Abstract
Pigmented naphthoquinone derivatives of shikonin are produced at specific times and in specific cells of Lithospermum erythrorhizon roots. Normal pigment development is limited to root hairs and root border cells in hairy roots grown on “noninducing” medium, whereas induction of additional pigment production by abiotic (CuSO4) or biotic (fungal elicitor) factors increases the amount of total pigment, changes the ratios of derivatives produced, and initiates production of pigment de novo in epidermal cells. When the biological activity of these compounds was tested against soil-borne bacteria and fungi, a wide range of sensitivity was recorded. Acetyl-shikonin and β-hydroxyisovaleryl-shikonin, the two most abundant derivatives in both Agrobacterium rhizogenes-transformed “hairy-root” cultures and greenhouse-grown plant roots, were the most biologically active of the seven compounds tested. Hyphae of the pathogenic fungi Rhizoctonia solani, Pythium aphanidermatum, and Nectria hematococca induced localized pigment production upon contact with the roots. Challenge by R. solani crude elicitor increased shikonin derivative production 30-fold. We have studied the regulation of this suite of related, differentially produced, differentially active compounds to understand their role(s) in plant defense at the cellular level in the rhizosphere.
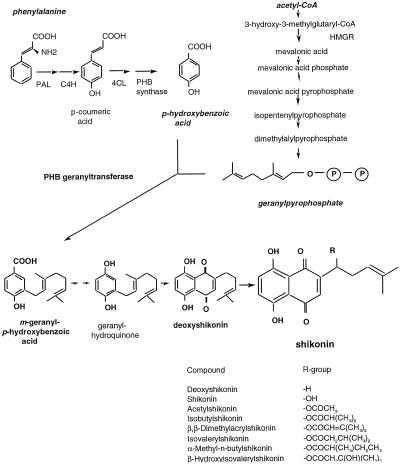
Plants communicate with their environment (the soil, climate conditions, neighboring plants, microorganisms, insects, etc.) by producing a diverse array of chemicals. These secondary metabolites are often characteristic of specific plants and plant families. Many members of the Boraginaceae family produce naphthoquinones in their roots. Naphthoquinones are colored substances derived from phenylpropanoid and isoprenoid precursors (Gaisser and Heide, 1996). Plants of the borage family are distributed worldwide and the naphthoquinones from many of these plants have been used in diverse cultures as colorants for cosmetics, fabrics, and foods (Jain and Mathur, 1965; Ballantine, 1969; Tabata and Fujita, 1985), and for medicinal applications, including antitumor, antiinflammatory, and antimicrobial agents (Papageorgiou, 1978; Tabata and Fujita, 1985). The chemicals involved in the antimicrobial activities studied to date are all derivatives of shikonin and its enantiomer alkannin from the European dye plant Alkanna tinctoria. Figure 1 illustrates the chemical structure of shikonin, its derivatives, and the proposed biosynthetic pathway. The pattern of shikonin derivatives may differ in any one plant, root culture, or cell-suspension culture (summarized from a literature survey; specific refs. are given in Table I).
Figure 1.
Proposed biochemical pathway for shikonin and shikonin derivative synthesis (after Gaisser and Heide, 1996). Derivatives of shikonin are formed by replacement of the R-group with the various fatty acid chains listed.
Table I.
Shikonin derivatives detected in plants, cell suspension, and callus cultures
| Source | DS | S | AS | IBS | DMAS | IVS | MBS | HIVS |
|---|---|---|---|---|---|---|---|---|
| Rootsa | X | X | X | X | ||||
| Rootsb | X | X | X | X | X | |||
| Rootsc | X | X | X | X | X | X | X | X |
| Callus/rootsd | X | X | X | X | X | X | X | |
| Callus/rootse | X | X | X | X | X | X | X | X |
| Cells/rootsf | X | X | X | X | X | X | X | X |
| Rootsg | X | X | X | X | X | X |
X's indicate that the derivative was present.
Because of its value as a pharmaceutical agent, most research to date has focused on understanding the production and regulation of shikonin with the goal of increasing production of the compound, but the biological significance of shikonin in the plant has been virtually ignored. Because shikonin and its derivatives have biological activity against microorganisms, it seems likely that these compounds may play a role in plant defense in the rhizosphere. Naphthoquinones have been shown to function in allelopathy (juglone; Binder et al., 1989), plant-insect interactions (plumbagin; Kubo and Klocke, 1986), and electron transport (phylloquinone [vitamin K1]; Hauska, 1988). Many of the products of the phenylpropanoid and isoprenoid pathways are produced in response to multiple stresses (for review, see Chappell, 1995; Dixon and Paiva, 1995) and frequently lead to the production of antimicrobial compounds (Bennett and Wallsgrove, 1994; Rhodes, 1994; Chappell, 1995; Herrmann, 1995). Regulation under numerous conditions has been studied using cell-suspension cultures (Table II), but nothing is known about the regulation of production of the specific derivatives of shikonin or of the regulation in specific cell types within the root. Shikonin accumulates in the cork layer of mature roots (Fujita and Yoshida, 1937) and its formation in significant quantities gives the roots a characteristic red or purple color. Production and accumulation in new roots has not been systematically studied, nor has the biological activity of these compounds in relation to soil-borne microorganisms.
Table II.
Factors affecting production of shikonin in cell-suspension cultures
| Increased Production | Decreased Production |
|---|---|
| Auxina | Light, white or bluea |
| Streptomycin (inhibitor of protein synthesis)b | 2,4-Da |
| Ascorbic acidb | p-Coumeric acidb |
| Suc > 5%b | Benzoic acidb |
| l-Pheb | p-Hydroxybenzoic acid (in Linsmaier and Skoog medium)b |
| Nitrate depletionc | Ammoniumc |
| Copper sulfated | Glyd |
| Endogenous polysaccharidese | Lack of acidic polysaccharidesf |
| Fungal elicitation (Penicillium)g | Glnh |
| Oligosaccharides of cell wall 12- to 22-mersi | Mevinolin (inhibitor of 3-hydroxy-3-methylglutaryl-CoA reductase)i |
| Methyl jasmonatej | 2-Aminoindan-2-phosphonic acid (inhibitor of PAL)j |
| p-Hydroxybenzoic acid (in M-9 medium)k |
Yazaki et al. (1987).
In view of the observations on the antimicrobial properties reported for shikonin and its derivatives, the production of numerous derivatives of shikonin in the roots of the plant, and the existing information on regulation of the pathways in common with many other plant defense responses, we investigated the regulation and biological activity of shikonin and its derivatives in hairy-root cultures of Lithospermum erythrorhizon. Because these compounds are brightly colored, the timing and cellular specificity of their production can be followed with simple equipment such as a dissecting microscope. The suite of compounds can be studied in the context of understanding the multiple actions of plants in fine tuning their response to their environment. The objectives of this study were: (a) to characterize the production of naphthoquinone pigments in L. erythrorhizon hairy-root cultures, (b) to analyze the effect(s) of shikonin and its derivatives on soil-borne microorganisms, and (c) to determine if the L. erythrorhizon hairy-root cultures could respond to microorganisms by modifying the production of shikonin and its derivatives.
MATERIALS AND METHODS
Plant Material
Seeds of Lithospermum erythrorhizon (Sieb. and Zucc.) were obtained from the Institut fur Pflanzengenetik und Kulturpflanzenforschung (Gatersleben, Germany), germinated, and grown in sterile potting soil (Pro Mix BX, Premier Horticulture, Red Hill, PA). The plants were maintained in the greenhouse at 60oC to 80oC with supplemental lighting from 1000-W high-pressure sodium lamps for 8 h daily from October through March and in ambient lighting the remainder of the year. Plants were watered three times per week. Plants were fertilized twice a week with Peters 15–16-17 Peat Lite Special (Scotts-Sierra Horticultural Products, Marysville, OH) at 200 ppm N.
Hairy-Root Production and Growth Conditions
Seeds of L. erythrorhizon were sterilized with 10% commercial bleach for 20 min, rinsed in five changes of sterile water, and germinated under sterile conditions on solid Murashige and Skoog medium (Murashige and Skoog, 1962). Seedlings were grown in GA7 hydroponic culture vessels (Magenta Corp., Chicago, IL), and stems were inoculated with Agrobacterium rhizogenes strain 15834 (ATCC) to produce hairy roots. Root cultures were obtained from 16 independent transformation events. All root culture lines appeared morphologically indistinguishable, and experiments described in this paper were performed on a single root line. Root cultures from the selected line were grown in the dark at 24°C on M medium (Bécard and Fortin, 1988) or M-9 (Fujita et al., 1981a) medium. Roots grown on solid or liquid M medium did not produce shikonin in sufficient quantities to be observable on visual inspection. M-9 served as an induction medium for shikonin because it contains 2.3 times the amount of copper sulfate as the M medium. Liquid root cultures were grown in the dark on a rotary shaker (model G-53, New Brunswick Scientific, Edison, NJ) at 90 rpm at 24°C. Solid root cultures were grown on medium solidified with 0.3% (w/v) Phytagel (Sigma).
Microscopy
Roots and fungal-growth plates were viewed and photographed using a Zeiss STEMI SV8 dissecting microscope and Kodak 160T slide film. Root hairs were viewed and photographed on a Zeiss inverted microscope and Kodak 160T slide film.
Bacterial and Fungal Inhibition Assays
Bacterial Growth Inhibition
Bacterial cultures were grown overnight at 24°C with shaking in Luria-Bertani broth (Luria and Burrows, 1957) to an optical density of 0.2 at 600 nm. A 100-μL aliquot of this liquid culture was spread onto 82-mm plates with solid Luria-Bertani medium. A filter disk saturated with 50 μg of shikonin or shikonin derivative (dissolved in chloroform and allowed to air dry) was placed on the bacterial lawn. The plates were incubated in the dark at 24°C. The clear zone from the filter disk to the bacteria was measured. Each experiment was performed three times for each isolate tested. The following bacterial strains were obtained from a collection of field isolates maintained in the laboratory of Dr. Leland S. Pierson III (Department of Plant Pathology, University of Arizona, Tucson): Bacillus subtilis 613R, Bacillus thuringiensis Gnatrol, Clavibacter michigenensis subsp. nebraskensis CN74-1, Agrobacterium radiobacter K84, Agrobacterium tumefaciens C58, Burkholdaria cepacea Deny, Escherichia coli ESS, Erwinia amylovora, Erwinia carotovora ATCC 15713, Pseudomonas aureofaciens 30-84, Pseudomonas fluorescens 2-79, Pseudomonas syringae B, Pseudomonas syringae pv phaseolicola, Ralstonia solanacearum, and Serratia marsecens. Erwinia herbicola was isolated from pea seedling roots (L.A. Brigham, unpublished data). A. rhizogenes ATCC 15834 was maintained by Paula Michaels at The Pennsylvania State University (University Park). Xanthomonas campestris pv pelargonii was from the collection of Dr. Gary Moorman (The Pennsylvania State University).
Fungal Growth Inhibition
Fungal isolates were maintained on V-8 medium (200 mL of commercial vegetable juice, 2 g of CaCO3, and 1 g of Glc per liter of water) solidified with 2% agar (Difco Laboratories, Detroit, MI) in the dark at 24°C. Inhibition assays were performed by dissolving shikonin in solid M-2 medium (modified Martin's medium: 10 g of Glc, 5 g of peptone, 1 g of KH2PO4 0.5 g of MgSO4, 22 g of agar per liter of double-distilled water) in 35-mm plastic Petri dishes. A 4-mm plug of fungal hyphae was placed on one side of the plate and hyphal length was measured at 24-h intervals. Each isolate was tested on all concentrations in three separate replicates. Fungal isolates Nectria hematococca 34-18 and N. hematococca T488 were from the laboratory cultures of Dr. Hans Vanetten (Department of Plant Pathology, University of Arizona, Tucson). All of the other fungal isolates were from Scott Rasmussen (Department of Plant Pathology, University of Arizona).
Elicitation of Shikonin Derivatives in Liquid Root Cultures
Fungal elicitors were prepared as described previously (Flores et al., 1988). Mycelium from a 2-week-old fungal culture was inoculated into a 2-L Erlenmeyer flask containing 1 L of Schenk and Hildebrandt medium and grown in the dark on a rotary shaker (model G-53, New Brunswick Scientific) at 90 rpm at 25°C for 3 weeks (Schenk and Hildebrandt, 1972). Mycelium was filtered off, resuspended in distilled water, homogenized, centrifuged at 18,000 rpm for 30 min, autoclaved at 121°C for 30 min, and stored at −20°C. A 2.5-mL aliquot of this filtrate was used per 50 mL of root-culture medium. Total shikonin was quantified by treatment of derivatives with 2.5% KOH for 10 min and spectrographic reading at 622 nm (Mizukami et al., 1977).
Identification and Quantification of Compounds
TLC Analysis
Shikonin derivatives were extracted from the roots and liquid media with chloroform. The chloroform layer was collected and evaporated to dryness. The residue was redissolved in chloroform and spotted onto TLC plates (channeled Kieselgel 60CF254, Merck, Darmstadt, Germany) using chloroform as the mobile phase. Standards were obtained from TCI America (Portland, OR). Specific compounds were extracted from the plates by scraping off and grinding the silica with a mortar and pestle, and dissolving in chloroform. The identification of the compounds was verified by comparison of retention time with known standards by HPLC.
HPLC Analysis
HPLC was performed on a C18 60-Å 4-μm column (3.9- × 300-mm, Nova-Pak, Waters) fitted to a HPLC device comprising a system controller (model 600E), a Waters Intelligent Sample Processor (model 712), and a photodiode array detector (model 990, all Waters). The isocratic solvent system was CH3CN:H2O:CH3COOH:Et3N (630:370:3:3, v/v) Chromatography was performed at a flow rate of 1.2 mL min−1, a pressure of 30 kg/cm2, and a column temperature of 23°C. A520 was monitored, and peaks were compared with known standards (TCI America).
RESULTS
Development of Naphthoquinone Pigments in Pot-Grown Plants and in Hairy-Root Cultures
Pigment Development under “Noninducing” Conditions
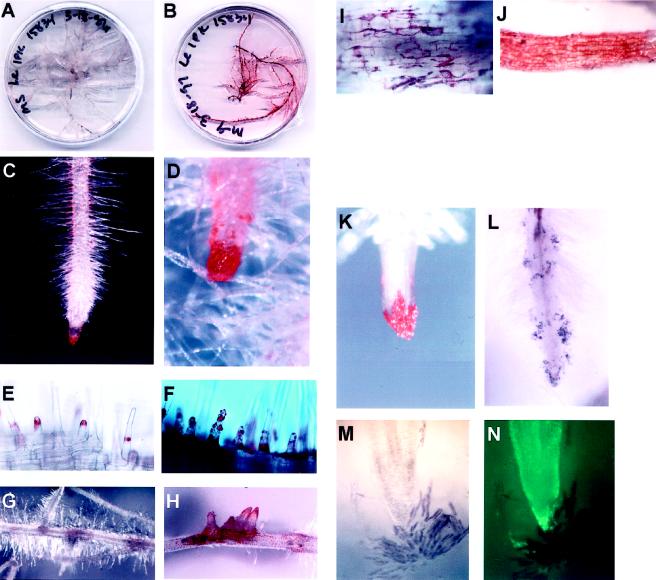
L. erythrorhizon hairy-root cultures grown on solid M medium in the dark at 24°C appeared white (Fig. 2A). Roots grew at a rate of 1 to 2 mm/d. Under magnification pigmentation was apparent in two cell types within these roots: the root hairs (Fig. 2, C and E) and the root border cells (Hawes and Brigham, 1992; Brigham et al., 1995; Hawes et al., 1998) (Fig. 2, C and K). Pigment granules in the root hairs were seen at the apex of the root hairs as they emerged (Fig. 2E). Pigmentation was not apparent before the hair had attained a length of at least 1 mm. As the root hair elongated, the pigment appeared to remain at the same distance from the epidermal surface as when it was first formed. The pigment was limited to a very specific region and appeared to be a ring, perhaps because the vacuole occurred at this region of the root hair. In the root-cap region, pigments were confined to the border cells alone (Fig. 2, K and M). Removing the border cells from the cap revealed that the pigment was formed in the border cells subsequent to separation from the cap, because the cap cells themselves were completely devoid of pigment. Border cells were left behind as the root grew through the medium and, in some cases, appeared to outline the root (Fig. 2L). When border cells were visualized under light microscopy in M medium, they appeared deep purple, as opposed to the white color of the underlying cap cells (Fig. 2M). Cells of the cap take up the vital stain fluorescence diacetate, whereas those of the detached border cells do not (Fig. 2N).
Figure 2.
L. erythrorhizon hairy-root cultures showing pigment formation in different cells under varying conditions. A, Transformed roots growing on M medium. B, Transformed roots growing on M-9 medium. Pigment diffusion from the roots into the medium was apparent at 3 weeks. C, Root tip grown on M medium showing normal pigment production pattern in border cells and root hairs. D, Root tip grown on M-9 medium showing increased pigmentation in border cells and root hairs. E, Pigment deposition patterns in M-grown roots. Emerging root-hair tips have a cap of pigment, which remains at about that same distance from the epidermis as the root hair grows. F, Root hairs of M-9-grown roots showing exudation of pigment in droplets all over the hairs. G, Lateral root formation in M-grown roots. Pigment is apparent in the region of root emergence. H, Lateral root formation in M-9-grown roots. Numerous roots emerge at a single point and pigmentation is more intense than in M-grown roots. I, Pigment deposition in a few of the cells near lateral root eruption. J, Pigment production in all epidermal cells of M-9-grown root. K, Border cell pigmentation on root cap of M-grown root. Pigments are confined to border cells. L, Border cell deposition along growing root in solid M medium. M, Light micrograph of normal (untransformed) root tip placed in water showing dispersion of border cells from the cap. Border cells are purple, cap is white. N, UV fluorescence of root stained with fluorescein diacetate showing living cells of root. Most border cells do not take up the stain.
Border cells with pigment also displayed less distinct cytoplasmic characteristics and failed to exhibit cytoplasmic streaming. Plasmolysis was often evident and in these cases pigmentation was confined to the region of the cytoplasm and the cell walls appeared clear (data not shown). The pigments appeared red when roots were grown in air, away from contact with the medium (Fig. 2K), and deep blue to purple when the cells of the roots were in contact with the medium (Fig. 2L). Shikonin derivatives chelate iron in the medium, turning the compounds purple in the process (L.A. Brigham, unpublished data). As the roots aged, pigmentation occurred at the points of lateral root emergence (Fig. 2G). Higher magnification revealed pigment granules that tended to accumulate in the cell walls (Fig. 2I). Shikonin production was also seen in the oldest parts of the root. The exact cellular location of the pigment was not obvious from visual inspection, because the older root segments also developed a brown coloration, probably as a result of the production of other phenolic compounds. Comparable patterns of red pigment formation were observed in roots grown in pots in the greenhouse. Purple pigmentation was never observed in these roots.
Induction of Shikonin Derivatives
Roots grown on the “inducing” medium (M-9) formed pigments sooner, in greatly increased amounts, and in more cell types than those grown on the noninducing medium (M) described above. L. erythrorhizon hairy-root cultures grown in the dark on M-9 medium at 24°C appeared fully pigmented, and the pigments had diffused into the medium, giving it a pinkish color (Fig. 2B). The root cap appeared to have greatly increased pigmentation and the border cells were not as distinct, probably because of increased mucilage production (Fig. 2D). The root hairs appeared to contain more pigment than the noninduced hairs, and the pigment was distributed throughout the cell (Fig. 2F). The amount of pigment production was increased to the point that it was exuded from the root hair and appeared in droplets on the surface of the hair. Lateral root formation occurred at shorter intervals than in the controls, and clusters of lateral roots were commonly observed emerging from a single site with greatly increased pigment production (Fig. 2H). In contrast to the noninduced state, in which these were the only two cell types that produced pigment, in the induced state pigment was produced in all of the epidermal cells of the root much earlier and before other phenolic products had turned the roots brown (Fig. 2J). The pigments appeared to accumulate in the cell wall and were seen throughout the apoplast.
Effects of Shikonin on Bacterial and Fungal Growth
Because shikonin has been used as an antimicrobial agent in human applications, and because shikonin and its derivatives are produced exclusively by the root of the plant, we tested whether shikonin was inhibitory to soil-borne microorganisms. Both bacterial and fungal isolates were assayed.
Bacterial Growth Inhibition
Bacterial isolates from plant roots covering a broad phylogenetic range were tested for inhibition of growth by the filter-disk method with 50 μg of standard shikonin per filter. The concentration was chosen within the range shown to inhibit human pathogens (Tanaka and Odani, 1972). Of the 31 strains tested, 10 were inhibited to some degree by shikonin. Table III lists a representative sample of the strains tested. Whereas no definite patterns emerge, it is clear that not all strains are sensitive to shikonin and some strains are more sensitive than others. As reported previously by Papageorgiou (1980), the E. coli strains were not affected. The two pathogenic strains of Erwinia were not sensitive, whereas the nonpathogenic E. herbicola was inhibited. All of the Agrobacterium strains were affected, but the nonpathogenic A. radiobacter was inhibited to a greater degree than the pathogenic strains. All gram-positive strains tested were inhibited. In summary, shikonin shows selective inhibition to some bacterial strains, including important root pathogens.
Table III.
Inhibition of bacterial isolates to shikonin, shikonin derivatives, and whole-root chloroform extractions
| Bacterial Isolate | Standards
|
Root
Culture Extractiona
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shikonin | DS | IBS | DMAS | IVS | MBS | AS | M | M-9 | IVS/MBS | AS | HIVS | |
| mm | ||||||||||||
| B. subtilis | 2.0 | 1.0 | 0.5 | 0.0 | 0.5 | 1.0 | 3.5 | 2.5 | 4.0 | 2.0 | 4.0 | 4.0 |
| B. thuringiensis | 2.0 | 0.5 | 0.0 | 0.0 | 0.1 | 0.1 | 2.0 | 2.5 | ndb | 0.0 | 2.0 | 3.0 |
| C. michigenensis subsp. nebraskensis | 4.0 | 4.0 | 1.0 | 1.0 | 0.5 | 1.5 | 4.0 | 4.0 | 2.0 | 1.0 | 3.0 | 4.0 |
| A. radiobacter | 3.0 | 1.0 | 0.5 | 0.0 | 0.0 | 1.0 | 3.0 | 3.0 | 4.0 | 1.0 | 2.0 | 2.0 |
| A. rhizogenes | 1.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.5 | 1.5 | 1.5 | 2.0 | 1.0 | 1.5 | 2.0 |
| A. tumefaciens | 0.5 | 0.0 | 0.1 | 2.0 | 0.1 | 0.1 | 1.5 | 3.0 | 3.0 | 2.0 | 2.0 | 3.0 |
| B. cepacea | 2.0 | 2.0 | 2.0 | 0.0 | 0.5 | 0.5 | 2.0 | 3.0 | 4.0 | 0.5 | 2.0 | 3.0 |
| E. coli | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| E. amylovora | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 |
| E. carotovora | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 |
| E. herbicola | 1.5 | 0.5 | 0.1 | 0.1 | 0.1 | 0.5 | 2.0 | 2.0 | 2.0 | 0.5 | 2.0 | 3.0 |
| P. aureofaciens | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | nd | nd | nd | nd |
| P. fluorescens | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | nd | nd | nd | nd |
| P. syringae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| P. syringae pv phaseolicola | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R. solanacearum | 1.0 | 1.0 | 0.1 | 0.1 | 0.1 | 0.5 | 3.0 | 3.0 | 2.0 | 0.5 | 2.0 | 2.0 |
| S. marsecens | 1.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 3 | 2.0 | nd | nd | nd | nd |
| X. campestris pv pelargonii | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Data are presented as the size of the zone of inhibition. In all cases, sd was less than 0.289.
Chloroform extraction of pigments from roots grown in liquid culture. Separation of components by TLC as described in Methods.
nd, Not determined.
Fungal Growth Inhibition and Morphological Changes
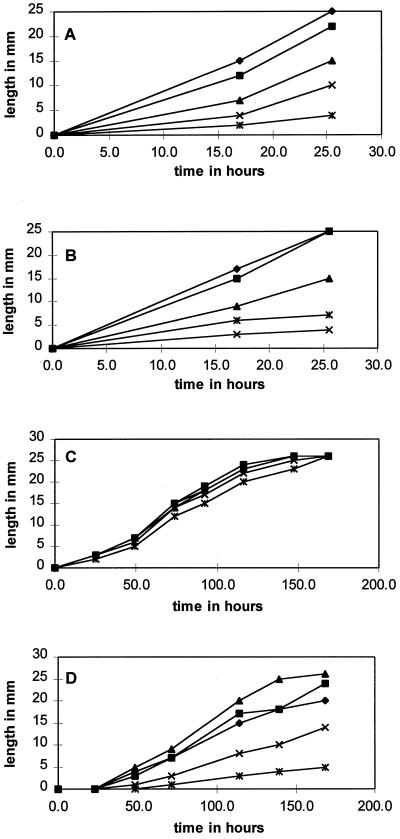
Hyphal growth inhibition was tested by a linear growth assay. Twelve fungal isolates were tested and showed a wide range of growth responses on medium containing 5, 50, 100, and 200 μg/mL shikonin standard. Growth was measured for each isolate until the hyphae of any treatment reached the edge of the 35-mm plate. The length of time for the control to reach the edge of the plate varied from 1 d (Pythium aphanidermatum) to 9 d (Colletotrichum destructivum). Figure 3 illustrates four different growth patterns. Pythium ultimum showed increasing sensitivity to increasing amounts of shikonin (Fig. 3A). P. aphanidermatum was increasingly inhibited by increasing concentrations of shikonin until the highest concentration (200 μg/mL), at which point it showed a slight increase in growth over the 100 μg/mL concentration (Student's t test, P = 0.06; Fig. 3B). Nectria hematococca 34-18 showed little inhibition of hyphal growth even at the highest shikonin concentration (Fig. 3C). Phytophthora parasitica grew faster than the control at 5 and 50 μg/mL (Student's t test, P = 0.01; Fig. 3D). Comparisons of growth patterns among all of the fungi tested were made by calculation of hyphal growth on each concentration of shikonin as a percentage of the growth of the control (Table IV).
Figure 3.
Fungal growth on medium containing no shikonin (control, ♦), 5 μg/mL shikonin (▪), 50 μg/mL shikonin (▴), 100 μg/mL shikonin (×), or 200 μg/mL shikonin (✷). A, P. ultimum. B, P. aphanidermatum. C, N. hematococca 34-18. D, P. parasitica.
Table IV.
Fungal growth on five different concentrations of shikonin (in μg/mL) as a percentage of growth of the control
| Fungi | 0 | 5 | 50 | 100 | 200 |
|---|---|---|---|---|---|
| % | |||||
| P. aphanidermatum | 100 | 100 | 60 | 16 | 28 |
| P. ultimum | 100 | 88 | 60 | 40 | 16 |
| Monosporascus cannonballus | 100 | 83 | 111 | 111 | 94 |
| F. oxysporum | 100 | 109 | 100 | 87 | 48 |
| F. proliferata | 100 | 100 | 92 | 72 | 52 |
| C. destructivum | 100 | 96 | 96 | 62 | 42 |
| R. solani | 100 | 100 | 60 | 16 | 0 |
| A. niger | 100 | 104 | 92 | 83 | 75 |
| N. haematococca 34-18 | 100 | 104 | 100 | 96 | 87 |
| N. haematococca T488 | 100 | 96 | 85 | 73 | 62 |
| P. parasitica | 100 | 120 | 130 | 70 | 25 |
| P. capsicii | 100 | 104 | 80 | 60 | 28 |
Data are presented as the percentage of growth compared with the control, and represent the averages of three separate experiments, each with three replicates. The coefficients of variation (V = [s × 100]/X; V, coefficient of variation; s, sd; X, mean) ranged from 2% for the controls to 16% for the treatments in which growth was most strongly inhibited.
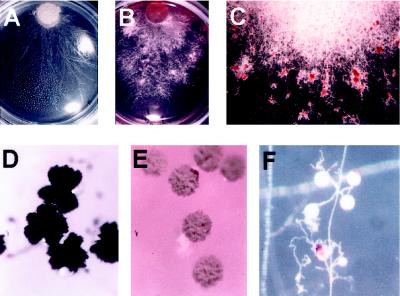
In addition to the variability of the effect of shikonin on hyphal growth rates, other changes were observed. Some fungal isolates changed the color of the medium containing shikonin from pink to varying shades of purple and blue, with each isolate producing a slightly different hue (data not shown). It is known that some fungi are capable of changing the pH of their surroundings, but pH measurements were not undertaken in this study. The hyphae of Rhizoctonia solani formed fairly uniform sheets over the control plates (Fig. 4A). However, at increasing concentrations of shikonin, the hyphal formations exhibited clumping and apparent sequestration of pigment within the clumps (Fig. 4, B and C). Aspergillus niger, which was only moderately inhibited by shikonin, showed significant changes in both morphology and pigment formation in its spores. Spores formed on plates with 5 μg/mL (Fig. 4D) were darker than the controls, and had a dumbbell shape similar to the controls, but with increasing amounts of shikonin, the spores showed less pigmentation. The spores formed on plates with 200 μg/mL shikonin (Fig. 4E) were beige and rounded. Glomus intraradices was not tested for growth inhibition because it was not known at the time if the obligate mycorrhizal fungus would infect L. erythrorhizon. However, co-cultivation of hairy roots of carrot, G. intraradices (Chabot et al., 1992), and L. erythrorhizon showed that this fungus also sequestered the pigments. Red pigment could be seen moving within the hyphae and, over several weeks, the pigments were deposited on the outside of a small percentage (less than 5%) of the fungal spores (Fig. 4F). Thus, as in the case of bacterial strains tested, fungal isolates exhibited differential sensitivity to shikonin. Several known pathogenic soil-borne fungi such as Rhizoctonia, Pythium, and Phytophthora were significantly inhibited by shikonin. In addition, morphological changes were often observed in hyphal growth patterns and spore formation.
Figure 4.
Changes in fungal morphology when grown on medium containing shikonin. A, R. solani hyphal growth on control plate showing an even growth pattern. B, R. solani hyphal growth on 100 μg/mL shikonin showing clumping of hyphae. C, R. solani hyphal growth on 200 μg/mL shikonin showing sequestration of shikonin pigment in the hyphal clumps. D, Spores of A. niger grown on 5 μg/mL shikonin. Spores are dark and dumbbell shaped. E, Spores of A. niger grown on 200 μg/mL shikonin. Spores are beige and round. F, Spores of G. intraradices grown on M medium in the presence of L. erythrorhizon hairy roots. Pigment is not apparent in the medium by visual inspection. Pigment is transported through the hyphae and deposited on a small fraction of the spores in any one area.
Production of Shikonin Derivatives
The production of different shikonin derivatives in roots sold commercially, in cell-suspension cultures, and in callus cultures has been analyzed by several researchers in China and Japan (Table I). Different subsets of the known shikonin derivatives are found in different species of Lithospermum, in different sources of individual species, and in different cell lines. Not all of the known compounds are found in a single plant or laboratory root culture. However, AS and HIVS were found in all samples in these studies. We wanted to determine if changes occurred in the production of derivatives under different conditions, including fungal challenge. All experiments were done on root cultures that originated from a single transformation event and were therefore genetically identical. Hairy-root cultures have been used as a tissue-culture tool for studies of secondary metabolite production in roots and have been found by numerous studies to “remain more or less stable in their growth and/or secondary metabolite productivity characteristics for a number of years” (Hamill and Lidgett, 1997). Roots were grown in liquid cultures of M or M-9 medium and challenged with R. solani elicitor preparations. Roots grown in M medium remained white as long as the medium was changed frequently. Roots grown in unchanged (depleted) medium eventually accumulated shikonin derivatives. As shown in Table V, roots grown in M-9 medium formed shikonin derivatives earlier and in greater quantities than those grown in M medium, and the ratios of HIVS to AS varied. HIVS and AS were detected in the greatest abundance in all of the roots sampled, although the relative proportions varied by treatment. IVS and MBS were not readily distinguishable using HPLC under the conditions we used, but one or both were detected in most cultures in the 10% to 15% range. Challenge with R. solani crude elicitor caused a reversal of the ratios of HIVS and AS compared with treatments on M medium. Shikonin derivatives from plants grown in pots in the greenhouse were analyzed and found to contain equal amounts (38% each) of HIVS and AS, 14% of IVS/MBS, and a small amount (2%) of DS. Shikonin was not detected in any of the samples analyzed.
Table V.
Relative ratios of shikonin and derivatives produced under different growth and elicitation conditions
| Media | HIVS | AS | DS | IBS | DMAS | IVS/MBS | Total Shikonina |
|---|---|---|---|---|---|---|---|
| μg/gb | |||||||
| M | 53.91 | 36.59 | 1.07 | 0.68 | 9.75 | 12 | |
| M-9 | 54.96 | 20.63 | 15.18 | 19 | |||
| M-Rsc | 37.21 | 40.60 | 124 | ||||
| M-9-Rs | 23.56 | 52.45 | 0.70 | 1.16 | 16.33 | 334 | |
| Nd | 38.87 | 38.70 | 2.017 | 13.90 |
Total chloform extraction was subjected to 2.5 KOH and A520 was read.
Fresh weight of roots.
M-Rs, R. solani elicitor.
N, Normal roots from pot-grown plants.
Effect of Shikonin Derivatives on Bacterial Growth
Because shikonin itself was shown to have an effect on specific bacterial and fungal soil isolates, further tests were done to determine their sensitivity to the shikonin derivatives and to the combinations of derivatives produced by the roots. As has been reported previously (Tanaka and Odani, 1972; Tabata et al., 1975), E. coli was not sensitive to shikonin or any of its derivatives. All organisms that were insensitive to shikonin were also insensitive to all of the derivatives tested, including the crude whole-root chloroform extract (Table III). However, the two Erwinia species that were insensitive to shikonin were insensitive to the derivatives, but were inhibited by an unknown component of the induced chloroform extract. In most cases, induced extract (M-9) produced the highest inhibition, followed by noninduced extracts. AS, HIVS, and shikonin were often as inhibitory as the whole-extract preparations and were the most-active single compounds. IBS and MBS were found the least inhibitory in most cases. As in the case of the fungal studies on shikonin, color changes in the bacterial plates were varied (data not shown), but pH was not determined. In summary, bacterial isolates showed a wide range of responses to shikonin derivatives singly and in combination. Most isolates were inhibited to the greatest degree by the two derivatives produced in highest abundance by the root cultures.
In Situ Fungal Inhibition and Plant Response to Fungi
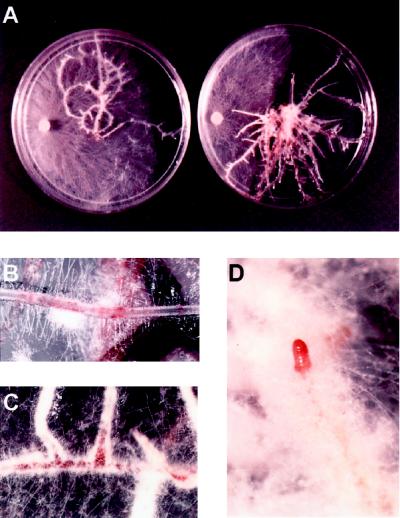
To analyze the dynamics of shikonin production in root cultures on fungi, we placed plugs of fungal hyphae on 4-week-old L. erythrorhizon root cultures on solid M and M-9 medium. One-half of them were grown in the light (to inhibit shikonin derivative production) and one-half were grown in the dark. Four isolates were chosen based on growth rates and response to the shikonin concentration assays (Table VI). The amount of shikonin derivatives the fungi were exposed to on the plates was not quantified. Pigment was apparent upon visual inspection in the roots grown on M-9 at this point. P. aphanidermatum was sensitive to both the existing shikonin derivatives on the M-9 plates and to increased shikonin derivative produced in the dark on both the M and M-9 plates. On the light-grown plates, shikonin production was inhibited and hyphal growth was twice that seen on the dark-grown M plates (Table VI; Fig. 5A). R. solani, which is very sensitive to high concentrations of shikonin (Table VI), was inhibited on the M-9 plates, but not significantly on the M plates, even though the fungus did induce pigment formation in these roots. N. hematococca T488, which is sensitive to shikonin at relatively low concentrations, showed marked inhibition on dark-grown plates whether preinduced or not. A. niger, the least-sensitive and slowest-growing isolate, showed no significant difference between light- and dark-grown plates, preinduced or not. This was the only one of the four isolates tested that did not induce localized pigment production in the roots (Table VI). Figure 5B shows an example of a region of hyphal contact with the root. The cells in the region of contact show considerable pigmentation. Areas normally pigmented, such as sites of lateral root emergence, had fewer hyphae than regions in between (Fig. 5C). However, all regions where hyphae made contact eventually produced pigment. Whereas most of the roots on the plate were eventually overcome by hyphal growth, the root tips were always devoid of hyphae. These tips, like those grown on M-9 medium, were moist and very red, showing increased pigment production (Fig. 5D). Whereas root epidermal cells in direct contact with hyphae showed increased pigment production compared with adjacent noncontact cells, the root caps showed increased production without direct hyphal contact.
Table VI.
Fungal growth on hairy-root cultures
| Fungi | Growth Rate | Hyphal
Growth
|
Inductionb | |||
|---|---|---|---|---|---|---|
| M
|
M-9
|
|||||
| Dark | Light | Dark | Light | |||
| da | mm | |||||
| P. aphanidermatum | 1 | 31 | 57 | 33 | 43 | Y |
| R. solani | 3 | 55 | 60 | 40 | 35 | Y |
| N. haematococca | 7 | 9 | 20 | 9 | 27 | Y |
| A. niger | 9 | 12 | 10 | 12 | 13 | N |
Data are from a single experiment with two replicates. The whole experiment was performed two times with similar results.
Days to reach the opposite side of a 35-mm plate.
Induction of shikonin derivatives in the root (Y, yes; N, no).
Figure 5.
In situ growth inhibition and stimulation of pigment production of R. solani in the presence of L. erythrorhizon hairy roots. A, Hairy roots grown in the light on M medium (left) and grown in the dark on M medium (right). The fungal plug is seen on the left in each plate. B, Pigment formation in root cells at the points of contact with hyphae. C, Accumulation of shikonin at lateral root eruptions on roots grown in M-9 medium showing inhibition of fungal growth. D, Root tip with greatly increased pigment production is free of hyphae, whereas the surrounding root surfaces are completely overcome.
Pigment production in root cultures was also induced with crude preparations of R. solani elicitor. Liquid cultures of L. erythrorhizon hairy roots were grown for 1 month in liquid M medium. One set of flasks received fresh M medium, and the other set received M-9 medium. Root cultures were allowed to grow for 1 d, then 2.5 mL (per 50 mL of medium) of fungal elicitor was added to one-half of the M flasks and one-half of the M-9 flasks. Pigment released into the media was evident visually at the end of 2 weeks. The media were collected at 3 weeks and the shikonin derivatives were extracted in chloroform and analyzed by HPLC. The roots treated with a combination of both M-9 and fungal elicitor showed the strongest response in total pigment produced (Table V). The roots treated with fungal elicitor in the inducing medium produced 3 times as much shikonin as those with fungal elicitor in M medium and almost 30 times as much as the noninduced roots. These results are consistent with the model that shikonin derivatives in the roots function as both preformed and inducible antimicrobial compounds.
DISCUSSION
The transformed roots of L. erythrorhizon appear to be a useful model in which to study the production and potential function(s) of a suite of antimicrobial compounds in plant roots (Rhodes et al., 1997). The derivatives of shikonin are produced in a highly regulated and visible manner in specific cell types of the growing root tip. The compounds have varying degrees of biological activity against a wide range of soil-borne microorganisms. Production of these compounds in changing ratios within the root varies with conditions of stress, including challenge by microorganisms.
The cellular pattern of shikonin derivative production depends on the developmental stage of the root and on environmental signals. In relatively “unstressed” root cultures pigment production is normally evident only in two cell types, the root hairs and border cells. The identification of pigments in these cells often requires magnification. As the radicle of L. erythrorhizon emerges from the seed, the root border cells are already pigmented, and as the root elongates, they remain at intervals along the root as a potential chemical barrier to microbial infection (Hawes and Brigham, 1992; Hawes et al., 1998). Even under unstressed conditions, the border cells in the root cultures are intensely pigmented, showing granules distributed throughout the cell (Tsukada and Tabata, 1984). The underlying cap cells are devoid of pigment. From observation in this study, it appears to be a rapid development, because no intermediate states of pigment production have been observed in border cells. The dynamics of the development of these compounds in border cells remains to be studied. The pigment in the root hairs appears to be localized to a region of the hair between the epidermal surface and the midpoint of the hair. The exact cellular location and the mechanism are unknown. The uniform placement of pigments in the root hairs at some distance from the root surface may also serve as a chemical barrier to microbial invasion. Preformed inhibitors are often distributed in plants in a tissue-specific manner, often in the outer layers of cells of plant organs (Osbourn, 1996). But, unlike many of the preformed inhibitors studied to date, the shikonin derivatives, in addition to being formed as part of normal development, are highly inducible.
In the unstressed roots described above, the pigments appeared to remain within the cells in which they were produced. In contrast, when the roots were specifically stressed by the addition of copper sulfate, the root hairs made significantly more pigments and the epidermal cells produced amounts sufficient to turn the roots “red.” In both these of cell types, the material is clearly extracellular. In root hairs, droplets formed on the surface; in epidermal cells, pigment accumulated in the apoplast. The mechanism by which this is accomplished is unknown. Tsukada and Tabata (1984) have described the formation of pigment within the cells of induced callus cultures as occurring on the rough ER in spherical bodies that break away and migrate to the plasma membrane, presumably releasing their contents. The droplets were found to be composed of 27.2% shikonin, 21.5% proteins, 28.6% lipids, and 22.7% “other” (Tsukada and Tabata, 1984). The caps of induced roots growing aerially in our study appeared moist, possibly because of the active export of these droplets. The amount of the compounds produced in each cell does not appear to be the driving mechanism, because, as we have observed, border cells from noninduced caps are almost completely filled with pigment that is not exported. Yazaki and Matsuoka (1997) have identified a clone from an induced cDNA library that may begin to shed light on this aspect of the regulation.
The ratio of compounds produced in different induced states differs from that in the noninduced state, suggesting that the plant can sense and respond to different environmental and physiological stimuli (Bennett and Wallsgrove, 1994). It has been reported that accumulation of glucosinolates in oilseed rape varies by challenge organism (insect, fungus, mechanical damage) and tissue type (for review, see Bennett and Wallsgrove, 1994). We found that challenge with a crude elicitor from R. solani changes the ratio of HIVS to AS produced by the roots irrespective of the media on which the roots were grown. The production of a set of very similar compounds raises the question of redundancy and whether the duplication is part of the survival strategy to preclude development of resistance by microorganisms. Of the seven compounds identified from L. erythrorhizon roots from potted plants, cell suspension, callus, and root cultures, we found that only three of the compounds were consistently produced in the root cultures and potted plants under the conditions in which they were grown in our laboratory. AS and HIVS, the most biologically active compounds, were the most abundant and were produced under all of the conditions, although in different amounts in relation to each other. The whole-root component mix almost always had the greatest activity against the bacteria tested, which may be attributable to the components acting synergistically, or to additional components soluble in the chloroform-extraction process. It is not known if the mechanisms of action in microorganisms are the same or different for the various compounds.
Microorganisms also influence the chemistry of the products they encounter. Certain fungi are known to change the pH of their surroundings (Bago et al., 1996). For example, Alternaria solani blocks the effects of saponin-based defense chemicals by lowering the pH of the infection site (for review, see Jackson and Taylor, 1996). Shikonin can function as a pH indicator in buffered aqueous solutions (Windholz, 1983). In the hyphal inhibition studies, in which shikonin was dissolved in the medium, there were almost as many color changes as there were fungi studied, indicating that each fungus changed the pH to a different degree. It is not known if pH influences the biological activity of shikonin for a specific microorganism, but alkaline conditions convert all shikonin derivatives to shikonin, which was the third most toxic of the derivatives tested in this study. pH also influences enzymatic optima, membrane permeability, and a host of other conditions that could ultimately determine the toxicity or safety of the environment for the fungus (Marschner, 1995). The composition of microbial populations in the rhizosphere of L. erythrorhizon is unknown, and further work is required to determine the nature of the rhizosphere communities in the various L. erythrorhizon populations.
The results from this study are consistent with the possibility that shikonin derivatives function as both preformed and inducible microbial inhibitors regulated in a cell-specific manner to maximize the effect of highly toxic substances with minimal expense and harm to the plant. The complexity of the rhizosphere is often decried as an almost insurmountable challenge by plant pathologists and physiologists, and thus research has concentrated on the effects of single compounds produced in a plant on specific microorganisms. The system described here may allow us to move toward understanding the complex relationships between multiple compounds influencing multiple organisms in the rhizosphere. Because the shikonin derivatives are produced from two pathways under intense investigation because of their contribution to antimicrobial products, this system may add to our understanding of how the plant regulates these pathways in subtle ways to influence microbial population dynamics in the rhizosphere. Studies are under way to examine the molecular mechanisms of cell-specific expression, regulation of compound ratios, and microbial sensitivity/tolerance mechanisms.
ACKNOWLEDGMENTS
We thank Drs. Leland S. Pierson and Derek Wood (University of Arizona, Tucson) for providing the bacterial strains used in these experiments, Dr. Hans Vanetten and Scott Rasmussen (University of Arizona) for providing most of the fungal isolates, Dr. Martha Hawes for providing laboratory space for the experiments performed at the University of Arizona, Tom Orum (University of Arizona) for providing assistance in the statistical analyses, and Anthony Omeis (Biology Greenhouse at The Pennsylvania State University) for propagating the L. erythrorhizon plants and caring for them in the greenhouse. The carrot hairy-root cultures were a gift from Dr. Roger Koide of The Pennsylvania State University. Dr. Gary Moorman graciously provided a critical reading of the manuscript, and Dr. Kazufumi Yazaki (Kyoto University, Japan) provided invaluable background information, discussion, and advice for this research.
Abbreviations:
- AS
acetyl-shikonin
- DMAS
β,β-dimethylacryl-shikonin
- DS
deoxy-shikonin
- HIVS
β-hydroxyisovaleryl-shikonin
- IBS
isobutyl-shikonin
- IVS
isovaleryl-shikonin
- MBS
α-methyl-n-butyl-shikonin
Footnotes
This research was supported by Department of Energy/National Science Foundation/U.S. Department of Agriculture Collaborative Research in Plant Biology award no. BIR-9220330, “Interdisciplinary Research Training Program in Advanced Root Biology.”
LITERATURE CITED
- Bago B, Vierheilig H, Piché Y, Azcón-Aguilar C. Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture. New Phytol. 1996;133:273–280. doi: 10.1111/j.1469-8137.1996.tb01894.x. [DOI] [PubMed] [Google Scholar]
- Ballantine JA. The isolation of two esters of the naphthoquinone alcohol, shikonin, from the shrub Jatropha glandulifera. Phytochemistry. 1969;8:1587–1590. [Google Scholar]
- Bécard G, Fortin JA. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988;108:211–218. doi: 10.1111/j.1469-8137.1988.tb03698.x. [DOI] [PubMed] [Google Scholar]
- Bennett RN, Wallsgrove RM. Secondary metabolites in plant defense mechanisms. New Phytol. 1994;127:617–633. doi: 10.1111/j.1469-8137.1994.tb02968.x. [DOI] [PubMed] [Google Scholar]
- Binder RG, Benson ME, Flath RA. Eight 1,4-naphthoquinones from Juglans. Phytochemistry. 1989;28:2799–2801. [Google Scholar]
- Brigham LA, Woo HH, Hawes MC. Root border cells as tools in plant cell biology studies. Methods Cell Biol. 1995;49:377–387. doi: 10.1016/s0091-679x(08)61467-3. [DOI] [PubMed] [Google Scholar]
- Chabot S, Bécard G, Piché Y. Life cycle of Glomus intraradix in root organ culture. Mycologia. 1992;84:315–321. [Google Scholar]
- Chappell J. The biochemistry and molecular biology of isoprenoid metabolism. Plant Physiol. 1995;107:1–6. doi: 10.1104/pp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H, Pickard J, Hoy M. Production of polyacetylenes and thiophenes in heterotrophic and photosynthetic root cultures of Asteraceae. In: Lamb J, Breteler H, Arnason T, Hansen L, editors. Chemistry and Biology of Naturally-Occurring Acetylenes and Related Compounds (NOARC). Amsterdam: Elsevier; 1988. pp. 233–254. [Google Scholar]
- Fujita Y, Hara Y, Ogino T, Suga C. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. I. Effects of nitrogen sources on the production of shikonin derivatives. Plant Cell Rep. 1981a;1:59–60. doi: 10.1007/BF00269272. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Hara Y, Suga C, Morimoto T. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. II. A new medium for the production of shikonin derivatives. Plant Cell Rep. 1981b;1:61–63. doi: 10.1007/BF00269273. [DOI] [PubMed] [Google Scholar]
- Fujita N, Maeda Y, Suga C, Morimoto T. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. III. Comparison of shikonin derivatives of cultured cells and Ko-shikon. Plant Cell Rep. 1983;2:192–193. doi: 10.1007/BF00270101. [DOI] [PubMed] [Google Scholar]
- Fujita N, Yoshida Y. Ueber die Anatomie von Radix Lithospermi. Yakugaku Zasshi. 1937;57:368–391. [Google Scholar]
- Fukui H, Tani M, Tabata M. Induction of shikonin biosynthesis by endogenous polysaccharides in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep. 1990;9:73–76. doi: 10.1007/BF00231552. [DOI] [PubMed] [Google Scholar]
- Fukui H, Yoshikawa N, Tabata M. Induction of shikonin formation by agar in Lithospermum cell suspension cultures. Phytochemistry. 1983;22:2451–2453. [Google Scholar]
- Gaisser S, Heide L. Inhibition and regulation of shikonin biosynthesis in suspension cultures of Lithospermum. Phytochemistry. 1996;41:1065–1072. [Google Scholar]
- Hamill JD, Lidgett AJ. Hairy root cultures: opportunities and key protocols for studies in metabolic engineering. In: Doran PM, editor. Hairy Roots: Culture and Applications. Sydney, Australia: Harwood Academic; 1997. pp. 1–29. [Google Scholar]
- Hauska G. Phylloquinone in photosystem I. Trends Biochem Sci. 1988;13:415–416. doi: 10.1016/0968-0004(88)90206-X. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Brigham LA. Impact of root border cells on microbial populations in the rhizosphere. Adv Plant Pathol. 1992;8:119–148. [Google Scholar]
- Hawes MC, Brigham LA, Wen F, Woo HH, Zhu Y. Function of root border cells in plant health: pioneers in the rhizosphere. Annu Rev Phytopathol. 1998;36:311–327. doi: 10.1146/annurev.phyto.36.1.311. [DOI] [PubMed] [Google Scholar]
- Herrmann KM. The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol. 1995;107:7–12. doi: 10.1104/pp.107.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AO, Taylor CB. Plant-microbe interactions: life and death at the interface. Plant Cell. 1996;8:1651–1668. doi: 10.1105/tpc.8.10.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AC, Mathur SK. Bull Natl Inst Sci India. 1965;28:52–56. [Google Scholar]
- Kim DJ, Chang HN. Increased shikonin production in Lithospermum erythrorhizon suspension cultures with in situ extraction and fungal cell treatment (elicitor) Biotech Lett. 1990;12:443–446. [Google Scholar]
- Kubo I, Klocke J. Insect ecdysis inhibitors. In: Green MB, Hedin PA, editors. Natural Resistance of Plants to Pests. Washington, DC: American Chemical Society; 1986. pp. 206–219. [Google Scholar]
- Kyogoku K, Terayama H, Tachi Y, Suzuki T, Komatsu M. Constituents of shikon. II. Comparison of contents, constituents, and antibacterial effect of fat soluble fraction between nanshikon and koshikon. Shoyakugaku Zasshi. 1973;27:31–36. [Google Scholar]
- Luria SE, Burrows JW. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957;74:461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H (1995) Mineral Nutrition in Higher Plants, Ed 2. Academic Press, London, pp 537–595
- Mizukami H, Konoshima M, Tabata M. Effect of nutrition factors on shikonin derivative formation in Lithospermum callus cultures. Phytochemistry. 1977;16:1183–1186. [Google Scholar]
- Mizukami H, Konoshima M, Tabata M. Variation in pigment production in Lithospermum erythrorhizon callus cultures. Phytochemistry. 1978;17:95–97. [Google Scholar]
- Morimoto I, Hirata Y. New naphthoquinone derivatives from Lithospermum erythrorhizon. Tetrahedron Lett. 1966;31:3677–3680. [Google Scholar]
- Morimoto I, Kishi T, Ikegami S, Hirata Y. Naphthoquinone derivatives from Lithospermum erythrorhizon Siebold et Zuccarini. Tetrahedron Lett. 1965;52:4737–4739. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Osbourn AE. Preformed anti-microbial compounds and plant defense against fungal attack. Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou V. Wound healing properties of naphthoquinone pigments from Alkanna tinctoria. Experientia. 1978;34:1499–1501. doi: 10.1007/BF01932375. [DOI] [PubMed] [Google Scholar]
- Papageorgiou V. Naturally occurring isohexenylnaphthazarin pigments: a new class of drugs. Planta Med. 1980;38:193–203. doi: 10.1055/s-2008-1074864. [DOI] [PubMed] [Google Scholar]
- Rhodes MJC. Physiological roles for secondary metabolites in plants: some progress, many outstanding problems. Plant Mol Biol. 1994;24:1–20. doi: 10.1007/BF00040570. [DOI] [PubMed] [Google Scholar]
- Rhodes MJC, Parr AJ, Walton NJ. Studies of secondary metabolic pathways in transformed roots. In: Doran PM, editor. Hairy Roots: Culture and Applications. Sydney, Australia: Harwood Academic; 1997. pp. 31–41. [Google Scholar]
- Schenk RU, Hildebrandt AC. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot. 1972;50:199–204. [Google Scholar]
- Tabata M, Fujita Y. Production of shikonin by plant cell cultures. In: Zaitlin M, Day P, Hollaender A, editors. Biotechnology in Plant Science. San Diego, CA: Academic Press; 1985. pp. 207–218. [Google Scholar]
- Tabata M, Mizukami H, Haoe S, Konoshima M. Anti-microbial activity of Lithospermum erythrorhizon callus cultures. Yakugaku Zasshi. 1975;95:1376–1379. doi: 10.1248/yakushi1947.95.11_1376. [DOI] [PubMed] [Google Scholar]
- Tabata M, Mizukami H, Hiraoka N, Konoshima M. Pigment formation in callus cultures of Lithospermum erythrorhizon. Phytochemistry. 1974;13:927–932. [Google Scholar]
- Tanaka Y, Odani T. Pharmacodynamic study on “shiunko.” I. Antibacterial effect of “shiunko.”. Yakugaku Zasshi. 1972;95:525–530. doi: 10.1248/yakushi1947.92.5_525. [DOI] [PubMed] [Google Scholar]
- Tani M, Fukui H, Shimomura M, Tabata M. Structure of endogenous oligogalacturonides inducing shikonin biosynthesis in Lithospermum cell cultures. Phytochemistry. 1992;31:2719–2723. [Google Scholar]
- Tani M, Takeda K, Yazaki K, Tabata M. Effects of oligogalacturonides on biosynthesis of shikonin in Lithospermum cell cultures. Phytochemistry. 1993;34:1285–1290. [Google Scholar]
- Tsukada M, Fukui H, Habara C, Tabata M. Comparative studies on naphthoquinone derivatives in various crude drugs of “zicao” (shikon) Shoyakugaku Zasshi. 1983;37:299–305. [Google Scholar]
- Tsukada M, Tabata M. Intracellular localization and secretion of naphthoquinone pigments in cell cultures of Lithospermum erythrorhizon. Planta Med. 1984;51:338–341. doi: 10.1055/s-2007-969725. [DOI] [PubMed] [Google Scholar]
- Windholz M, ed (1983) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, Ed 9. Merck & Co., Rahway, NJ
- Yazaki K, Matsuoka H. cDNA cloning and functional analysis of LEDI-2, a gene preferentially expressed in the dark in Lithospermum erythrorhizon cell suspension cultures (abstract no. 1348) Plant Physiol. 1997;114:S-261. [Google Scholar]