Abstract
Background
Randomized controlled trials of how best to administer fresh frozen plasma (FFP) in the presence of ongoing severe traumatic hemorrhage are difficult to execute and have not been published. Meanwhile, coagulopathy remains a common occurrence during major trauma resuscitation and hemorrhage remains a major cause of traumatic deaths, suggesting that current coagulation factor replacement practices may be inadequate.
Methods
We used a pharmacokinetic model to simulate the dilutional component of coagulopathy during hemorrhage and compared different FFP transfusion strategies for the prevention or correction, or both, of dilutional coagulopathy. Assuming the rates of volume replacement and loss are roughly equal, we derived the hematocrit and plasma coagulation factor concentration over time based on the rate of blood loss and replacement, the hematocrit and coagulation factor concentration of the transfusate, and the hematocrit and plasma factor concentration at the time when FFP transfusion begins.
Results
Once excessive deficiency of factors has developed and bleeding is unabated, 1–1.5 units of FFP must be given for every unit of packed red blood cells (PRBC) transfused. If FFP transfusion should start before plasma factor concentration drops below 50% of normal, an FFP:PRBC transfusion ratio of 1:1 would prevent further dilution.
Conclusion
During resuscitation of a patient who has undergone major trauma, the equivalent of whole-blood transfusion is required to correct or prevent dilutional coagulopathy.
Abstract
Contexte
Les essais contrôlés randomisés sur la meilleure façon d'administrer du plasma frais congelé (PFC) en présence d'une hémorragie traumatique sévère persistante sont difficiles à réaliser et on n'en a pas publié. Entre-temps, la coagulopathie demeure courante pendant la réanimation à la suite d'un traumatisme majeur et l'hémorragie demeure une cause importante de décès traumatiques, ce qui indique que les pratiques en vigueur de remplacement des facteurs de coagulation pourraient avoir des lacunes.
Méthodes
Nous avons utilisé un modèle pharmacocinétique pour simuler la composante dilutionnelle de la coagulopathie pendant une hémorragie et comparé différentes stratégies de transfusion de PFC pour prévenir ou corriger la coagulopathie dilutionnelle, ou les deux. Si l'on suppose que les taux de remplacement de volume et de perte sont à peu près égaux, nous avons dérivé l'évolution dans le temps de l'hématocrite et de la concentration plasmatique de facteurs de coagulation en fonction du taux de perte et de remplacement du sang, de l'hématocrite et de la concentration de facteurs de coagulation transfusés, ainsi que l'hématocrite et la concentration plasmatique de facteurs au début de la transfusion de PFC.
Résultats
Lorsqu'un déficit excessif de facteurs a fait son apparition et que le saignement n'est pas enrayé, il faut administrer de 1 à 1,5 unité de PFC par unité de culot globulaire transfusée. Si la transfusion de PFC doit commencer avant que la concentration plasmatique de facteurs tombe au-dessous de 50 % de la normale, un ratio de transfusion PFC:culot globulaire de 1:1 éviterait une dilution plus importante.
Conclusion
Au cours de la réanimation d'un patient qui a subi un traumatisme majeur, l'équivalent d'une transfusion de sang total s'impose pour corriger ou éviter la coagulopathie dilutionnelle.
Dilution of coagulation factors commonly occurs during resuscitation of a patient with trauma. Some of this dilution is the result of benign neglect, because it is usually neither necessary nor desirable to transfuse with coagulation factors when blood loss is mild or moderate. It is, however, not uncommon for serious dilution to occur when blood loss becomes massive and inadequately controlled, and when factor replacement falls behind. Patients with trauma are also prone to have had volume contraction before resuscitation begins (thus exacerbating the dilution problem), hypothermia, disseminated intravascular coagulation (DIC), thrombocytopenia, metabolic derangements and fibrinolysis.1,2 Even in patients who have not received aggressive fluid resuscitation initially at the scene,3 a common scenario in the hospital would be for multiple units of packed red blood cells (PRBCs) to have been administered by the time coagulation abnormalities are reported. Fresh frozen plasma (FFP) is then ordered and, as the units are thawed, more PRBCs and fluids are infused. It is, therefore, not entirely surprising that coagulopathy is common in patients with severe trauma, and uncontrolled bleeding is the second leading cause of traumatic mortality, being responsible for 40% of deaths,4 and accounts for most of those occurring within the first 48 hours. In one study,2 33%–55% of patients with major trauma had an admission blood sample that indicated an activated partial thromboplastin time (APTT) that was greater than 55 seconds and a prothrombin time (PT) greater than 18 seconds. Those clotting times are equivalent to a level of coagulation activities, as determined in vitro at 37°C, of less than 40% of normal,5 and below the minimum level for some factors (e.g., I, II, VIII, IX, X, XI, von Willebrand's factor monomer) for adequate hemostasis in the face of hemorrhage.5
The best strategy for FFP administration in the presence of sustained hemorrhage is difficult to study, especially in the trauma setting. Not surprisingly, blinded randomized controlled trials on the subject appear nonexistent. There is, however, no shortage of empirical strategies published in reviews and textbooks. These include the following: 1 unit of FFP for every 4–10 units of PRBCs6,7,8,9 and a minimum of 2 units of FFP if the PT is greater than or equal to 1.8 х control.10 Our own experience and the fact that coagulopathy is so common in patients with trauma suggest that these FFP dosings6,7,8,9,10 may be inadequate. Inspired by earlier work on hemodilution and blood salvage,11,12,13,14 we have developed a model for simulating the course of dilutional coagulopathy during FFP and PRBC transfusion in a patient with ongoing hemorrhage. Our simulation only concerns the dilutional aspect of this coagulopathy. It does not address the important issues of DIC, hypothermia, acidosis or thrombocytopenia.
Methods
We have designed a model for calculating plasma factor concentration as a function of time during ongoing hemorrhage and blood product transfusion, in the absence of DIC and loss to extravascular compartments (Fig. 1). We assume herein that transfusion volume equals ongoing blood loss, a situation pertinent to a subset of, but not all, patients with major trauma. This model therefore does not apply to the other common scenario of exsanguination.
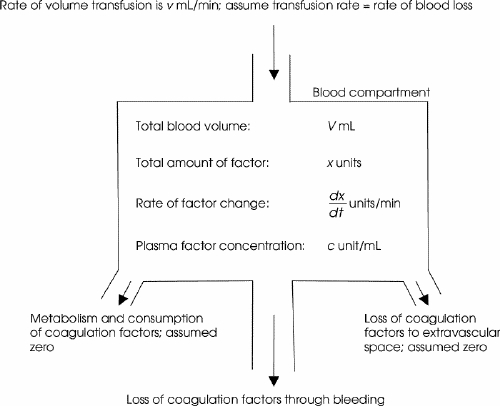
FIG. 1. A compartmental model for calculation of plasma coagulation concentration due to dilution when the blood transfusion rate matches blood loss over clinically relevant time intervals. This model does not apply to the important situation when blood loss greatly exceeds the rate of transfusion.
Our simulation model is based on a common scenario: a patient sustains injuries resulting in major blood loss and is still bleeding. By the time FFP transfusion commences (time t = 0), a certain degree of coagulation factor deficiency is present. We first define the following nomenclature that appears in Figure 1:
· Hematocrit of patient's blood: h (normal is ~ 0.4)
· Patient's blood volume: V mL
· Plasma volume: (1–h)V mL
· Concentration of coagulation factors in patient's plasma: c unit/mL
· Total amount of coagulation factor in patient's plasma: x = (1–h)Vc units
· Concentration of coagulation factor in patient's plasma is the total amount of coagulation factor divided by plasma volume:
· Overall concentration of coagulation factor in the infused blood products: ci unit/mL
· Hematocrit of infused blood product: hi
· Rate of blood loss and matching transfusion: v mL/min
· Rate of loss of coagulation factor through blood loss: v(1–h)c units/min
· Rate of replenishment of coagulation factor through transfusion: vci units/min
· Rate of replenishment of red cells through transfusion: vhi mL/min
Note that c is the patient's in-vivo plasma coagulation factor concentration, and ci is the overall concentration of coagulation factors in the combination of blood products given.
The rate of increase in the hematocrit is the rate of the addition of red cells minus the rate of loss:
Integrating both sides of Equation 1 from time 0 (see Appendix 1), when FFP transfusion begins and hematocrit is h 0, to t, when hematocrit is h (Equation 2):
The rate of factor increase in plasma is the rate of factor replacement minus the rate of loss through hemorrhage (Fig. 1):
Solving for c in Equation 3 (see Appendix 1),
Equations 2 and 4 give the changes in the patient's in-vivo hematocrit and plasma factor concentration, respectively, with time during resuscitation. By defining a patient's hematocrit (h 0) and plasma factor concentration (c 0) at time (t) zero (when FFP transfusion begins) and the rate of blood loss as a fraction of total blood volume (v/V) per second, one can calculate how different transfusion strategies (Table 1) affect the patient's blood composition.
Table 1
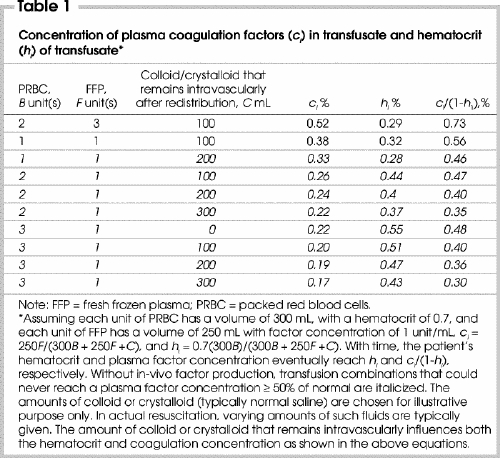
So far we have used a generic term c for plasma factor concentration. In reality, the pharmacokinetics of coagulation factors are different, as are their minimum plasma concentrations required for hemostasis. Nevertheless, because several of the factors require a minimum of 0.4 of normal level for adequate hemostasis,5,15 it would be appropriate to transfuse FFP and aim for target plasma factor concentrations of greater than 0.4 for all factors. For the rest of this paper, we have picked a more conservative target of 0.5, because the typical PT at 0.5 dilution is 16.5 seconds,5 a value generally considered at or above the threshold that triggers FFP transfusion during major bleeding.9,15,16,17,18 By comparison, at 0.4 dilution, the PT approaches 19 seconds,5 which is probably excessive in a setting of severe hemorrhage at multiple sites. Moreover, while a 0.4 concentration in isolated factors may not adversely affect hemostasis, it is not clear whether a 60% reduction in most or all factors does not seriously affect hemostasis in a hemorrhagic setting, hence the choice of 0.5 as our target.
Equations 2 and 4 were plotted using a spreadsheet program, under bleeding rates expressed as the fraction of total blood volume (v/V) lost per minute, and using various combinations of PRBC, FFP and crystalloids/colloids (Table 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6). The crystalloid (typically normal saline) volumes indicated are those left intravascularly after redistribution (e.g., some 25%–30% of infused saline remains intravascularly after 1 hour). The amount of colloid or intravascular crystalloid that remains influences both the hematocrit and coagulation factor concentration. For a 70-kg person with a blood volume of 5 L, v/V of 0.005/min and 0.02/min translate to bleeding rates of 25 mL/min (1.5 L/h) and 100 mL/min (6 L/h), respectively.
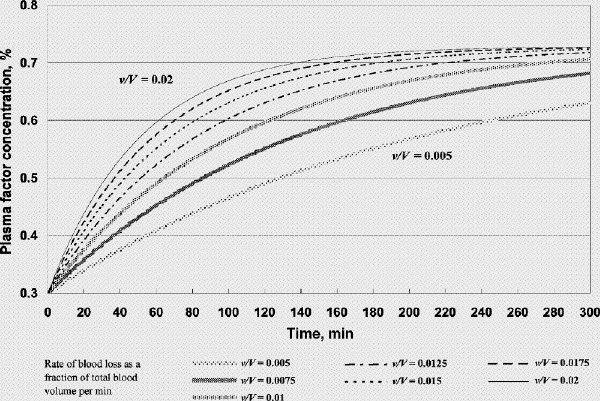
FIG. 2A. Factor concentration during resuscitation; transfusate PRBC 2 units (U): FFP 3 U: crystalloid 100 mL (factor concentration 0.3 at time 0). B. Hematocrit during resuscitation; transfusate PRBC 2 U: FFP 3 U: crystalloid 100 mL. FFP = fresh frozen plasma, PRBC = packed red blood cells, v/V = rate of blood loss as a fraction of total blood volume per minute.
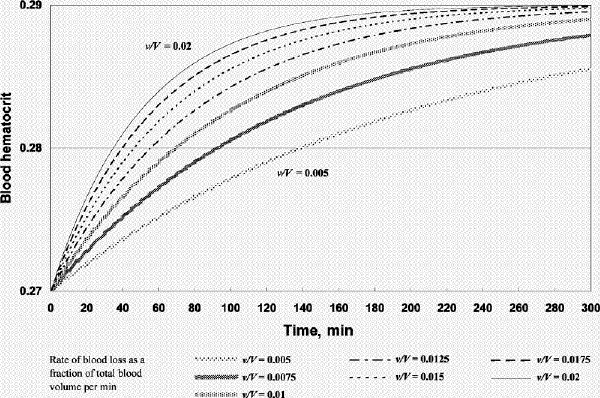
Figure 2. Continued.
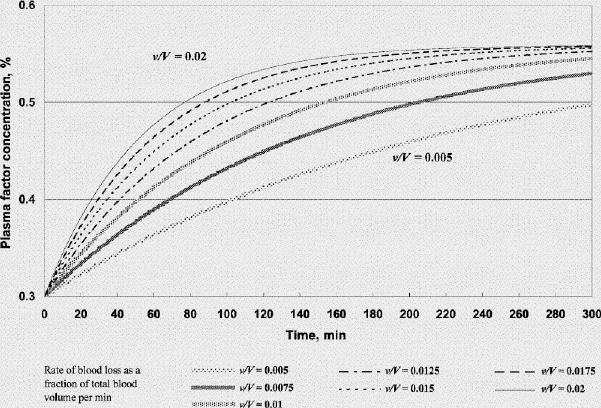
FIG. 3A. Factor concentration during resuscitation; transfusate PRBC 1 U: FFP 1 U: crystalloid 100 mL (factor concentration 0.3 at time 0). B. Hematocrit during resuscitation; transfusate PRBC 1U: FFP 1 U: crystalloid 100 mL. FFP = fresh frozen plasma, PRBC = packed red blood cells, v/V = rate of blood loss as a fraction of total blood volume per minute.
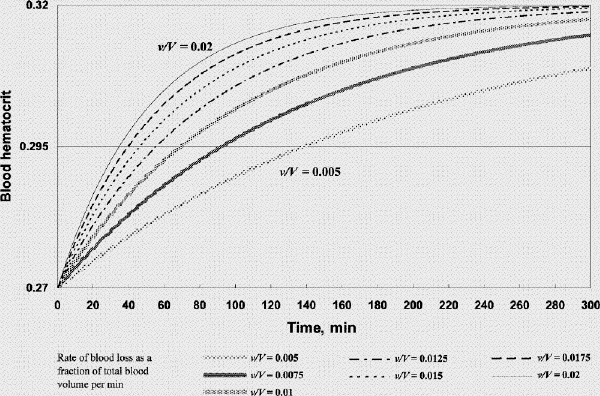
Figure 3. Continued.
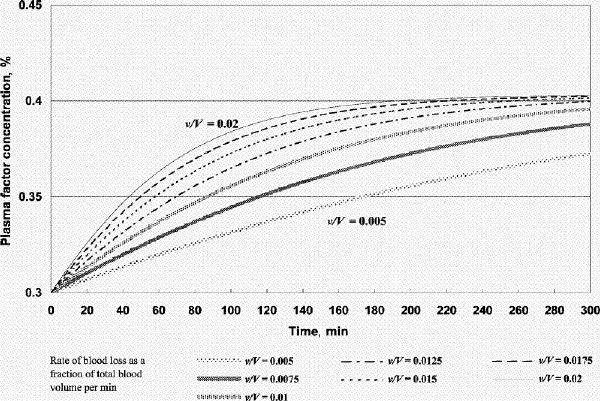
FIG. 4A. Factor concentration during resuscitation; transfusate PRBC 3 U: FFP 1 U: crystalloid 100 mL (factor concentration 0.3 at time 0). B. Hematocrit during resuscitation; transfusate PRBC 3 U: FFP 1U: crystalloid 100 mL. FFP = fresh frozen plasma, PRBC = packed red blood cells, v/V = rate of blood loss as a fraction of total blood volume per minute.
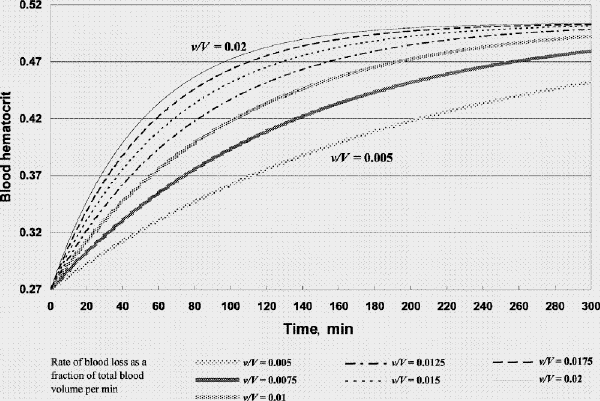
Figure 4. Continued.
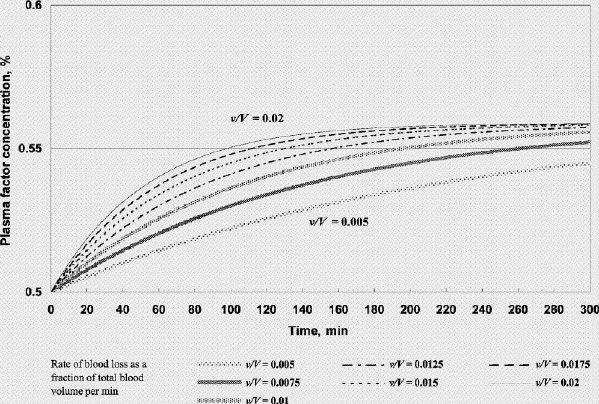
FIG. 5. Factor concentration during resuscitation; transfusate PRBC 1 U: FFP 1 U: crystalloid 100 mL (factor concentration 0.5 at time 0). FFP = fresh frozen plasma, PRBC = packed red blood cells, v/V = rate of blood loss as a fraction of total blood volume per minute.
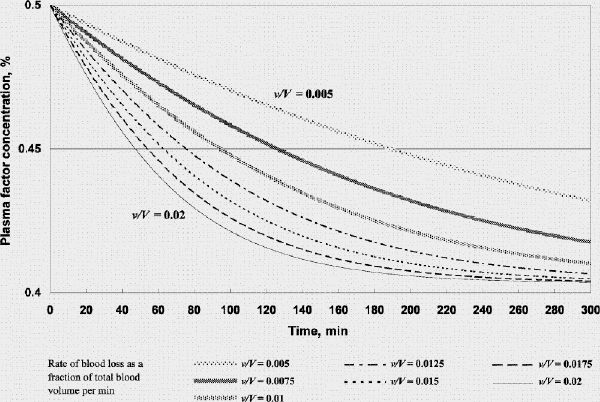
FIG. 6. Factor concentration during resuscitation; transfusate PRBC 3 U: FFP 1 U: crystalloid 100 mL (factor concentration 0.5 at time 0). FFP = fresh frozen plasma, PRBC = packed red blood cells, v/V = rate of blood loss as a fraction of total blood volume per minute.
Results
Using our model we simulated 2 scenarios. The first is that of a hemorrhaging patient who has received fluid and PRBC resuscitation such that the hematocrit and plasma factor concentration at t = 0 are 0.27 and 0.3 (PT ⊕ 21.6 s, APTT ⊕74 s),5 respectively (Fig. 2, Fig. 3, Fig. 4). The second involves the same patient but h and c at time t = 0 are 0.27 and 0.5 (PT ⊕ 16.5 s, APTT ⊕ 44.2 s),5 respectively (Fig. 5, Fig. 6). The initial hematocrit and plasma factor concentration values at t = 0 have been chosen arbitrarily, and the reader is free to substitute any initial values and bleeding rates into the model or to simulate any scenario for analysis (template available on request). Please note the use of different scales in the figures. As far as the model is concerned, it does not matter whether the initial coagulopathic state is caused by dilution alone, or in combination with DIC, and whether FFP had already been given. It is a starting state from which we see how we could reverse the course of coagulopathy. FFP transfusion after t = 0 will, however, only be aimed at correcting the dilution problem. In other words, if DIC continues, more FFP (and other hemostatic products) than that which our model predicts would be required.
Figure 2A shows that if one were to transfuse 1.5 units of FFP for every unit of PRBC plus a small amount of crystalloid after the patient's plasma factor concentration has been diluted to 0.3, the plasma factor concentration gradually increases such that eventually it exceeds 0.5. For v/V = 0.005/min and 0.02/min, it will take 130 minutes and 31 minutes, respectively, for the plasma factor concentration to reach 0.5 (while the patient's hematocrit reaches slightly above 0.28). Figure 3A and 3B show that, for the same bleeding rates, an PRBC:FFP transfusion ratio of 1:1 would result in the factor concentration reaching 0.5 in 300 minutes and 75 minutes, respectively, while the hematocrit reaches above 0.3. However, if one were to transfuse 1 unit of FFP for every 3 units of PRBC, the factor concentration would never reach 0.5 (Fig. 4A), while the hematocrit is pushed well above 0.3 (Fig. 4B). In the latter scenario, the unnecessarily excessive rise in hematocrit can be avoided at the expense of coagulation function if one administers more crystalloid (Table 1).
Figure 5 shows that if FFP transfusion is started in time during resuscitation (when the plasma factor concentration drops to 50%), factor concentration can be maintained at above 0.5 if one were to administer 1 unit of FFP for each unit of PRBC. However, a transfusion strategy of 1 unit of FFP for every 3 units of PRBC with a small volume of crystalloid would result in a decline of the plasma factor concentration. The decline is accelerated for higher rates of bleeding (Fig. 6) and when large amounts of crystalloid (to avoid an unnecessarily high hematocrit) are used.
Discussion
We have shown that once significant coagulopathy has developed and if hemorrhage continues, 1–1.5 units of FFP must be given per unit of PRBC transfused just to correct the dilutional component of the coagulopathy alone. Even when this is done, correction of coagulopathy takes time. Faster correction by using higher FFP:PRBC (lower PRBC:FFP) ratios could result in anemia. Our results (not all shown) also show that transfusion at PRBC:FFP ratios of 3:1 perpetuates dilutional coagulopathy and could result in high hematocrit values. On a theoretical basis, this exercise confirms our suspicion, and is in agreement with our clinical experience, that PRBC:FFP ratios of 4–10:16,7,8,9,10 are inadequate.
Our model is “conservative.” It does not include the deleterious effects of hypothermia, DIC and metabolic derangements on coagulopathy, and it uses 0.5 as the target plasma factor concentration, which is probably low in the face of major hemorrhage. Our model also ignores loss of coagulation factors to extravascular tissues, metabolism and normal consumption. Overall, our model underestimates the degree of coagulopathy and the FFP requirement by ignoring these issues.
In comparison with the approaches to FFP use in major bleeding advocated by some,6,7,8,9,10 however, our simulation results are “liberal.” We believe that prevailing recommendations regarding FFP use during trauma resuscitation6,7,8,9,10 could have been influenced by earlier work, suggesting that dilutional coagulopathy develops late in elective surgical patients.17,19,20,21 However, elective patients do not normally suffer an initial blood-volume contraction (isovolemic hemodilution results in less dilution for the same volume of blood loss), significant hypothermia or DIC. Furthermore, massive transfusion studies performed before the late 1980s used whole blood, and their results may not be pertinent to today's practice of using PRBC.22
This study could be criticized for the lack of supporting clinical data. Our results are, however, in broad agreement with those of retrospective studies.2,23,24,25,26 Implicit endorsements of increased FFP use could also be found in the claim that whole blood is superior to PRBC for resuscitation of patients with trauma27 and in the success of the “staged laparotomy” strategy.1 After a minimal operation to control surgical bleeding and enteric spillage, the severely injured patient is admitted to the intensive care unit for re-warming and for aggressive correction of coagulation indices, among other parameters, before repair of internal injuries is performed.1 This approach may result in salvage rates of 20%–60% in patients who otherwise would have died.1 In comparison, the recommendations6,7,8,9,10 given in various reviews appear not to be supported by theory or by any good clinical studies, retrospective or prospective. Thus, our simulation should help lay the ground for future clinical studies to determine the best FFP dosing strategies during major transfusion resuscitation. Unfortunately, robust clinical data on this subject are difficult to collect and may be a long time coming, if at all.
Assumptions made in the development of this resuscitation model
The plasma concentrations of several coagulation factors increase when the body is stressed.5 By starting at a low plasma factor concentration (e.g., 30%, 50%), however, our model bypasses such initial stress responses. Likewise, our model begins after the hypercoagulable phase induced by mild hemodilution has passed. In other words, we apply our model beginning at time t = 0, when the body's maximum compensatory responses have already occurred and have fallen short. It is noteworthy that most of the factors do not have a significant reserve for immediate mobilization or increased production when stressed.
Our model assumes that transfusion equals blood loss. In practice, while those rates will not match at every instant, it is reasonable to assume that in an important subset of patients the average rate of transfusion should match that of loss over clinically relevant time intervals. Likewise, because the rate of blood loss is variable, the results reflect average overall bleeding rate over such time intervals. Figures 2–6 present smooth curves of factor concentrations. With fluctuating rates of blood loss, the actual course of factor concentration in relation to time would be irregular and confined within the curves that define the maximum and minimum rates.
Because our focus here is dilutional coagulopathy, the contribution of cryoprecipitate, if given, is ignored in our model. Cryoprecipitate contains mainly fibrinogen, von Willebrand's factor and factor VIII, and cannot be relied upon for the correction of dilution across the board.
Platelets are mobilized at times of stress. It is our clinical impression, and that of others,28 that with the use of PRBC instead of whole blood, dilutional coagulopathy develops no later than dilutional thrombocytopenia. After acute-phase platelet mobilization is nearly exhausted, the pharmacokinetics of platelets may also be described by our model. Thrombocytopenia seldom poses a serious problem, however, because it takes less time for the platelet count to be reported and for platelets to be processed and transfused.
Use of simulation
Double-blind placebo-controlled trials are difficult to conduct on patients with hemorrhagic trauma. Simulation has been used already to study hypothermia29 and dilutional coagulopathy22 in these patients. In the latter exercise, Hirshberg and colleagues22 simulated exsanguinations, whereas we simulated the situation in which transfusion matches blood loss over relevant time intervals. Both situations are pertinent in resuscitation of patients with trauma. Interestingly, the results are remarkably similar in that both recommend that FFP and PRBC should be given in such ratios as to resemble fresh whole blood.
Closing comments
This simulation was performed to address the often-heard comment that many trauma patients' coagulopathy is refractory. When not distracted by the mathematics, the reader may find it intuitive that coagulopathy cannot be corrected or prevented in major sustained hemorrhage if the transfusate is equivalent to blood that is deficient in coagulation factors.30 The mathematics, therefore, has merely proven the obvious. Our results showing that it is only possible to correct a severe coagulopathy by transfusing at least the equivalent of whole blood are sobering food for thought for any clinician faced with a trauma patient bleeding at multiple sites and showing no sign of improved hemostasis despite “textbook” coagulation management, and for any clinician still waiting for clinical data to show how we could reduce the serious and well-recognized impact of coagulopathy on trauma morbidity and mortality.
Coagulopathy after major trauma is incompletely understood. Early recognition of significant coagulopathy and its timely prevention or correction, or both, require a proactive approach, but this can be difficult because laboratory results are often delayed, point-of-care coagulation timers are not reliable, microvascular bleeding may be occult, clinical signs are subtle and FFP takes time to thaw. Hypothermia and DIC must be quickly eliminated. As is consistent with our experience, our model suggests that the resolution and the prevention of deterioration of established dilutional coagulopathy in major traumatic ongoing hemorrhage require the transfusion of 1–1.5 units of FFP for each unit of PRBC. On the other hand, if significant coagulopathy has not yet developed and is not looming, an aggressive FFP transfusion strategy is not required and, indeed, should be discouraged.
Theoretically, the increased use of FFP in trauma could result in increased use of blood products. Conversely, appropriately aggressive FFP use could lead to decreased net bleeding, morbidity and mortality. This uncertainty can only be resolved by clinical studies. The availability of recombinant activated factor VII will likely change transfusion practice, but its use in patients with trauma has yet to be approved or clearly defined.31 Meanwhile, we hope that this exercise has raised awareness of the problem of coagulopathy and, in our opinion, the underuse of FFP, in the resuscitation of patients with major trauma.
Appendix 1.

Competing interests: None declared.
Correspondence to: Dr. Anthony M.H. Ho, Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Shatin, NT, Hong Kong SAR, PRC; fax 852 2637 2422; hoamh@cuhk.edu.hk
Accepted for publication Sept. 1, 2004
References
- 1.Gentilello LM, Pierson DJ. Trauma critical care. Am J Respir Crit Care Med 2001;163:604-7. [DOI] [PubMed]
- 2.Faringer PD, Mullins RJ, Johnson RL, Trunkey DD. Blood component supplementation during massive transfusion of AS-1 red cells in trauma patients. J Trauma 1993;34:481-7. [DOI] [PubMed]
- 3.Bickell WH, Wall MJ Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med 1994;331: 1105-9. [DOI] [PubMed]
- 4.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma 1995;38:185-93. [DOI] [PubMed]
- 5.Reiss RF. Hemostatic defects in massive transfusion: rapid diagnosis and management. Am J Crit Care 2000;9:158-65. [PubMed]
- 6.Stern SA, Bobek EMK. Resuscitation: management of shock. In: Ferrera PC, Colucciello SA, Marx JA, Verdile V, editors. Trauma management: an emergency medicine approach. St. Louis (MO): Mosby; 2001. p. 75-102.
- 7.Montenegro LM, Jobes DR. Complications of massive transfusion. In: Atlee JL, editor. Complications in anesthesia. Philadelphia: WB Saunders; 1999. p. 668-73.
- 8.Robb WJ. Massive transfusion in trauma. AACN Clin Issues 1999;10:69-84. [DOI] [PubMed]
- 9.Turner DAB. Emergency anaesthesia. In: Aitkenhead AR, Rowbotham DJ, Smith G, editors. Textbook of anaesthesia, 4th ed. Edinburgh (UK): Churchill Livingstone; 2001. p. 619-28.
- 10.Murphy WG, Davies MJ, Eduardo A. The haemostatic response to surgery and trauma. Br J Anaesth 1993;70:205-13. [DOI] [PubMed]
- 11.Cohen JA, Brecher ME. Preoperative autologous blood donation: Benefit or detriment? A mathematical analysis. Transfusion 1995;35:640-4. [DOI] [PubMed]
- 12.Hay SN, Monk TG, Brecher ME. Intraoperative blood salvage: a mathematical perspective. Transfusion 2002;42:451-5. [DOI] [PubMed]
- 13.Ho AMH, Dion P, Karmakar MK, Cheng G, Chung DC, Tay BA. A pharmacokinetic model for factor VIII dosing during active haemorrhage in patients with haemophilia A. Anaesthesia 2001;56:785-90. [DOI] [PubMed]
- 14.Waters JH, Lee JS, Karafa MT. A mathematical model of cell salvage efficiency. Anesth Analg 2002;95:1312-7. [DOI] [PubMed]
- 15.Reiner AP. Massive transfusion. In: Spiess BC, Counts RB, Gould SA, editors. Perioperative transfusion medicine. Baltimore (MA): Williams & Wilkins; 1998. p. 351-64.
- 16.Miller RD. Transfusion therapy. In: Anesthesia. 5th ed. Philadelphia: Churchill Livingstone; 2000. p. 1613-44.
- 17.Murray DJ, Olson J, Strauss R, Tinker JH. Coagulation changes during packed red cell replacement of major blood loss. Anesthesiology 1988;69:839-45. [DOI] [PubMed]
- 18.Stehling L. Blood transfusion and component therapy. In: Gregory GA, editor. Pediatric anesthesia. 4th ed. New York: Churchill Livingston; 2002. p. 117-44.
- 19.Laks H, Handin RI, Martin V, Pilon RN. The effects of acute normovolemic hemodilution on coagulation and blood utilization in major surgery. J Surg Res 1976;20:225-30. [DOI] [PubMed]
- 20.Umlas J, Sakhuja R. The effect on blood coagulation of the exclusive use of transfusions of frozen red cells during and after cardiopulmonary bypass. J Thorac Cardiovasc Surg 1975;70:519-23. [PubMed]
- 21.Murray DJ, Pennell BJ, Weinstein SL, Olson JD. Packed red cells in acute blood loss: dilutional coagulopathy as a cause of surgical bleeding. Anesth Analg 1995;80:336-42. [DOI] [PubMed]
- 22.Hirshberg A, Dugas M, Banez EI, Scott BG, Wall MJ Jr, Mattox KL. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454-63. [DOI] [PubMed]
- 23.Hewson JR, Neame PB, Kumar N, Ayrton A, Gregor P, Davis C, et al. Coagulopathy related to dilution and hypotension during massive transfusion. Crit Care Med 1985;13:387-91. [DOI] [PubMed]
- 24.Phillips TF, Soulier G, Wilson RF. Outcome of massive transfusion exceeding two blood volumes in trauma and emergency surgery. J Trauma 1987;27:903-10. [DOI] [PubMed]
- 25.Mitchell KJ, Moncure KE, Onyeije C, Rao MS, Siram S. Evaluation of massive volume replacement in the penetrating trauma patient. J Natl Med Assoc 1994;86:926-9. [PMC free article] [PubMed]
- 26.Cinat ME, Wallace WC, Nastanski F, West J, Sloan S, Ocariz J, et al. Improved survival following massive transfusion in patients who have undergone trauma. Arch Surg 1999;134:964-8. [DOI] [PubMed]
- 27.Bernstein DP. Transfusion therapy in trauma. In: Capan LM, Miller SM, Turndorf H, editors. Trauma anesthesia and intensive care. New York: J.B. Lippincott; 1991. p. 167-205.
- 28.Hiippala S. Replacement of massive blood loss. Vox Sang 1998;74:399-407. [DOI] [PubMed]
- 29.Hirshberg A, Sheffer N, Barnea O. Computer simulation of hypothermia during “damage control” laparotomy. World J Surg 1999;23:960-5. [DOI] [PubMed]
- 30.Ho AMH, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg 2005;190:479-84. [DOI] [PubMed]
- 31.Ho AMH, Dion PW, Karmakar MK. Use of recombinant activated factor VII in patients with severe coagulopathy and bleeding [letter]. Anesthesiology 2003;98:1025-6. [DOI] [PubMed]


