Abstract
Introduction
Anorectal malignant tumours are increasing in frequency for unknown reasons. Surgery is the principal treatment, and the role of adjuvant therapy has not been defined. We therefore decided to review the experience of the Princess Margaret Hospital in Toronto, a large tertiary care cancer hospital, with respect to the surgical management of anorectal melanoma.
Methods
We reviewed the charts of all registered patients with anorectal malignant melanoma (AMM) treated with surgery or radiotherapy, or both, at the hospital between 1980 and 1999, paying particular attention to survival, and local and distant recurrences.
Results
There were 14 patients, all of whom were followed up to the time of death or for a minimum of 28 months for surviving patients. The mean ages at diagnosis were 56 years for men and 68 years for women. Clinical staging was as follows: local, 10 patients; locoregional, 3 patients and metastatic disease, 1 patient. Local therapy included local resection alone in 7 cases and abdominoperineal resection in 7. Seven patients received pelvic irradiation at some time during their disease, using different doses and fractionation schemes. Three of them had concomitant chemotherapy and radiotherapy with no tumour regression. In all 3 patients the lesions was reclassified as AMM and the patient underwent surgery. The other 4 patients had a short course of radiotherapy for palliation after the original lesion recurred. The overall median survival was 12 (range from 3–51) months. Two patients remained alive at last follow-up. Patients managed by local excision had a median survival of 12 (range from 3–51) months, and those managed by abdominoperineal resection had a median survival of 7 (range 5–51) months. Of the 10 patients treated initially with local excision, 6 required reoperation. Three underwent salvage abdominoperineal resection. Six patients were alive 1 year after treatment (median survival 32.5 mo [range from 21–51 mo]). Eight patients had a rapid evolution of their disease with a median survival of 5.5 (range from 3–12) months. Eleven of the 12 patients who died had metastatic disease.
Conclusions
Systemic dissemination is almost universal in patients with AMM. The overall survival was poor regardless of local treatment. There was a 60% failure rate of local excision, which necessitated further surgery. Improving local control is important since some patients will survive up to 3 years.
Abstract
Introduction
Les tumeurs malignes anorectales sont de plus en plus fréquentes pour des raisons inconnues. La chirurgie est le principal traitement et l'on n'a pas défini le rôle de la thérapie d'appoint. C'est pourquoi nous avons décidé de passer en revue l'expérience de prise en charge chirurgicale du mélanome anorectal à l'Hôpital Princess Margaret à Toronto, important hôpital de soins tertiaires en oncologie.
Méthodes
Nous avons étudié le dossier de tous les patients inscrits qui avaient un mélanome malin anorectal (MMA) et que l'hôpital a traités entre 1980 et 1999, par chirurgie, par radiothérapie, ou par les deux moyens, en accordant une attention spéciale à la survie et aux récidives locales et éloignées.
Résultats
Sur 14 patients, tous ont fait l'objet d'un suivi jusqu'au moment du décès ou pendant au moins 28 mois chez les survivants. Au moment du diagnostic, les hommes avaient en moyenne 56 ans et les femmes, 68 ans. Le stade clinique était le suivant : local chez 10 patients, locorégional chez 3 patients et métastases chez 1 patient. La thérapie locale a inclus une résection locale seulement dans sept cas et une résection abdominopérinéale dans sept autres. Sept patients ont reçu une irradiation pelvienne à un moment donné au cours de leur maladie, à des doses et des fractionnements différents. Trois d'entre eux ont reçu une chimiothérapie et une radiographie concomitantes sans que la tumeur diminue. Chez les trois patients, on a reclassé les lésions comme MMA et dans chaque cas, le patient a subi une intervention chirurgicale. Les quatre autres patients ont suivi un bref traitement de radiothérapie palliative après la réapparition de la lésion originale. La survie médiane globale s'est établie à 12 mois (plage de 3 à 51). Deux patients étaient toujours vivants au moment du dernier suivi. Les patients traités par exérèse locale présentaient une survie médiane de 12 mois (plage de 3 à 51) et chez ceux qu'on a traités par résection abdominopérinéale, elle s'est établie à 7 mois (plage de 5 à 51). Sur les 10 patients qui ont subi à l'origine une exérèse locale, 6 ont dû être opérés de nouveau. Trois ont subi une résection abdominopérinéale de sauvegarde. Six patients étaient vivants un an après le traitement (survie médiane de 32,5 mois, [plage de 21 à 51]). Dans huit cas, la maladie a évolué rapidement et la survie médiane s'est établie à 5,5 mois (plage de 3 à 12). Onze des 12 patients qui sont morts avaient des métastases.
Conclusions
La dissémination systémique est presque universelle chez les patients qui ont un MMA. La survie globale était médiocre sans égard au traitement local. Le taux d'échec des exérèses locales a atteint 60 %, ce qui a nécessité une autre intervention chirurgicale. Il importe d'améliorer le contrôle local puisque certains patients survivent jusqu'à trois ans.
The anal canal is the most common site for the development of primary melanoma in the gastrointestinal tract,1,2 yet the melanocytic type represents only 0.1% to 4.6% of all anal malignant tumours.3 Its incidence is rising, although the reasons for this increase are unclear.2,4 Typically, affected patients are older (sixth to eighth decade), Caucasian2,5 and female.2,3,6 The presenting symptom is usually rectal bleeding or a mass. Therefore, the tumour is usually discovered fortuitously during a rectal examination for other anorectal disorders.3 Anorectal malignant melanomas (AMMs) are typically more than 1 mm thick when diagnosed. In a recent review, the 5-year survival for anorectal melanoma was estimated to be 19.8%,2 with the majority of patients dying of systemic dissemination.
Surgery remains the mainstay of treatment, and the role of adjuvant therapy, such as chemotherapy or immunotherapy, remains unknown. Surgical issues such as the need for a lymphadenectomy remain controversial, leaving the choice of 2 surgical options: local excision or abdominoperineal resection. The majority of reports in the literature have not found a significant survival advantage when comparing radical and conservative surgery.3,7,8,9,10 Some authors, however, have reported increased survival with abdominoperineal resection11,12 or pelvic exenteration13 in selected patients, such as those with thinner or lower stage cancers.
Recent studies propose a conservative surgical approach, since cure is rarely achieved and many patients are elderly. The role if any, of pelvic radiation has not been systematically examined.
We undertook this study to review the experience at a single large Canadian tertiary care cancer centre in an attempt to document the effect of different treatment modalities, including surgery and radiation therapy on the outcome.
Patients and methods
Charts were identified from the database of the Princess Margaret Hospital, in Toronto, which collects diagnostic information for each new registered patient visit. Patients were excluded if they were seen only once or if the diagnosis was not AMM. Each chart was reviewed and all dates of death were confirmed with the public provincial registry.
From January 1980 to December 1999, 14 patients with anorectal melanoma (5 men, 9 women) were identified. They were all referred to our centre within 2 months of the initial diagnosis. The histologic diagnosis, when performed elsewhere, was confirmed by an oncologic pathologist. All patients were staged clinically and with cross-sectional imaging (computed tomography [CT] or ultrasonography of the liver). The mean ages at diagnosis were 56 (range from 32–81) years for men and 68.2 (range from 44–92) years for women. Patients were followed up until the time of death, or for a minimum of 28 months for 2 patients who were alive at the last follow-up. Survival was defined as the interval between diagnosis at the Princess Margaret Hospital and death. Because of the small number of patients, statistical analyses were not performed; when appropriate, the mean, median and range are given.
Results
The presenting symptom in all patients was bleeding or sensation of a mass in the anorectal area, or both. The diagnosis was established after proctoscopy and biopsy in all instances. Information concerning the size of the primary lesion and details of the surgical margins could not be retrieved in a consistent manner since most biopsies were done elsewhere.
For the purpose of this analysis, the clinical staging was defined as follows: local disease (tumour involving the anorectum only); locoregional disease (involvement of the inguinal or femoral lymph nodes); and metastatic disease (involvement of distant organs or lymph nodes beyond the femoral triangle). Ten of 14 patients had localized disease on clinical examination at their first hospital visit and 3 had locoregional disease. Only 1 patient had overt metastatic disease at the time of referral.
The initial histologic diagnosis was correct in all but 3 cases. These 3 lesions did not show melanin pigmentation and were labelled as poorly differentiated tumours. This was interpreted as primary anal carcinoma in 2 cases and a rectal cancer in the remaining patient. The 2 patients who were initially diagnosed with “anal carcinoma” received chemoradiation as the primary treatment rather than surgery. The patient diagnosed with “rectal tumour” was given preoperative chemoradiotherapy. The actual diagnosis was recognized after the radiation treatment, on review of the histologic slides, as the tumours did not respond to the therapy as expected. These patients were then referred for a second opinion.
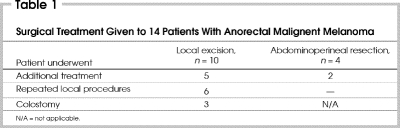
All patients underwent surgical excision (Table 1). The initial surgery consisted of a local excision in 10 patients and abdominoperineal resection in 4 patients. Of the 10 patients initially treated with local excision, 6 required reoperation. A permanent colostomy for recurrent disease was necessary for 3 of the 6 patients who had an abdominoperineal resection after 1 local recurrence or several of them. The effect of radiotherapy on the rate of relapse after local excision could not be assessed.
Table 1
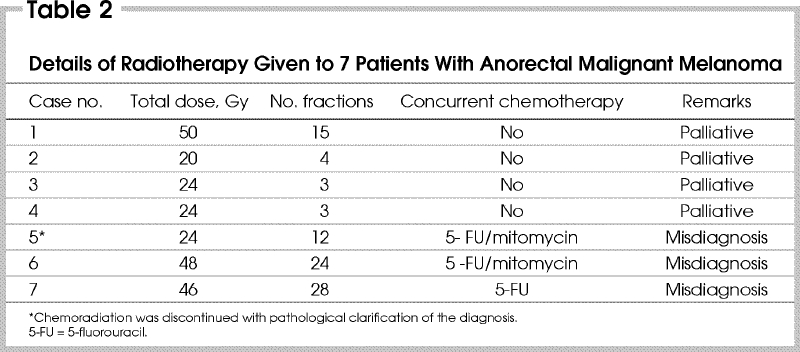
Seven patients received pelvic irradiation at some time during their disease, using different doses and fractionation schemes (Table 2). Three of these patients, as previously mentioned, received concomitant chemotherapy and radiotherapy without any tumour regression. These 3 lesions were re-examined and reclassified as AMM and all 3 patients were then referred for surgery. The remaining 4 patients had a short course of radiation alone for palliation, after the original lesion had recurred.
Table 2
The overall median survival was 12 (range from 3–51) months. There were only 2 survivors at the end of data collection. Patients who underwent initial local excision had a median survival of 6 (range from 3–39) months, whereas the median survival after abdominoperineal resection was 12 (range from 5–51) months. The patients who had pelvic radiation had a median survival of 16.5 (range 5–36) months. Those who did not receive radiation had a median survival of 5 (range from 3–51) months.
Overall, there was a wide distribution of survival even though most patients had clinically localized disease. There were 6 patients with a median survival of 32.5 (range from 21–51) months and 8 patients with a median survival of 5.5 (range from 3–12) months. Eleven of the 12 patients who died had systemic dissemination of disease with involvement of distant nodes, liver, lung and brain as the most common sites.
Discussion
The prognosis of AMM is poor. Most series, including ours, report lesions presenting at a late stage with symptoms similar to benign anorectal disorders, such as bleeding or a mass. In a minority of patients the lesion is discovered incidentally during a screening test. Most of the current knowledge and decision-making is therefore based on these advanced neoplasms.
In this series, we elected to stage patients according to their clinical regional nodal status. The primary tumour thickness could not be retrieved in the majority of patients. Approximately one-third presented with detectable regional nodal disease, and 1 patient had metastatic disease at diagnosis, a distribution similar to that of other series.
Although the presence of metastatic disease heralds a poor outcome in the literature, the absence of palpable nodal disease does not constitute a useful clinical predictive tool for good outcome. The biologic behaviour of the primary lesion is an important factor, as shown by the fact that patients with localized disease survived a few months to a few years. It would be particularly useful to be able to predict which patients would have a very rapid disease course. Variables such as tumour thickness and other histologic features may be able to stratify these patients in the future.
This review also draws attention to potential errors in diagnosis that can occur with amelanotic lesions. These lesions are typically diagnosed as poorly differentiated tumours of the anorectum, another rare clinical entity. We urge caution with this diagnosis and suggest that further immunodiagnostic studies be done in every case to rule out melanoma.
In the literature, there is consensus that surgical management offers the only chance for cure as well as palliation. Thibault and colleagues14 reviewed 50 patients with AMM seen at the Mayo Clinic over 54 years. They reported that the 5-year survival and recurrence rates for local excision and abdominoperineal resection did not differ. Similar conclusions were drawn in a number of other studies.2,8,10,11,15,16,17 In a large retrospective study of patients with AMM at Memorial Sloan-Kettering Cancer Center, Brady and colleagues18 reported a more favourable, but not statistically significant 5-year disease-free survival in patients who underwent APR. However, they recommended that abdominoperineal resection should be used in small tumours without evidence of metastasis. In our study, survival after local excision and abdominoperineal resection was in the same range (3–51 mo). Given these considerations, we believe there is no significant survival advantage with a more radical surgical approach.
In reviewing 63 cases of AMM in the Netherlands, Roumen19 concluded that even though survival was not affected by the surgical modality, the rate of local recurrence was slightly higher in those who had local excision. This is in keeping with our findings and underscores the need for continued surveillance in patients who have had this procedure. In the same Dutch study, local recurrences did not lead to local problems, as most of these patients did not survive. However, in our series, the majority of patients with local recurrence lived long enough to require further operations, and half of them eventually underwent an abdominoperineal resection. A method of estimating survival would help select the surgical procedure, since local surgical treatment is often followed by relapse in patients who survive more than a year. The difficulty in obtaining wide margins and the extensive lymphatic permeation often associated with this disease are the likely contributing factors to the high local recurrence rate.
The role, if any, of adjuvant therapy is unknown. Data in the literature on the effect of radiotherapy alone or in combination with surgery are sparse.14,19,20 Bujko and colleagues20 reported 3 cases in which radiotherapy was used and was associated with long-lasting control of local symptoms. They observed a high rate of recurrence in the inguinal lymph nodes and recommended that the groin lymph nodes be included in the radiation field. In another case report, Gupta and colleagues21 proposed that supplementing interstitial brachytherapy after local resection of anorectal melanoma may help to prevent local recurrence. The addition of radiotherapy in our patients did not appear to change the local control rate, but the data did not allow us to make any useful recommendations in this regard. In the 3 patients who were irradiated preoperatively, the radiation treatment had little effect on the local tumour burden. The 4 patients who had palliative radiotherapy had metastatic disease and a short survival thereafter.
In the current study, comparison of the survival of patients who had surgery alone or surgery in combination with radiotherapy, demonstrated no significant observable difference. However, in view of the above case reports and the high failure rate after local excision alone, the role of adjuvant radiotherapy in improving local control needs to be evaluated. Of the 12 patients who failed treatment, 11 died of metastatic disease, with distant nodes, liver, lung and brain as the most common sites. This pattern of failure attests to the aggressive nature of AMM.
It is doubtful that much progress can be made to cure this disease without improving early detection. In several patients, small foci of dysplastic melanocytic lesions were seen surrounding the malignant lesion, indicating that a premalignant stage may make this strategy possible. Also, the screening efforts undertaken for colorectal cancer may help detect this disease earlier, and surgeons and other practitioners should be sufficiently aware of its existence to avoid delays in diagnosis.
Poster presentation at the Society of Surgical Oncology meeting, Mar. 16–19, 2000.
Competing interests: None declared.
Correspondence to: Dr. Jean Couture, Hopital Charles LeMoyne, 3120 Blvd. Taschereau, Greenfield Park QC J4V 2H1; fax 450 466-5768; jean.couture@rrsss16.gouv.qc.ca
Accepted for publication Jan. 8, 2003.
References
- 1.Bolivar JC, Harris JW, Branch W. Melanoma of the anorectal region. Surg Gynecol Obstet 1982;154:337-41. [PubMed]
- 2.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer 1998;83:1664-78. [DOI] [PubMed]
- 3.Ben-Izhak O, Levy R, Weill S. Anorectal malignant melanoma. A clinicopathologic study, including immunohistochemistry and DNA flow cytometry. Cancer 1997; 79: 18-25. [DOI] [PubMed]
- 4.Cagir B, Whiteford MH, Tropham A. Changing epidemiology of anorectal melanoma. Dis Colon Rectum 1999;42:1203-8. [DOI] [PubMed]
- 5.Quan SH. Anorectal melanoma. In: Cohen AM, Winawer SJ, editors. Cancer of the colon, rectum and anus. New York: McGraw-Hill; 1995. p. 1069-71.
- 6.Weinstock MA. Epidemiology and prognosis of anorectal melanoma. Gastroenterology 1993;104:174-8. [DOI] [PubMed]
- 7.Wanebo HJ, Woodruff JM, Farr GH. Anorectal melanoma. Cancer 1981;47:1891-900. [DOI] [PubMed]
- 8.Cooper PH, Mills SE, Allen MS Jr. Malignant melanoma of the anus: report of 12 patients and analysis of 255 additional cases. Dis Colon Rectum 1982;25:693-703. [DOI] [PubMed]
- 9.Phade VR, Lawrence WR. Anorectal melanoma. Br J Surg 1981;68:667-8. [DOI] [PubMed]
- 10.Ross M, Pezzi C, Pezzi T. Patterns of failure in anorectal melanoma. A guide to surgical therapy. Arch Surg 1990;125: 313-6. [DOI] [PubMed]
- 11.Slingluff CL Jr, Vollmer RT, Seigler HF. Anorectal melanoma: clinical characteristics and results of surgical management in twenty-four patients. Surgery 1990;107:1-9. [PubMed]
- 12.Remingo PA, Der BK, Forsberg RT. Anorectal melanoma report of 2 cases. Dis Colon Rectum 1976;19:350-6. [DOI] [PubMed]
- 13.Baskis AM, Sugarbaker EV, Chretien PB. Anorectal melanoma: the role of posterior pelvic exenteration. Dis Colon Rectum 1982;25:772-7. [DOI] [PubMed]
- 14.Thibault C, Sagar P, Nivatvongs S. Anorectal melanoma — an incurable disease? Dis Colon Rectum 1997;40:661-8. [DOI] [PubMed]
- 15.Ceccopieri B, Marcomin AR, Vitagliano F. Primary anorectal malignant melanoma: report of two cases. Tumori 2000;86:356-8. [DOI] [PubMed]
- 16.Luna-Perez P, Rodriguez DF, Macouzet JG. Anorectal malignant melanoma. Surg Oncol 1996;5:165-8. [DOI] [PubMed]
- 17.Ward MW, Romano G, Nicholls RJ. The surgical treatment of anorectal malignant melanoma. Br J Surg 1986;73:68-9. [DOI] [PubMed]
- 18.Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum 1995;38:146-51. [DOI] [PubMed]
- 19.Roumen RM. Anorectal melanoma in the Netherlands: a report of 63 patients. Eur J Surg Oncol 1996;6:598-601. [DOI] [PubMed]
- 20.Bujko K, Nowacki MP, Liszka-Deleck P. Radiation therapy for anorectal melanoma — a report of three cases. Acta Oncol 1998;37:497-9. [DOI] [PubMed]
- 21.Gupta R, Sharma SC, Bose SM. Adjuvant interstitial brachytherapy in a case of anorectal melanoma. Trop Gastroenterol 2000;21:86-7. [PubMed]