Abstract
Introduction
With the improved quality and widespread availability of diagnostic abdominal imaging, incidental intra-abdominal lesions (incidentalomas) are being increasingly identified. Our objective was to characterize the clinical features of asymptomatic patients with incidentally discovered pancreatic lesions and to assess the accuracy of preoperative radiologic diagnosis against the final histologic diagnosis.
Methods
This cohort study is based on prospectively collected data from a surgical pancreatic database. Preoperative imaging of patients with pancreatic incidentalomas was retrospectively and independently assessed by 2 radiologists blinded to the final histologic diagnosis. Seven patients who were asymptomatic and had incidentally discovered pancreatic masses underwent complete resection of the mass. The clinical features and patient survival data were analyzed. The accuracy of preoperative imaging was assessed by comparing the preoperative diagnosis to the final histologic diagnosis.
Results
Lesions most commonly occurred in females (6 patients) and in the tail or body of the pancreas (5 patients). The histologic type of the masses included neuroendocrine tumour (3), serous cystadenoma (2), intraductal papillary mucinous tumour (1) and papillary cystic and solid tumour (1). Preoperative imaging was unreliable in predicting the histologic type of the resected mass.
Conclusions
Our findings suggest that preoperative imaging does not always predict the surgical histologic type of pancreatic incidentalomas. Unless the diagnosis of serous cystadenoma is certain, surgical resection should be considered in low-risk patients in whom pancreatic masses are found incidentally.
Abstract
Introduction
La qualité améliorée de l'imagerie abdominale de diagnostic et sa disponibilité généralisée permettent de repérer de plus en plus de lésions intra-abdominales (incidentalomes) par hasard. Notre objectif consistait à définir les caractéristiques cliniques des patients asymptomatiques chez lesquels on a découvert par hasard des lésions au pancréas et à évaluer l'exactitude du radiodiagnostic préopératoire en fonction du diagnostic histologique final.
Méthodes
Cette étude de cohorte repose sur des données recueillies de façon prospective provenant d'une base de données chirurgicale sur le pancréas. Deux radiologistes ont évalué de façon rétrospective et indépendamment l'un de l'autre, sans connaÎtre le diagnostic histologique final, les résultats d'imagerie préopératoire de patients présentant des incidentalomes pancréatiques. Sept patients asymptomatiques chez lesquels on avait découvert par hasard une masse au pancréas ont subi une exérèse complète de la masse. On a analysé les caracéristiques cliniques et les données sur la survie des patients. On a évalué l'exactitude de l'imagerie préopératoire en comparant le diagnostic préparatoire au diagnostic histologique final.
Résultats
Les lésions étaient plus fréquentes chez les femmes (6 patients) et dans la queue ou le corps du pancréas (5 patients). Le type histologique des masses comprenait une tumeur neuroendocrinienne (3), un cystadénome séreux (2), une tumeur mucineuse papillaire intracanalaire (1) et un kyste papillaire et solide (1). L'imagerie préopératoire n'était pas fiable pour prédire le type histologique de la masse réséquée.
Conclusions
Nos constatations indiquent que l'imagerie préopératoire ne prédit pas toujours le type histologique chirurgical des incidentalomes du pancréas. Sauf si l'on est certain du diagnostic de cystadénome séreux, il faudrait envisager la résection chirurgicale dans le cas des patients à faible risque chez lesquels on constate par hasard la présence de masses au pancréas.
Incidentally discovered abdominal masses (incidentalomas) are increasingly being detected.1,2,3,4,5 Common incidentalomas occur within the liver, kidney and adrenal glands. In a Mayo Clinic study of computed colonography, 108 (41%) of 264 patients had extracolonic findings,6 which included 30 intra-abdominal masses (2 were malignant renal tumours). Incidental adrenal masses are found on 0.4% to 4.9% of CT scans, and it is well established that these lesions can be followed with serial imaging if they are not large, hormonally active or overtly malignant.1,3,4 The majority of renal cell carcinomas are now identified incidentally, and this incidental discovery appears to be altering the natural history of the disease.2,7,8 Incidentally discovered hepatic lesions have also been described and have been found to be of little clinical relevance in 83% of cases.5
There has also been an increase in the occurrence of unusual tumours of the pancreas identified incidentally in asymptomatic patients. These tumours include serous cystadenomas, mucinous cystadenomas, mucinous cystadenocarcinomas, nonfunctional neuroendocrine tumours, papillary cystic and solid tumours, and intraductal papillary mucinous neoplasms.9,10,11,12,13,14 It is postulated that this increase is secondary to the widespread availability and use of abdominal imaging.9 Apart from serous cystadenomas, all of these lesions are either malignant or have malignant potential.10,15,16,17 A description of the clinical features of asymptomatic patients with pancreatic incidentalomas and their management is lacking. In this study, we reviewed a single surgeon's 13-year experience of pancreatic incidentalomas to determine the clinical features and assess the accuracy of preoperative radiologic diagnosis compared to the final pathological diagnosis of incidental pancreatic masses in these asymptomatic patients.
Methods
All 53 patients who underwent a pancreatic resection between 1987 and 2000 were prospectively enrolled into a computerized database at Sunnybrook and Women's College Health Sciences Centre in Toronto. The database was prospectively updated. We identified 7 patients who were asymptomatic and had incidentally identified pancreatic tumours; the remaining 46 had symptomatic lesions. Patient demographics, intraoperative decision-making, postoperative care, final pathological diagnosis, and outcomes data were extracted from the database and from the hospital charts.
The original radiologic studies (computed tomography [CT], magnetic resonance imaging [MRI] or ultrasonography [US]) that identified the pancreatic lesions were reviewed. In 6 of the 7 patients CT was done at our institution using a helical CT scanner with 7-mm collimation, pitch value of 1 and 50% overlapped slices to reconstructions with 3.5-mm interscan spacing. A rapid power-injected bolus of 2 mL/kg body weight, up to a maximum of 150 mL of nonionic contrast material at a rate of 3 mL/s was used. All patients received oral contrast medium (Hypaque 4%) before the CT. The seventh patient underwent CT at another institution and the scan was unavailable for review. MRI images were obtained on a General Electric 1-T unit. Standard pancreatic protocols were employed that included the acquisition of fast spin echo T 1- and T 2-weighted axial images; fat-suppressed spoiled gradient-recalled (SPGR) T 1-weighted axial sequences and axial SPGR T 1-weighted postgadolinium-DTPA sequences that included 10-minute delayed imaging of the pancreas. Fundamental transabdominal US was performed with the use of various US units. In each case Doppler views of the lesion were used to demonstrate any abnormal vascularity within the pancreatic lesion. Two experienced, fellowship-trained radiologists (M.R. and M.A.) having a special interest in abdominal imaging retrospectively, independently reviewed all pertinent examinations, blinded to the final pathological diagnosis. If a single, radiologic diagnosis was not agreed upon, then both diagnoses were noted.
Results
The pancreatic lesions in the 7 study patients were discovered during evaluation for pelvic pain (3), breast cancer staging (1), a broad-based cancer screening test by a family doctor (1), renal colic (1) and vaginal bleeding (1). In the case of the patient with vaginal bleeding, the doctor had ordered pelvic US but abdominal US was done instead, in error.
The average age of the patients was 43 years. Six patients were female and 5 presented within the last 3 years of the study. The most common tumour location was the distal pancreas (5 patients) (Table 1). The median size of the tumours was 2.5 cm (range from 2–6.7 cm). The final pathological diagnosis represents a spectrum of less common pancreatic lesions, including neuroendocrine tumours, intraductal papillary mucinous tumours, papillary cystic and solid tumours and serous cystadenomas (Table 2). The most common pathological diagnosis was nonfunctional neuroendocrine tumour (3 patients). Operations for incidentalomas included 2 Whipple procedures and 5 distal pancreatectomies or splenectomies. The postoperative complication of pancreatic fistula was noted in 3 cases. All fistulas resolved with nonoperative measures within 14, 41 and 110 days, respectively. One of these fistulas developed after a Whipple procedure and the others after distal pancreatectomy. Two of 4 stapled distal pancreatectomies leaked, but the single oversewn distal pancreatectomy did not. There were no postoperative deaths. The median follow-up was 18 months (range from 14–133 mo). No patients were lost to follow-up. At the time of last follow-up all patients were alive without recurrent disease.
Table 1
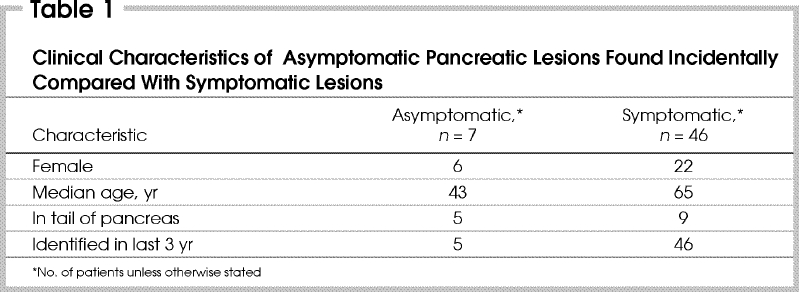
Table 2
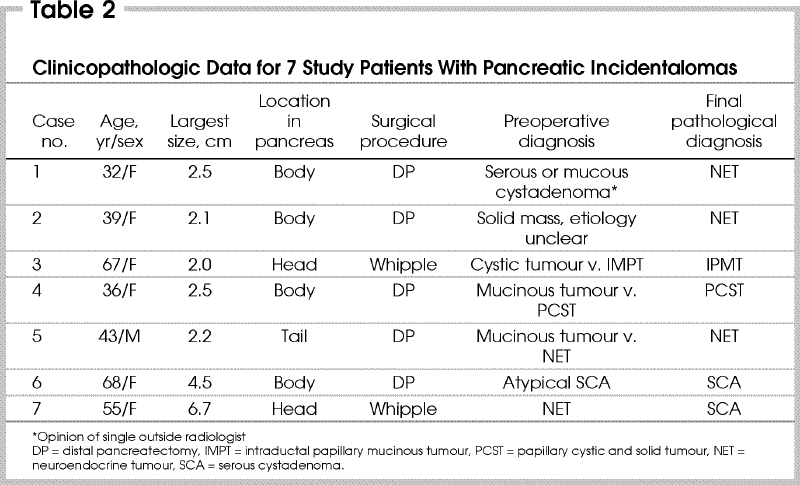
Review of the initial abdominal imaging from these 7 patients by abdominal imaging specialist radiologists, blinded to the final pathological diagnosis failed to consistently agree with the final histopathology (Table 2). The final histologic diagnosis was correctly identified by at least 1 radiologist in 4 out of 7 cases, but there was an accurate consensus on the correct diagnosis in only 1 case. In 1 case, the imaging was performed at an outside institution and was not available for review by our 2 radiologists. In this case, the outside radiologist offered a differential diagnosis of either serous or mucinous cystadenoma. This lesion on final pathological diagnosis was a neuroendocrine tumour (although it did have cystic components).
The cohort of patients with incidentally discovered pancreatic lesions differed from the cohort of patients who had resected symptomatic lesions. No pancreatic lesions incidentally identified in asymptomatic patients were adenocarcinomas. In contrast, ductal adenocarcinoma was the most common histologic diagnosis in symptomatic patients from the database. Also, patients with incidentally discovered lesions were much younger (43 v. 65 yr), more likely to be female (86% v. 32%) and to have distal lesions (71% v. 20%) than patients with symptomatic lesions.
Discussion
Incidentally discovered, clinically significant abdominal lesions are increasing in prevalence.3 Initially described in the adrenal gland, these lesions have also been encountered in the kidney and liver.1,5,6 Both renal and adrenal lesions can frequently be followed conservatively unless they are deemed to be frankly or potentially malignant.2,4 In the case of liver lesions, most are clinically insignificant.5,6 Indeed, there are clearly established algorithms for incidentally discovered adrenal, renal and liver tumours in the literature. In contrast, although a variety of pancreatic tumours occur as incidental asymptomatic masses, pancreatic incidentalomas have not been described as a unique clinical entity, and there are no clearly established treatment algorithms.9,10,11,12,13,14,15,16,18,19,20,21,22,23
There has recently been an increase in the reported incidence of unusual tumours of the pancreas.9,12,13,14 Many such tumours have only been well described within the last 2 decades.14,18,19 In the first 10 years of this study only 2 such tumours were resected whereas 5 were resected in the last 3 years. Sheehan and colleagues9 have noted a similar trend, and it has been postulated that this change is secondary to an increase in the quantity and quality of abdominal imaging.
The management of pancreatic incidentalomas in our study was surgical. There were no deaths although pancreatic fistulas did account for morbidity in 3 of the 7 patients. Although some authors have demonstrated good results for enucleation of cystic pancreatic lesions and neuroendocrine tumours,24,25 that was not the approach in our series. Similarly, some have emphasized that splenic preserving distal pancreatectomy may have merit and that this operation can be done without added morbidity.26,27
At our centre, we found that the preoperative radiologic diagnosis of incidentally discovered pancreatic lesions did not always correlate with the final pathologic diagnosis. Four out of our 7 patients had an accurate diagnosis when a differential diagnosis was based on the preoperative radiologic findings. Accurate preoperative diagnosis is particularly challenging for some cystic tumours of the pancreas. The classic CT appearance of a serous cystadenoma is a multilobular microcystic mass with no cyst greater than 2 cm in diameter, possible central calcification and absence of signs of distant or lymph-node metastasis.28,29,30,31,32 In contrast, mucinous cystic tumours have rare central septa with cysts greater than 2 cm and possible peripheral calcifications.28,29,30,31,32 Cystadenocarcinomas may have evidence of metastatic disease and solid excrescences. Unfortunately, these classic patterns are often not present, and the ability to definitively identify a serous cystadenoma varies greatly, from 23% to 93% of cases.23,28,30 In addition, it may be challenging to differentiate cystic lesions of the pancreas from papillary cystic and solid tumour, intraductal mucinous papillary tumour and tumours with cystic degeneration.27,33,34 Moreover, cystic neoplasms may be misdiagnosed as being benign, such as pseudocysts.35
Predicting the malignant potential of nonfunctional neuroendocrine tumours of the pancreas can also be difficult.32 Radiologic evidence of invasion, lymph-node involvement, size greater than 2 cm or metastatic spread often indicates malignant potential, but if these clinical factors are absent, the malignant potential is often unclear.32 Furthermore, the working diagnosis derived from preoperative imaging was unreliable in correctly predicting the surgical histopathology in our experience.
The cohort of asymptomatic patients who had incidentally discovered pancreatic lesions had different clinical characteristics compared with the cohort of patients with symptomatic lesions in our pancreatic database. The majority of patients with incidentally discovered lesions were female, had distal pancreatic lesions, and none had ductal adenocarcinoma.
The histologic diagnoses of the cohort of asymptomatic patients with incidentally identified pancreatic masses is similar to those in the literature (Table 3 10,11,16,17,18,19,20,21,32,36). The most prevalent asymptomatic resected pancreatic tumour in our experience was the nonfunctional neuroendocrine tumour. In recent series examining endocrine tumours of the pancreas, an increase in nonfunctional neuroendocrine tumours has been recognized (18%–66% of all cases).14 Some investigators believe that nonfunctional neuroendocrine tumours have a greater propensity to metastasize than their functional counterparts and suggest that all nonfunctional tumours be considered malignant.16 We suggest that the surgical management of such patients should be considered given that long-term survival can be achieved with surgical resection, even in the presence of locally advanced or lymph-node disease.14,16,20
Table 3
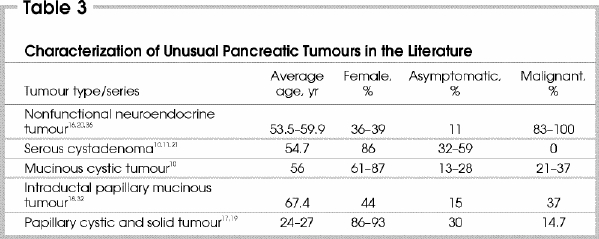
Cystic neoplasms of the pancreas were the second most common tumours encountered as incidentalomas in our series. This differs from the findings in the literature where serous cystadenomas are the most common lesions identified, with 32% to 59% of patients presenting asymptomatically.10,11,21,22,23 These tumours may be either serous or mucinous in origin. The true prevalence of these tumours is unclear but appears to be increasing.9 Serous cystadenoma, or microcystic adenoma, is thought to be a benign disease with little or no malignant potential.37 An algorithm that allows for observation of lesions when the diagnosis of serous cystadenoma has been clearly established is well accepted.11 Unfortunately, diagnosis based on radiologic imaging alone is difficult, and the overall accuracy is about 50%.23,28,30 In addition, some authors have challenged the concept of the benign serous cystadenoma, and serous cystadenocarcinoma has been reported.18,38
Mucinous tumours or macrocystic adenomas may be either cystadenomas or cystadenocarcinomas. Mucinous cystadenoma is not a malignant lesion but has the potential for malignant transformation with rapid progression and should thus be viewed as premalignant or potentially malignant. Surgical resection is recommended in patients with appropriate surgical risk.10,11 Cystadenocarcinoma is a malignant tumour and should be treated with aggressive surgical intervention.
Intraductal papillary mucinous tumour (IPMT) and papillary cystic and solid tumours may also present as incidentalomas. IPMT is a newly described pancreatic neoplasm defined by the World Health Organization in 1996. This is a premalignant or frankly malignant condition with a histologic spectrum ranging from adenoma to infiltrating carcinoma. The proportion of tumours that are malignant varies from 21% to 37%.12,13 Surgical resection is recommended in all appropriate-risk patients.12,15,18,33,39,40 There is some controversy regarding the extent of surgical resection. Some authors consider this a disease of the entire ductal system and have recommended total pancreatectomy.41 In other recent series, consideration of complete resection of the tumour with intraoperative frozen section to analyze the margin has been presented as the procedure of choice.15,33,39,41
Papillary cystic and solid tumour of the pancreas is very rare, with about 289 documented cases.17 Malignant disease has been documented in 14.7% of cases.17 Surgical resection, even in the face of limited metastatic disease, has resulted in long-term survival and should be considered the standard of practice.17
In our study, incidental pancreatic masses were identified in a group of patients with distinct clinical features compared with those for symptomatic patients with pancreatic lesions. For pancreatic incidentalomas the spectrum of pancreatic disease does not seem to include ductal adenocarcinoma. The identification of pancreatic incidentalomas appears to be increasing secondary to the broad application of high-resolution imaging. When a pathologic diagnosis is evident from the preoperative imaging in pancreatic incidentalomas, management algorithms are clear. Unfortunately, a histologic diagnosis cannot always be accurately predicted from preoperative imaging. In good-risk, asymptomatic patients we advocate surgical intervention unless serous cystadenoma can be confidently diagnosed.34
Acknowledgments
We acknowledge the efforts of Linda Last in the preparation of the manuscript.
Competing interests: None declared.
Correspondence to: Dr. Sherif S. Hanna, Toronto Sunnybrook Regional Cancer Centre, 2075 Bayview Ave., Toronto ON M4N 3M5; fax 416 217-1338; sherif.hanna@swchsc.on.ca
Accepted for publication Apr. 15, 2003.
References
- 1.Brunt LM, Moley JF. Adrenal incidentaloma. World J Surg 2001;25:905-13. [DOI] [PubMed]
- 2.Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma — age and stage characterization and clinical implications: study of 1092 patients (1982–1997). Urology 2000;56:58-62. [DOI] [PubMed]
- 3.Westbrook JI, Braithwaite J, McIntosh JH. The outcomes for patients with incidental lesions: serendipitous or iatrogenic? AJR Am J Roentgenol 1998;171:1193-6. [DOI] [PubMed]
- 4.Kolomecki K, Pomorski L, Kuzdak K, Narebski J, Wichman R. The surgical treatment of adrenal gland tumors — incidentaloma. Neoplasma 1999;46:124-7. [PubMed]
- 5.Little JM, Kenny J, Hollands MJ. Hepatic incidentaloma: a modern problem. World J Surg 1990;14:448-51. [DOI] [PubMed]
- 6.Hara AK, Johnson CD, MacCarty RL, Welch TJ. Incidental extracolonic findings at CT colonography. Radiology 2000;215:353-7. [DOI] [PubMed]
- 7.Bretheau D, Koutani A, Lechevallier E, Coulange C. A French national epidemiologic survey on renal cell carcinoma. Oncology Committee of the Association Française d'Urologie. Cancer 1998;82:538-44. [DOI] [PubMed]
- 8.Aso Y, Homma Y. A survey on incidental renal cell carcinoma in Japan. J Urol 1992;147:340-3. [DOI] [PubMed]
- 9.Sheehan M, Latona C, Aranha G, Pickleman J. The increasing problem of unusual pancreatic tumors. Arch Surg 2000;135: 644-8. [DOI] [PubMed]
- 10.Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg 1999;230:152-61. [DOI] [PMC free article] [PubMed]
- 11.Rattner DW, Fernandez-del Castillo C, Warshaw AL. Cystic pancreatic neoplasms. Ann Oncol 1999;10(Suppl 4):104-6. [PubMed]
- 12.Sohn TA, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg 2001;234:313-21. [DOI] [PMC free article] [PubMed]
- 13.Loftus EV Jr, Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology 1996;110:1909-18. [DOI] [PubMed]
- 14.Madura JA, Cummings OW, Wiebke EA, Broadie TA, Goulet RL Jr, Howard TJ. Nonfunctioning islet cell tumors of the pancreas: a difficult diagnosis but one worth the effort. Am Surg 1997;63:573-7. [PubMed]
- 15.Falconi M, Salvia R, Bassi C, Zamboni G, Talamini G, Pederzoli P. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg 2001;88:376-81. [DOI] [PubMed]
- 16.Matthews BD, Heniford BT, Reardon PR, Brunicardi FC, Greene FL. Surgical experience with nonfunctioning neuroendocrine tumors of the pancreas. Am Surg 2000;66:1116-22. [PubMed]
- 17.Mao C, Guvendi M, Domenico DR, Kim K, Thomford NR, Howard JM. Papillary cystic and solid tumors of the pancreas: a pancreatic embryonic tumor? Studies of three cases and cumulative review of the world's literature. Surgery 1995;118:821-8. [DOI] [PubMed]
- 18.Siech M, Tripp K, Schmidt-Rohlfing B, Mattfeldt T, Gorich J, Beger HG. Intraductal papillary mucinous tumor of the pancreas. Am J Surg 1999;177:117-20. [DOI] [PubMed]
- 19.Pettinato G, Manivel JC, Ravetto C, Terracciano LM, Gould EW, di Tuoro A, et al. Papillary cystic tumor of the pancreas. A clinicopathologic study of 20 cases with cytologic, immunohistochemical, ultrastructural, and flow cytometric observations, and a review of the literature. Am J Clin Pathol 1992;98:478-88. [DOI] [PubMed]
- 20.Bartsch DK, Schilling T, Ramaswamy A, Gerdes B, Celik I, Wagner HJ, et al. Management of nonfunctioning islet cell carcinomas. World J Surg 2000;24:1418-24. [DOI] [PubMed]
- 21.O'Dell ML, Handler MS, Wetzel LH. Incidental detection of a microcystic adenoma of the pancreas. South Med J 1991;84:776-9. [DOI] [PubMed]
- 22.Pike J, Ehsani MT. Microcystic adenoma of the pancreas — an incidental finding. Australas Radiol 1989;33:404-5. [DOI] [PubMed]
- 23.Procacci C, Graziani R, Bicego E, Bergamo-Andreis IA, Guarise A, Valdo M, et al. Serous cystadenoma of the pancreas: report of 30 cases with emphasis on the imaging findings. J Comput Assist Tomogr 1997;21:373-82. [DOI] [PubMed]
- 24.Talamini MA, Moesinger R, Yeo CJ, Poulose B, Hruban RH, Cameron JL, et al. Cystadenomas of the pancreas: Is enucleation an adequate operation? Ann Surg 1998;227:896-903. [DOI] [PMC free article] [PubMed]
- 25.Yeo CJ, Wang BH, Anthone GJ, Cameron JL. Surgical experience with pancreatic islet-cell tumors. Arch Surg 1993;128:1143-8. [DOI] [PubMed]
- 26.Yamaguchi K, Noshiro H, Yokohata K, Nakano K, Watanabe M, Ohtani K, et al. Is there any benefit of preservation of the spleen in distal pancreatectomy? Int Surg 2001;86:162-8. [PubMed]
- 27.Aldridge MC, Williamson RC. Distal pancreatectomy with and without splenectomy. Br J Surg 1991;78:976-9. [DOI] [PubMed]
- 28.Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, et al. CT of primary cystic pancreatic neoplasms: Can CT be used for patient triage and treatment? AJR Am J Roentgenol 2000;175:99-103. [DOI] [PubMed]
- 29.Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr 1999;23:906-12. [DOI] [PubMed]
- 30.Johnson CD, Stephens DH, Charboneau JW, Carpenter HA, Welch TJ. Cystic pancreatic tumors: CT and sonographic assessment. AJR Am J Roentgenol 1988;151:1133-8. [DOI] [PubMed]
- 31.Soyer P, Rabenandrasana A, Van Beers B, Barge J, Sibert A, Laissy JP, et al. Cystic tumors of the pancreas: dynamic CT studies. J Comput Assist Tomogr 1994;18:420-6. [DOI] [PubMed]
- 32.Schindl M, Kaczirek K, Kaserer K, Niederle B. Is the new classification of neuroendocrine pancreatic tumors of clinical help? World J Surg 2000;24:1312-8. [DOI] [PubMed]
- 33.Cuillerier E, Cellier C, Palazzo L, Deviere J, Wind P, Rickaert F, et al. Outcome after surgical resection of intraductal papillary and mucinous tumors of the pancreas. Am J Gastroenterol 2000;95:441-5. [DOI] [PubMed]
- 34.Sarr MG, Kendrick ML, Nagorney DM, Thompson GB, Farley DR, Farnell MB. Cystic neoplasms of the pancreas: benign to malignant epithelial neoplasms. Surg Clin North Am 2001;81:497-509. [DOI] [PubMed]
- 35.Tandan VR, Gallinger S. Management of cystic lesions of the tail of the pancreas. Can J Surg 1995;38:347-50. [PubMed]
- 36.Martin I, Hammond P, Scott J, Redhead D, Carter DC, Garden OJ. Cystic tumours of the pancreas. Br J Surg 1998;85:1484-6. [DOI] [PubMed]
- 37.Pyke CM, van Heerden JA, Colby TV, Sarr MG, Weaver AL. The spectrum of serous cystadenoma of the pancreas. Clinical, pathologic, and surgical aspects. Ann Surg 1992;215:132-9. [DOI] [PMC free article] [PubMed]
- 38.Formentini A, Birk D, Siech M, Mattfeldt T, Fortnagel G, Beger HG. Serous cystadenocarcinoma of the pancreas and serous cystadenoma associated with ductal adenocarcinoma. HPB 2000;2:41-8.
- 39.Paye F, Sauvanet A, Terris B, Ponsot P, Vilgrain V, Hammel P, et al. Intraductal papillary mucinous tumors of the pancreas: pancreatic resections guided by preoperative morphological assessment and intraoperative frozen section examination. Surgery 2000;127:536-44. [DOI] [PubMed]
- 40.Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg 1998;228:685-91. [DOI] [PMC free article] [PubMed]
- 41.de Stadt JV, Closset J, Gelin M. Intraductal papillary mucinous tumors (IPMT). Ann Surg 1999;230:828-9. [DOI] [PMC free article] [PubMed]


