Abstract
Venous thromboembolism is the most common preventable cause of death in surgical patients. Thromboprophylaxis, using mechanical methods to promote venous outflow from the legs and antithrombotic drugs, provides the most effective means of reducing morbidity and mortality in these patients. Despite the evidence supporting thromboprophylaxis, it remains underused because surgeons perceive that the risk of venous thromboembolism is not high enough to justify the potential hemorrhagic complications of anticoagulant use. The risk of venous thromboembolism is determined by patient characteristics and by the type of surgery that is performed. In this paper we identify the risk factors for venous thromboembolism and provide a scheme for stratifying surgical patients according to their risk. We describe the mechanism of action of the various forms of thromboprophylaxis and outline the evidence supporting thromboprophylaxis in different surgical settings. Finally, we recommend optimal forms of thromboprophylaxis in patients who undergo various types of surgery. Intermittent pneumatic compression, with or without elastic stockings, can be used for thromboprophylaxis in patients who undergo neurosurgical procedures; for patients who undergo vascular or cardiovascular procedures, long-term acetylsalicylic acid should be used for thromboprophylaxis. Low-molecular-weight heparin (LMWH) or warfarin is the choice for patients with spinal cord operations and all patients with major trauma who do not have contraindications to anticoagulation should receive thromboprophylaxis with LMWH.
Abstract
La thromboembolie veineuse est la cause de décès évitable la plus courante chez les patients en chirurgie. La thromboprophylaxie pratiquée par des moyens mécaniques pour promouvoir l'écoulement veineux des jambes et l'administration d'antithrombotiques constitue le moyen le plus efficace de réduire la morbidité et la mortalité chez ces patients. En dépit des données probantes à l'appui de la thromboprophylaxie, elle est toujours sous-utilisée parce que les chirurgiens croient que le risque de thromboembolie veineuse n'est pas assez important pour justifier les complications hémorragiques que pourrait entraÎner l'utilisation d'anticoagulants. Le risque de thromboembolie veineuse est déterminé par les caractéristiques du patient et par le type d'intervention chirurgicale pratiquée. Dans cette communication, nous décrivons les facteurs de risque de thromboembolie veineuse et présentons un programme de stratification des patients en chirurgie en fonction du risque. Nous décrivons le mode d'action des diverses formes de thromboprophylaxie et présentons un aperçu des données probantes qui appuient la thromboprophylaxie dans différents contextes chirurgicaux. Nous recommandons enfin des formes optimales de thromboprophylaxie chez les patients qui subissent divers types d'interventions chirurgicales. On peut recourir à la compression pneumatique intermittente, avec ou sans bas élastiques, comme thromboprophylaxie chez les patients qui subissent des interventions neurochirurgicales. Dans le cas de ceux qui subissent une intervention vasculaire ou cardiovasculaire, il faudrait utiliser l'acide acétylsalicylique à long terme comme thromboprophylaxie. L'héparine de faible poids moléculaire (HFPM) ou la warfarine représentent le traitement de choix dans le cas des patients qui subissent une intervention chirurgicale à la moelle épinière et tous les patients qui ont subi un traumatisme majeur et qui ne présentent pas de contre-indication à l'anticoagulation devraient recevoir une thromboprophylaxie à l'HFPM.
Venous thromboembolism (VTE), which encompasses pulmonary embolism (PE) and deep vein thrombosis (DVT), is a major cause of morbidity and mortality in hospitalized patients. It is estimated that 100 000 patients die from PE each year in the United States.1 In the majority of these patients, the diagnosis was not suspected before death, highlighting the fact that fatal PE can be the first manifestation of asymptomatic DVT. Unrecognized DVT also can lead to long-term morbidity from post-phlebitic syndrome and may predispose patients to recurrent VTE.2
Because VTE in hospitalized patients often is asymptomatic, it is inappropriate to rely on early diagnosis. Furthermore, noninvasive tests, such as compression ultrasonography, have limited sensitivity for a diagnosis of asymptomatic DVT. Thromboprophylaxis is, therefore, the most effective strategy to reduce morbidity and mortality from VTE in surgical patients. Despite this evidence, thromboprophylaxis is underused in clinical practice because surgeons believe that the risk of VTE is too low to justify the potential hemorrhagic complications resulting from the use of anticoagulants.3 Focusing on surgical patients, in this paper we shall (a) list the risk factors for VTE in surgical patients, (b) provide a scheme for stratifying patients according to their risk of VTE, (c) outline the various forms of thromboprophylaxis and describe their mechanism of action, (d) review the evidence for thromboprophylaxis in different clinical settings, and (e) provide recommendations as to the optimal forms of thromboprophylaxis for each patient group.
Risk factors for venous thromboembolism in surgical patients
The risk of VTE is determined by patient characteristics and the clinical setting. They include, major medical illnesses, obesity, risk factors such as previous VTE, cancer, age over 60 years, prolonged immobilization, lower limb paralysis, use of hormonal therapy (oral contraceptives or hormone replacement therapy) and comorbid conditions, such as stroke, congestive heart failure or recent myocardial infarction. Biochemical abnormalities also may predispose patients to VTE. These risk factors can be inherited or acquired. Inherited abnormalities include deficiencies of antithrombin, protein C or protein S, activated protein C resistance, which usually is caused by the Factor V (Leiden) mutation, and the prothrombin gene mutation. Acquired abnormalities include antiphospholipid antibody syndrome, myeloproliferative disorders, particularly essential thrombocythemia and polycythemia rubra vera, and paroxysmal nocturnal hemoglobinuria.
Clinical settings associated with a high incidence of VTE include major orthopedic surgery of the lower limbs, particularly elective hip or knee arthroplasty or surgery for hip fracture, surgery for cancer, neurosurgery, acute spinal cord injury and multiple trauma.2
Risk stratification for venous thromboembolism
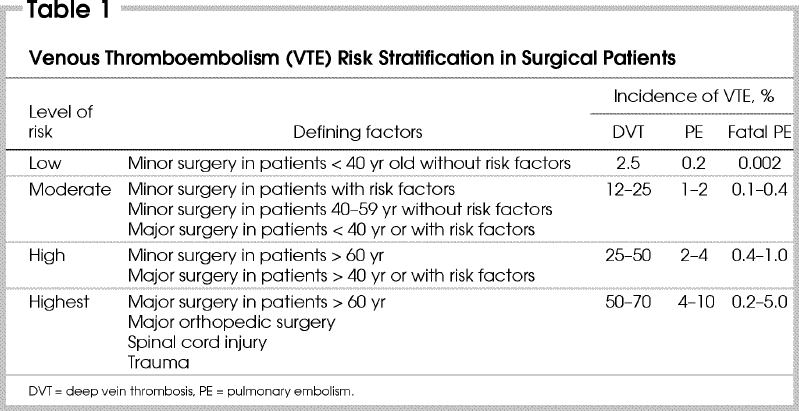
Patients can be stratified for risk of VTE according to their age, presence or absence of other risk factors for VTE and the type of surgery that they are to undergo (Table 1). Those at low risk do not need specific therapy apart from early mobilization, whereas those at moderate or higher risk should receive thromboprophylaxis.
Table 1
Thromboprophylactic measures and their mechanism of action
Both mechanical and pharmacologic agents can be used for thromboprophylaxis. Mechanical methods serve to prevent venous stagnation in the lower limbs by promoting venous outflow, whereas pharmacologic methods act by attenuating coagulation. Compression elastic stockings and intermittent pneumatic compression are the mechanical methods used for prophylaxis, whereas anticoagulants, such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH) and warfarin, or antiplatelet agents, particularly acetylsalicylic acid, are the pharmacologic agents used for this purpose. A recent addition to this list is synthetic pentasaccharide, known as fondaparinux, which has been licensed in the United States for thromboprophylaxis in high-risk orthopedic patients.
Anticoagulants
UFH and LMWH act as anticoagulants by binding to antithrombin and accelerating the rate at which it inhibits clotting factors, particularly thrombin and activated Factor X (Factor Xa). The interaction of UFH and LMWH with antithrombin is mediated by a unique pentasaccharide sequence found on one-third or one-fifth of the chains of UFH and LMWH, respectively.4,5 Fondaparinux, a synthetic analogue of this naturally-occurring pentasaccharide sequence, also acts as an anticoagulant by binding antithrombin.6
LMWH is produced by depolymerizing UFH to generate heparin chains with a mean molecular weight one-third that of UFH (i.e., 5000 Da and 15 000 Da, respectively). The shorter LMWH chains have better bioavailability after subcutaneous injection than the longer chains of UFH, and LMWH has a longer half-life than UFH. LMWH also is associated with a lower incidence of heparin-induced thrombocytopenia.5
The anticoagulant profile of LMWH differs from that of UFH.5 To catalyze Factor Xa inhibition by antithrombin, heparin needs only to bind to antithrombin via its pentasaccharide sequence; an interaction that induces conformation changes in the reactive centre loop of antithrombin and accelerates its rate of Factor Xa inactivation. In contrast, to catalyze thrombin inactivation by antithrombin, heparin must bind to both antithrombin and thrombin, thereby bridging inhibitor and enzyme together. Only heparin chains comprising the pentasaccharide and at least 13 additional saccharide units, corresponding to a molecular weight of 5400 Da or higher, are of sufficient length to provide this bridging function. Because at least half the chains of LMWH are too short to provide this bridging function, LMWH has greater inhibitor activity against Factor Xa than thrombin. In contrast, all the chains of UFH are long enough to bridge antithrombin to thrombin, endowing it with equal inhibitory activity against Factor Xa and thrombin.
With a molecular weight of about 1500 Da, fondaparinux is too short to bridge antithrombin to thrombin. Consequently, fondaparinux catalyzes Factor Xa inhibition by antithrombin but has no effect on the rate of thrombin inactivation. Fondaparinux exhibits excellent bioavailability after subcutaneous injection and is given once daily.6
UFH, LMWH and fondaparinux usually are started postoperatively (Table 2) to reduce the risk of spinal hematoma, a rare, but devastating, complication of spinal puncture for spinal or epidural anesthesia.2 When these agents are given in prophylactic doses, anticoagulation monitoring is unnecessary. Warfarin also is used for thromboprophylaxis, but it must be monitored so that the dose can be titrated to achieve an International Normalized Ratio (INR) of 2–3.
Table 2

Antiplatelet drugs
Acetylsalicylic acid inhibits platelets by permanently acetylating cyclooxygenase-1, the enzyme involved in the first step in the synthesis of thromboxane A2, a potent platelet agonist. Because it blocks platelet and megakaryocyte cyclooxygenase-1, its effects persist for the lifetime of the platelets. With a platelet lifespan of about 10 days and 10% replacement of circulating platelets per day, half of the antiplatelet effect of acetylsalicylic acid is reversed within 5–6 days of stopping the drug.
Thienopyridines, which include ticlopidine and clopidogrel, irreversibly inhibit platelet ADP receptors. Both agents must undergo hepatic transformation to generate metabolites that inhibit these receptors. Consequently, their onset of action is delayed unless loading doses are given.
Clopidogrel is replacing ticlopidine because of safety and convenience advantages. Unlike ticlopidine, neutropenia, thrombocytopenia and thrombotic thrombocytopenic purpura are rare complications of clopidogrel therapy. Furthermore, clopidogrel can be given once daily, whereas ticlopidine must be given twice daily. Clopidogrel or ticlopidine is a reasonable alternative for patients allergic to acetylsalicylic acid.
Thromboprophylaxis in various clinical settings
The risk of VTE varies depending on the type of surgery and on patient characteristics. Data on thromboprophylaxis in patients who undergo general surgery (including gynecologic and urologic surgery), orthopedic surgery and neurosurgery will be presented. Information on thromboprophylaxis in patients with acute spinal cord injury and trauma also will be provided because they often require surgical consultation.
General surgery
From a pooled analysis of events observed in control patients included in randomized trials comparing various methods of prophylaxis with placebo, the incidence of venographically confirmed DVT in general surgery patients not receiving prophylaxis is about 20%.2 Of these thrombi, 6%–7% involve the proximal deep veins (i.e., the popliteal or more proximal veins), a site from which embolization is more likely. The incidence of clinically overt PE and fatal PE in this patient population is at least 1.6% and 0.9%, respectively.2
Low-dose UFH (5000 U subcutaneously 2–3 times daily starting 2 h before the procedure) and LMWH are both effective at reducing the occurrence of DVT in general surgery patients. According to meta-analyses and large clinical trials, low-dose UFH reduces the incidence of DVT from about 25% to 8%, and lowers the incidence of clinically overt and fatal PE by 50% and 90%, respectively. Large trials comparing low-dose UFH with LMWH in general surgical patients and meta-analysis of these trials suggest that the 2 agents are equally effective.7 LMWH, however, has certain advantages. It can be given once daily and it is less likely to cause heparin-induced thrombocytopenia. The only disadvantage of LMWH is that it costs more than low-dose UFH, a feature that has limited its use for this indication in many North American centres.
Some studies reported fewer wound hematomas and less bleeding with LMWH, while others have shown the opposite effects. These discrepancies appear to be related to the dose of LMWH used. Doses of LMWH in excess of 3400 U/d produce more bleeding than low-dose UFH, whereas lower doses of LMWH are associated with less bleeding without loss of efficacy. When used in doses less than 3400 U, the first dose of LMWH can be given 2 hours before operation. Initiation of therapy preoperatively may prevent DVT during or immediately after surgery.
Elastic stockings reduce DVT and enhance the protection afforded by low-dose UFH or LMWH.8 Whether elastic stockings reduce proximal DVT or PE is unknown. In high-risk patients, however, it is reasonable to combine the use of elastic stockings with low-dose UFH or LMWH.
A meta-analysis by the Antiplatelet TrialistsÍ Collaboration suggests that perioperative acetylsalicylic acid therapy reduces the incidence of DVT and PE by 37% and 71%, respectively, reductions that are highly significant.9 These results must be interpreted with caution, however, because most of the individual trials included in this meta-analysis showed no significant benefit of acetylsalicylic acid or demonstrated that it was less effective than other forms of thromboprophylaxis. Consequently, acetylsalicylic acid cannot be recommended as the sole form of thromboprophylaxis in general surgery patients.
Although data are limited, warfarin given postoperatively is likely to be an effective form of prophylaxis. The delayed onset of action of warfarin and the need for INR monitoring are major drawbacks that limit the use of warfarin in this setting.
Based on the risk stratification scheme shown in Table 1, low-risk patients do not require prophylaxis other than early mobilization. For those at moderate to high risk, low-dose UFH or LMWH is appropriate, and either agent can be combined with the use of elastic stockings in patients at highest risk. Warfarin given postoperatively is likely to be a suitable alternative to parenteral anticoagulants in high-risk patients.
General surgery in cancer patients
Patients who undergo general surgery for cancer have a 29% incidence of venographically-detected DVT compared with 19% in those without cancer.2 Low-dose UFH and LMWH apper to be equally effective and safe in this patient group, and either agent can be used. Because patients with underlying cancer are at higher risk, it is reasonable for them to use elastic stockings in conjunction with these agents.
Major orthopedic surgery
Patients who undergo major orthopedic surgery, which includes elective hip or knee arthroplasty or surgery for hip fracture, have a high incidence of postoperative VTE. Consequently, primary prophylaxis is mandatory. The optimal duration of thromboprophylaxis in these patients is controversial, but recent studies suggest that extended prophylaxis may be beneficial.
Elective hip arthroplasty
Without prophylaxis, the incidence of venographically-detected DVT is 51% in patients who have elective hip arthroplasty. Of these thrombi, half involve the proximal veins.2 Although 2 separate meta-analyses indicated that low-dose UFH and acetylsalicylic acid are more effective than placebo at reducing the incidence of postoperative DVT in this setting, both regimens are less effective than LMWH or warfarin. Consequently, neither low-dose UFH nor acetylsalicylic acid is recommended.
Postoperative LMWH or warfarin are the regimens most widely used for thromboprophylaxis in North America. LMWH is started 12–24 hours after operation and is given once or twice daily thereafter. Warfarin is started the evening after operation, and the dose is titrated to achieve an INR of 2–3. The risk of bleeding at the surgical site is slightly higher with LMWH than with warfarin because LMWH produces more rapid anticoagulation.10
Preoperative low-dose UFH followed by postoperative UFH in doses adjusted to maintain the activated partial thromboplastin time (aPTT) at, or just above, the upper range of normal (so-called adjusted-dose UFH) is effective and safe. This regimen is not widely used, however, because frequent laboratory monitoring is needed to ensure appropriate UFH dosing.
Neither elastic stockings nor intermittent pneumatic compression reduces the incidence of proximal DVT in patients who undergo elective hip arthroplasty, although both modalities lower the rate of calf DVT. Because of their limited efficacy, mechanical methods should not be used as the sole form of thromboprophylaxis, but they can be combined with LMWH or warfarin. Fondaparinux also is effective in patients undergoing elective hip arthroplasty. Cost considerations may limit its use, however, because fondaparinux is more expensive than LMWH.6
Elective knee arthroplasty
Without prophylaxis, the incidence of venographically-detected DVT is 61% in patients who undergo elective knee arthroplasty. About 25% of these thrombi involve the proximal veins.2 Low-dose UFH and acetylsalicylic acid reduce the incidence of DVT, but these agents are less effective than LMWH or warfarin. Although LMWH and warfarin are the prophylactic agents of choice, both are less effective for preventing DVT in patients who undergo elective knee arthroplasty than they are in those who undergo elective hip replacement. This primarily reflects their relative inability to reduce the rate of distal DVT in knee surgery patients.
Studies that compared LMWH with warfarin in patients undergoing elective knee arthroplasty demonstrated lower rates of venographically-detected DVT with LMWH than with warfarin. Even with LMWH, however, rates of DVT remain at 25%–45%. Because it produces more rapid anticoagulation than warfarin, LMWH causes more bleeding at the surgical site.10
Geerts and associates2 have shown that intermittent pneumatic compression is an effective form of thromboprophylaxis in knee surgery patients. The utility of intermittent pneumatic compression is limited, however, because it is cumbersome and can only be applied when patients are in hospital. Intermittent pneumatic compression is a reasonable alternative to LMWH or warfarin in patients at high risk for bleeding.
Surgery for hip fracture
Without prophylaxis, the incidence of venographically-detected DVT is about 51% in patients who undergo surgery for hip fracture. About 50% of the thrombi involve the proximal veins, a situation analogous to that in elective hip arthroplasty.2 These findings suggest that direct trauma to the proximal veins at the time of surgery may be an important trigger for DVT in both settings.
Thromboprophylaxis in patients with hip fracture is problematic. Patients tend to be elderly, and have multiple medical problems that increase their risk of bleeding. Furthermore, surgery often is delayed, and prolonged immobilization predisposes these patients to VTE. Two small trials showed a 27% incidence of DVT despite low-dose UFH. Pooled results from 5 trials with LMWH and 5 studies with low- intensity warfarin (INR of 1.2–1.5) showed similar rates of DVT to that achieved with low-dose UFH. Acetylsalicylic acid reduces the risk of fatal PE and DVT, but is less effective than LMWH or warfarin and, therefore, is not recommended.11 Perhaps the most promising agent is fondaparinux. When compared with LMWH, fondaparinux produced a reduction in the incidence of both proximal and distal DVT. At present, however, LMWH or warfarin is most often used for thromboprophylaxis in patients undergoing surgery for hip fracture. Neither intermittent pneumatic compression nor elastic stockings have been well evaluated in this setting, but intermittent pneumatic compression is a reasonable alternative to anticoagulants in patients at high risk for postoperative bleeding.
Optimal duration of thromboprophylaxis
The optimal duration of thrombo- prophylaxis in patients subjected to major orthopedic surgery remains controversial. Six randomized clinical trials showed a reduction in the rate of venographically-detected DVT when thromboprophylaxis is extended from approximately 1 week to 1 month after elective hip arthroplasty.12 Because most venographically- detected thrombi are asymptomatic, their clinical relevance is uncertain. Cohort studies have shown that the rate of symptomatic VTE after 7–10 days of in-hospital thromboprophylaxis with either warfarin or LMWH is about 3.5%–4.0% in the first 3 months after elective hip or knee arthroplasty.2 A meta-analysis of the venographic studies suggested that extended thromboprophylaxis reduces symptomatic VTE from 3.3%–1.3%.12 Consequently, there is a trend toward extended prophylaxis with LMWH or warfarin. Based on all available information, we believe it is reasonable to give thromboprophylaxis for at least 10 days and, at a minimum, provide extended prophylaxis for patients who are not fully mobile or have risk factors for VTE.
Vascular and cardiothoracic surgery
Little information is available on the risk of VTE in patients who undergo vascular or cardiothoracic surgery. However, the incidence in this population is likely to be 25%–30%, a rate similar to that in general surgery patients. Although not well studied in vascular or cardiothoracic surgery patients, low-dose UFH and LMWH are reasonable choices for thromboprophylaxis.
Acetylsalicylic acid improves graft patency in patients who undergo coronary artery bypass grafting. It should be given in a dose of 325 mg once daily for at least 1 year, and patients with underlying coronary artery disease or ongoing risk factors for coronary artery disease should receive long-term acetylsalicylic acid therapy. Most patients who have peripheral vascular reconstructive surgery have generalized atherosclerotic disease. These patients also should receive long-term acetylsalicylic acid therapy to reduce their risk of cardiovascular events.13 Clopidogrel or ticlopidine is a reasonable alternative in patients with acetylsalicylic acid intolerance.
Elective neurosurgery
Venographic studies have demonstrated an incidence of DVT ranging from 24%–33% in patients using elastic stockings but no pharmacologic thromboprophylaxis.2 About 5% of these thrombi involve the proximal veins.2 Patients with malignant brain tumours are at particularly high risk for VTE. Because of concerns about bleeding, mechanical methods are used more often than anticoagulants in neurosurgical patients. Intermittent pneumatic compression reduces the incidence of DVT from 23%–6%.2 Although elastic stockings alone have been reported to be as effective as the combination of elastic stockings and intermittent pneumatic compression, recent studies have questioned the effectiveness of elastic stockings in patients who have surgery for brain tumours.2
Low-dose UFH or LMWH reduces the incidence of DVT in neurosurgical patients. Compared with elastic stockings alone, the combination of LMWH and elastic stockings reduced DVT and proximal DVT rates from 33% and 13%, respectively, to 17% and 5%, respectively.14 If low-dose UFH or LMWH is used, treatment should be started postoperatively to reduce the risk of intraoperative bleeding.
In summary, intermittent pneumatic compression, with or without elastic stockings, can be used for thromboprophylaxis. Other options are postoperative low-dose UFH or LMWH, with or without concomitant elastic stockings.
Acute spinal cord injury
Patients with acute spinal cord injury have the highest rate of DVT among all hospital admissions.15 Rates of symptomatic DVT and PE are 15% and 5%, respectively in this patient group.2 Despite increased awareness about the importance of VTE as a complication, PE remains the third most common cause of death in spinal cord injury patients.
Low-dose UFH has been compared with adjusted-dose UFH or LMWH in spinal cord injury patients. Based on small randomized trials, low-dose UFH is less effective than adjusted-dose UFH or LMWH, and LMWH is more effective than adjusted-dose UFH.2 Intermittent pneumatic compression alone is ineffective in this patient population. Spinal cord injury patients remain at risk for thrombosis for at least 3 months, particularly if the injury is complete. Consequently, these patients benefit from extended prophylaxis using LMWH or warfarin in doses sufficient to produce an INR of 2–3.
Trauma
Patients with trauma, particularly those with multiple trauma, are at high risk for VTE. A prospective cohort study13 of such patients found a rate of venographically detected DVT and proximal DVT of 58% and 18%, respectively. Advanced age, lower limb or pelvic fractures, spinal cord injury, prolonged immobilization and the use of femoral venous catheters are factors associated with an increased risk of DVT. In a randomized trial comparing low-dose UFH with LMWH, rates of DVT and proximal DVT were reduced from 44% and 15%, respectively, with low-dose UFH to 31% and 6%, respectively, with LMWH. Overall rates of major bleeding were less than 2% in both groups.13 Based on these data, all patients with major trauma who do not have contraindications to anticoagulation should receive thromboprophylaxis with LMWH. Thromboprophylaxis should be continued until hospital discharge, or longer, if patients remain immobilized.
Recommendations
General recommendations
It is imperative that every hospital should develop a thromboprophylaxis strategy. Acetylsalicylic acid is not recommended as the sole method of thromboprophylaxis because other methods are more effective. Anticoagulants should be used with caution in patients having spinal puncture or epidural catheter insertion for regional anesthesia or analgesia.
Specific recommendations
General surgery
· Low-risk patients do not need specific prophylaxis, but early ambulation is essential.
· All other patients should receive prophylaxis with low-dose UFH or LMWH. Intermittent pneumatic compression, with or without concomitant elastic stockings, can be used in place of an anticoagulant in those at risk for bleeding.
Orthopedic surgery
· Patients who undergo elective hip or knee arthroplasty or surgery for hip fracture should receive prophylaxis with LMWH (starting 12 h before the procedure, for 12–24 h after, or at half-dose 4–6 h after the procedure followed by full-dose the next day) or warfarin (started after operation and titrated to achieve an INR of 2–3).
· Intermittent pneumatic compression or elastic stockings combined with LMWH or warfarin may provide additional protection. They are unlikely to be as effective as LMWH or warfarin but provide a reasonable alternative in patients at high risk for bleeding.
· Prophylaxis with LMWH or warfarin should be given for at least 10 days. Patients with risk factors for VTE (i.e., cancer, previous VTE, hormone use or biochemical abnormalities) or those who are not mobile should receive prophylaxis with LMWH or warfarin for 30 days.
Vascular or cardiothoracic surgery
· Low-risk patients need no specific prophylaxis except early ambulation.
· Patients requiring prolonged hospitalization should receive postoperative prophylaxis with low-dose UFH or LMWH. Intermittent pneumatic compression, with or without concomitant use of elastic stockings, can be used in place of an anticoagulant in those at risk for bleeding.
· Acetylsalicylic acid (325 mg once daily) should be given for at least 1 year to patients who undergo coronary artery bypass grafting. Patients with underlying coronary artery disease and patients who have peripheral vascular reconstructive surgery because of atherosclerotic disease should receive long-term acetylsalicylic acid. Clopidogrel or ticlopidine is a reasonable alternative in patients with acetylsalicylic acid intolerance.
Neurosurgery
· Intermittent pneumatic compression, with or without concomitant elastic stockings, is the recommended form of thromboprophylaxis. Postoperative low-dose UFH or LMWH is an acceptable alternative.
· The combination of elastic stockings or intermittent pneumatic compression with low-dose UFH or LMWH may be more effective than either modality alone and can be considered for high-risk patients.
Acute spinal cord injury
· LMWH is the best form of prophylaxis. Thromboprophylaxis should be continued into the rehabilitation phase. LMWH or warfarin (in doses titrated to achieve an INR of 2–3) can be used for extended prophylaxis.
· Intermittent pneumatic compression or elastic stockings may be considered if anticoagulants are contraindicated early after injury.
Trauma
· Trauma patients with risk factors for VTE should receive thromboprophylaxis with LMWH provided there are no contraindications to anticoagulation.
· Initial prophylaxis with intermittent pneumatic compression or elastic stockings, or both, should be given if there is a contraindication to anticoagulation.
Acknowledgments
We are indebted to Mrs. S. Crnic for her help preparing the manuscript.
Dr. O'Donnell is the recipient of the R.K. Fraser Fellowship in Thrombosis. Dr. Weitz is a Career Investigator of the Heart and Stroke Foundation of Canada and holds the Heart and Stroke Foundation of Ontario/J.F. Mustard Chair in Cardiovascular Research and the Canada Research Chair in Thrombosis from the Government of Canada.
Correspondence to: Dr. Jeffrey Weitz, Henderson Research Centre, 711 Concession St., Hamilton ON L8V 1C3; fax 905 575-2646; jweitz@thrombosis.hhscr.org
Accepted for publication June 11, 2002.
References
- 1.Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death: the changing mortality in hospitalized patients. JAMA 1986;255:2039-42. [PubMed]
- 2.Geerts W, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, et al. Prevention of venous thromboembolism. Chest 2001;119:132S-175S. [DOI] [PubMed]
- 3.Stratton MA, Anderson FA, Bussey HI, Caprini J, Comerota A, Haines ST, et al. Prevention of venous thromboembolism: adherence to the 1995 American College of Chest Physicians Consensus Guidelines for Surgical Patients. Arch Intern Med 2000;160:334-40. [DOI] [PubMed]
- 4.Hirsh J. Heparin. N Engl J Med 1991;324:1565-74. [DOI] [PubMed]
- 5.Weitz JI. Low-molecular-weight heparin. N Engl J Med 1997;337:688-98. [DOI] [PubMed]
- 6.Turpie AG. Pentasaccharide Org31540 /SR90107A clinical trials update: lessons for practice. Am Heart J 2001;142:S9-15. [DOI] [PubMed]
- 7.Nurmohamed MT, Rosendaal FR, Buller HR, Dekker E, Hommes DW, Vandenbroucke JP, et al. Low-molecular-weight heparin versus standard heparin in general and orthopedic surgery: a meta-analysis. Lancet 1992;340:152-6. [DOI] [PubMed]
- 8.Amarigiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev 2000;(3):CD001484. [DOI] [PubMed]
- 9.Antiplatelet TrialistsÍ Collaboration. Collaborative overview of randomized trials of antiplatelet therapy: III. Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. BMJ 1994;308:235-46. [PMC free article] [PubMed]
- 10.Hull R, Raskob G, Pineo G, Rosenbloom D, Evans W, Mallory T, et al. A comparison of subcutaneous low molecular weight heparin with warfarin sodium for prophylaxis against deep vein thrombosis after hip or knee implantation. N Engl J Med 1993;329:1370-6. [DOI] [PubMed]
- 11.Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial [see comments]. Lancet 2000;355:1295-302. Comments in: ACP Journal Club 2001;134:13; Lancet 2000;355:1288-9. [PubMed]
- 12.Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet 2001;358:9-15. [DOI] [PubMed]
- 13.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against VTE after major trauma. N Engl J Med 1996;335:701-7. [DOI] [PubMed]
- 14.Agnelli G, Piovella F, Buoncristiani P, Severi P, Pini M, DÍAngelo A, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of VTE after elective neurosurgery. N Engl J Med 1998;339:80-5. [DOI] [PubMed]
- 15.Consortium for Spinal Cord Medicine. Prevention of thromboembolism in spinal cord injury. J Spinal Cord Med 1997;20:259-83. [DOI] [PubMed]


