 min) (p < 0.01) and 33 minutes with the stapler (range from 12–88 min) (p < 0.06). As Corman and associates9 pointed out, the rapidity of the BAR technique would be significant when constructing multiple anastomoses. An added advantage of the BAR method is the uniform technique used throughout the colon and small bowel. This is not the case with the use of staplers, where different instruments are used in various configurations. The BAR also obviates the need for an additional enterotomy, and since it can fashion an end-to-end anastomosis, less mobilization of the bowel is necessary, leading to less trauma to the tissues and decreased operative time. The device is also less expensive than multiple applications of staplers in performing anastomoses.
The BAR has also been used in upper gastrointestinal surgery with good results. Gullichsen and associates23 showed its safety in jejunojejunal anastomoses when compared with sutured anastomoses. Thiede and colleagues24 reported on the use of the BAR in the upper gastrointestinal tract, including the stomach, and for side-to-side enteral anastomoses, in a multicentre European trial. The study showed an acceptable clinical leak rate of 0.58% and no ileus postoperatively secondary to the BAR.
In recent reports, use of the BAR has been described in laparoscopic procedures. Some have used the device to construct extracorporeal anastomoses with ease and good results.25,26 Additionally, a transanal inserter has been devised to allow intracorporeal construction of an anastomosis in laparoscopic anterior resection.27 Another device has been devised to construct low rectal anastomoses in open surgery.7,28 Although this provides a method of inserting and closing the BAR in a short rectal stump, it is still necessary to tie a pursestring around the BAR deep in the pelvis. In addition, the patients with low rectal anastomoses experienced tenesmus and increased frequency of evacuations until the fragmented BAR passed.
One criticism of the BAR method has been the steep learning curve of the technique. It has been reported by several authors that intraoperative difficulties with the BAR occur mostly during the first several cases10,12,24,29 and are often the result of choosing a device that is too large. We also found this to be the case, and subsequently we found the BAR easy to use in all types of intestinal anastomoses, given the uniformity of the technique. We currently employ the BAR in the majority of anastomoses and have adopted it in laparoscopic-assisted intestinal resections.
Conclusions
Our review has shown that the BAR is safe in elective lower gastrointestinal surgery, with acceptable incidences of anastomotic leak, bowel obstruction and anastomotic stricture, as has been shown by other prospective series reported in the literature. The BAR has the advantage of being a rapid and uniform method that requires less mobilization of tissues, and that can also be used in the upper gastrointestinal tract and laparoscopic bowel surgery. It also seems to be safe in emergency surgery and in high-risk anastomoses, although concomitant steroid use may be associated with a higher risk of leakage.
We believe the BAR method is a viable alternative to the conventional methods of bowel anastomoses (hand-sewing and stapling) in low-risk intestinal surgery. Complications related to its use appear equivalent to other conventional methods. Its use in high-risk anastomoses needs to be further investigated.
Presented at the meeting of the Canadian Society of Colon and Rectal Surgeons, Montréal, Que., Sept. 25, 1999.
Competing interests: None declared.
Correspondence to: Dr. Paul Belliveau, Division of General Surgery, Rm. 482A, Hotel Dieu Hospital, 166 Brock St., Kingston ON K7L 5G2; fax 613 546-4854; bellivep@kgh.kari.net
Accepted for publication Sept. 9, 2002.
References
- 1.Denans FN. Nouveau procédé pour la guérison des plaies des intestins. Recueil de la Société Royale de Médecine de Marseille (Séance de 24 fév. 1826, rédigée par MP Roox). Tome I. Marseille: Imprimerie d'Archard; 1827. p. 127-31.
- 2.Amat C. Appareils à sutures: les viroles de Denans; les pointes de Bonnier; les boutons de Murphy. Arch Med Pharmacie Militaires Paris 1895;25:273-85.
- 3.Murphy JB. Cholecysto-intestinal, gastro-intestinal, enterointestinal anastomosis, and approximation without sutures. Med Rec 1892;42:665-76.
- 4.Hardy TG Jr, Pace WG, Maney JW, Katz AR, Kaganov AL. A biofragmentable ring for sutureless bowel anastomosis: an experimental study. Dis Colon Rectum 1985;28:484-90. [DOI] [PubMed]
- 5.Hardy TG Jr, Aquilar PS, Stewart WR, Katz AR, Maney JW, Costanzo JT, et al. Initial clinical experience with a biofragmentable ring for sutureless bowel anastomosis. Dis Colon Rectum 1987;30:55-61. [DOI] [PubMed]
- 6.Hardy TG Jr, Stewart WR, Aquilar PS, Maney JW, Katz AR, Costanzo JT, et al. Biofragmentable ring for sutureless bowel anastomosis: early clinical experience. Contemp Surg 1987;31:39-44. [DOI] [PubMed]
- 7.Chen TC, Yang MJ, Chang CP. New anastomotic gun for biofragmentable anastomotic ring in low anterior resection. Dis Colon Rectum 1995;38:1214-6. [DOI] [PubMed]
- 8.Irvin TT, Hunt TK. Reappraisal of the healing process of anastomosis of the colon. Surg Gynecol Obstet 1974;138:741-6. [PubMed]
- 9.Corman ML, Prager ED, Hardy TG Jr, Bubrick MF. Comparison of the Valtrac biofragmentable anastomosis ring with conventional suture and stapled anastomosis in colon surgery: results of a prospective, randomized clinical trial. Dis Colon Rectum 1989;32:183-7. [DOI] [PubMed]
- 10.Bubrick MP, Corman ML, Cahill CJ, Hardy TG Jr, Nance FC, Shatney CH, et al. Prospective, randomized trial of the biofragmentable anastomosis ring. Am J Surg 1991;161:136-43. [DOI] [PubMed]
- 11.Gullichsen R, Havia T, Ovaska J, Rantala A. Colonic anastomosis using the biofragmentable anastomotic ring and manual suture: a prospective, randomized study. Br J Surg 1992;79:578-80. [DOI] [PubMed]
- 12.Choi HJ, Kim HH, Jung GJ, Kim SS. Intestinal anastomosis by use of the biofragmentable anastomotic ring: Is it safe and efficacious in emergency operations as well? Dis Colon Rectum 1998;41: 1281-6. [DOI] [PubMed]
- 13.Di Castro A, Biancari F, Brocato R, Adami EA, Truosolo B, Massi G. Intestinal anastomosis with the biofragmentable anastomosis ring. Am J Surg 1998;176: 472-4. [DOI] [PubMed]
- 14.Cahill CJ, Betzler M, Gruwez JA, Jeekel J, Patel JC, Zederfeldt B. Sutureless large bowel anastomosis: European experience with the biofragmentable anastomosis ring. Br J Surg 1989;76:344-7. [DOI] [PubMed]
- 15.Valle M, Biancari F, Caviglia A, D'Andrea V, Baselice PF. The biofragmentable anastomosis ring in elective colon resections. Int Surg 1998;83:58-9. [PubMed]
- 16.Golub R, Golub RW, Cantu R Jr, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg 1997;184:364-72. [PubMed]
- 17.Pahlman L, Ejerblad S, Graf W, Kader F, Kressner U, Lindmark G, et al. Randomized trial of a biofragmentable bowel anastomosis ring in high-risk colonic resection. Br J Surg 1997;84:1291-4. [DOI] [PubMed]
- 18.Croston JK, Jacobs DM, Kelly PH, Feeney DA, Johnston GR, Strom RL, et al. Experience with the biofragmentable anastomotic ring in bowel preoperatively irradiated with 6000 rad. Dis Colon Rectum 1990;33:222-6. [DOI] [PubMed]
- 19.Smith AD, Bubrick MP, Mestitz ST, Crouch FM, Johnston GR, Feeney DA, et al. Evaluation of the biofragmentable anastomotic ring following preoperative irradiation to the rectosigmoid in dogs. Dis Colon Rectum 1988;31:5-9. [DOI] [PubMed]
- 20.Maney JW, Katz AR, Li LK, Pace WG, Hardy TG. Biofragmentable bowel anastomosis ring: comparative efficacy studies in dogs. Surgery 1988;103:56-62. [PubMed]
- 21.Gullichsen R, Ovaska J, Havia T, Yrjana J, Ekfors T. What happens to the Valtrac anastomosis of the colon? A follow-up study. Dis Colon Rectum 1993;36:362-5. [DOI] [PubMed]
- 22.Dyess DL, Curreri PW, Ferrara JJ. A new technique for sutureless intestinal anastomosis: a prospective, randomized, clinical trial. Am Surg 1990;56:71-5. [PubMed]
- 23.Gullichsen R, Ovaska J, Rantala A, Havia T. Small bowel anastomosis with the biofragmentable anastomosis ring and manual suture: a prospective, randomized study. World J Surg 1992;16:1006-9. [DOI] [PubMed]
- 24.Thiede A, Geiger D, Dietz UA, Debus ES, Engemann R, Lexer GC, et al. Overview on compression anastomoses: biofragmentable anastomosis ring multicenter prospective trial of 1666 anastomoses. World J Surg 1998;22:78-87. [DOI] [PubMed]
- 25.Polglase AL, Skinner SA, Johnson WR. Laparoscopic assisted right hemicolectomy with Valtrac BAR (Biofragmentable Anastomotic Ring) ileotransverse anastomosis. Aust N Z J Surg 1993;63:481-4. [DOI] [PubMed]
- 26.Sackier JM, Slutzki S, Wood C, Negri M, Moor EV, Halevy A. Laparoscopic endocorporeal mobilization followed by extracorporeal sutureless anastomosis for the treatment of carcinoma of the left colon. Dis Colon Rectum 1993;36:610-2. [DOI] [PubMed]
- 27.Thaler K, Schoenleben F, Scheidbach H, Koeckerling F, Hohenberger W, Schneider I. Laparoscopic-assisted colon anastomosis using the Valtrac ring: an animal study. Dis Colon Rectum 1999;42:1196-9. [DOI] [PubMed]
- 28.Chen TC, Ding KC, Yang MJ, Chang CP. New device for biofragmentable anastomotic ring in low anterior resection. Dis Colon Rectum 1994;37:834-6. [DOI] [PubMed]
- 29.McCue JL, Phillips RK. Sutureless intestinal anastomoses. Br J Surg 1991;78:1291-6. [DOI] [PubMed]