Abstract
Objective
To determine in patients with localized primary melanoma of the trunk or extremities the optimal excision margin that achieves the highest disease-free survival and overall survival and the lowest local recurrence rate.
Data sources
Trials comparing 2 different excision margins were identified by searching MEDLINE from 1966 to May 2002 using the term “melanoma,” subheading “surgery,” and limiting the search to human studies and randomized controlled trials (RCTs). Additional studies were found using the MeSH term “surgical procedures, operative,” combining with “melanoma,” and limiting to human studies. We searched EMBASE and the Cochrane Library in May 2002 using similar terminology. No language restriction was applied.
Study selection
We selected studies for the overview using the following inclusion criteria: design — an RCT with wide excision versus narrower excision (margin width was not specified a priori); population — adult patients (> 18 yr) with cutaneous melanoma of the trunk or extremities without evidence of metastasis; intervention — surgical excision of the primary melanoma; and outcomes — at least 1 of overall survival, disease-free survival, local recurrence, wound complications and necessity for skin grafting.
Data extraction
Information was abstracted for each outcome reported in the studies, and results were pooled by consensus. Statistical analysis was performed using RevMan 4.1 (The Cochrane Collaboration) software program. Relative risk and risk difference were reported with 95% confidence intervals. The number needed to harm was calculated for the need for skin grafting by taking the inverse of the risk difference.
Data synthesis
Three trials and their follow-up studies met the inclusion criteria and included 2087 adults with localized cutaneous melanoma of the trunk or extremities. No statistically significant differences were found between wide surgical excision (margins ranging from 3–5 cm) and narrower surgical excision (margins ranging from 1–2 cm) with respect to mortality, disease-free survival or local recurrence rate.
Conclusions
Surgical excision margins no more than 2 cm around a melanoma of the trunk or extremities are adequate; overall survival, disease-free survival and recurrence rate are not adversely affected compared with a wider excision. There is more data to support a 2-cm margin than a 1-cm margin as the minimum margin of excision. Surgical margins should be no less than 1 cm around the primary melanoma.
Abstract
Objectif
Déterminer, chez des patients atteints d'un mélanome primitif local du tronc ou des membres, la marge d'excision optimale produisant le taux le plus élevé de survie sans maladie et de survie globale, ainsi que le taux le plus faible de récurrence locale.
Sources de données
On a trouvé des études comparant deux marges d'excision différentes en effectuant dans MEDLINE une recherche portant sur la période de 1966 à mai 2002 au moyen des termes «melanoma», sous-rubrique «surgery», et en limitant la recherche aux études sur les êtres humains et aux études contrôlées randomisées (ECR). On a trouvé d'autres études en utilisant l'expression MeSH «surgical procedures, operative», combinée à «melanoma» et en limitant la recherche aux études sur les êtres humains. Nous avons effectué une recherche dans EMBASE et la Cochrane Library en mai 2002 en utilisant une terminologie semblable. Aucune restriction linguistique n'a été appliquée.
Sélection des études
Nous avons choisi les études pour la vue d'ensemble en utilisant les critères d'inclusion suivants : conception — ECR comportant une marge d'excision large par rapport à une marge plus étroite (la largeur de la marge n'a pas été précisée au départ); population — patients adultes (> 18 ans) ayant un mélanome cutané du tronc ou des membres sans indication de métastases; intervention — excision chirurgicale du mélanome primitif; et évolution de l'état de santé — au moins un cas de survie globale, de survie sans maladie, de récurrence locale, de complications liées à la plaie et de besoin d'une greffe de peau.
Extraction des données
On a résumé l'information portant sur l'évolution de chaque cas signalé dans les études et regroupé les résultats par consensus. On a procédé à une analyse statistique au moyen du logiciel RevMan 4.1 (The Cochrane Collaboration). Le risque relatif et le risque différentiel comportaient un intervalle de confiance à 95 %. On a calculé le nombre nécessaire pour qu'il y ait préjudice dans le cas du besoin d'une greffe de peau en calculant l'inverse du risque différentiel.
Synthèse de données
Trois études cliniques et leurs études de suivi qui satisfaisaient aux critères d'inclusion portaient sur 2087 adultes ayant un mélanome cutané localisé du tronc ou des membres. On n'a pas constaté de différences statistiquement significatives entre l'excision chirurgicale large (marge variant de 3 à 5 cm) et l'excision chirurgicale plus étroite (marge variant de 1 à 2 cm) pour ce qui est des taux de mortalité, de survie sans maladie ou de récurrence locale.
Conclusions
Des marges d'excision chirurgicale d'au plus 2 cm autour d'un mélanome du tronc ou des membres suffisent et n'ont pas d'effets indésirables sur les taux de survie globale, de survie sans maladie et de récurrence par rapport à une excision plus large. Il y a plus de données à l'appui d'une marge de 2 cm que d'une marge de 1 cm comme marge minimale. Les marges chirurgicales doivent avoir au moins 1 cm autour du mélanome primitif.
Surgical excision remains the mainstay of treatment for primary cutaneous melanoma. However, how much surrounding normal skin should be excised around a primary cutaneous melanoma is controversial. Historically, specimens larger than 5 cm in dimension were removed because of the contention that a large excision will provide optimal outcomes in terms of local recurrence rate and survival.1 Furthermore, because increased numbers of melanocytes have been found in the clinically normal skin surrounding many melanomas and melanocytic activation has been reported up to 4 cm from the primary tumour in clinically normal skin, wide excision is a logical choice.2,3 In some cases, extremely wide excisions were recommended because recurrences were found up to 15 cm from the original site of primary tumour excision.4 Acceptance of such a wide surgical margin was not based on good scientific outcome data, and these radical excisions were morbid, especially since many required skin grafting.
Other studies have suggested that more conservative excision margins are equivalent to large excisions with respect to survival yet allow a much less morbid procedure.5,6,7 In addition, Olsen8 reported similar local recurrence rates in patients who had a narrow excision and those who had at least 5 cm excision margins. The suggestion from these nonrandomized studies that narrower excisions are equivalent to wider excisions can only be confirmed in randomized controlled trials (RCTs). A major advantage of narrower excisions, if overall survival and recurrence rates are similar, would be less morbidity and fewer skin grafting procedures, which require the use of additional resources, more operative time, more intensive wound care, and usually have a less desirable cosmetic outcome.
Our primary objective was to determine the optimal margin of surgical excision that achieves the highest disease-free survival, overall survival and the lowest local recurrence rate in adult patients with a primary cutaneous melanoma of the trunk or extremities and no evidence of metastatic disease. Secondary objectives were to determine the complication rate in terms of local wound infection and dehiscence rate, and the need for skin grafting with various margins of excision.
Methods
This systematic review and meta-analysis was completed using the approach described by The Cochrane Collaboration.9
Data sources
All RCTs or quasi-randomized clinical trials comparing 2 different margins of excision in adult patients with confined primary cutaneous melanoma of the trunk or extremities were searched on MEDLINE from 1966 to May 2002 using the terms “melanoma, surgery,” limited to human studies. Additional studies were found using the MeSH term “Surgical Procedures, Operative,” combined with “Melanoma,” and limited to human studies. EMBASE and the Cochrane Library were searched in May 2002 using similar terminology. No language restriction was applied. No unpublished articles or abstracts published or presented at meetings were included.
Study selection
Two authors (P.I.H. and L.A.D.) reviewed the titles of the articles and the abstracts if available as identified from the search. Any article deemed appropriate or possibly meeting the inclusion criteria from the title or abstract was retrieved. These articles were screened by both authors, and the following criteria were used to select studies: (1) study design — an RCT with one excision margin versus another excision margin (there were no strict a priori definitions of wide or narrow margins; any study that compared one excision margin width versus another automatically met the criterion of a wide versus a narrower excision margin); (2) population — adult patients (> 18 yr) with cutaneous melanoma of the trunk or extremities without evidence of metastasis; (3) intervention — surgical excision of the primary melanoma; (4) outcome — at least 1 of overall survival, disease-free survival, local recurrence, wound complications and need for skin grafting. Studies were excluded if they were repeat reports of a previously published trial.
Data extraction
Data forms were developed and used to extract data from each article that met the inclusion criteria. The 2 authors independently abstracted the data for each article. Demographic data and the main outcome measures were extracted. Overall survival and disease-free survival were converted to mortality and any recurrence, in keeping with The Cochrane Collaboration format of attaining a risk reduction of an adverse event rather than of a favourable event.
Studies were assessed for methodologic quality using the “Users' guides to the medical literature” published by the Journal of the American Medical Association.10,11
Statistical analysis
Analysis was performed using RevMan 4.1 (The Cochrane Collaboration, Oxford, UK) software program. Relative risk (RR) and risk difference (RD) are reported using the fixed-effects model with 95% confidence intervals (CIs). An RR or equal to 1 or an RD equal to 0 indicates no difference in outcome between wide excision and narrower excision. An RR less than 1 or a negative RD indicates that the outcome favours wide excision, and RR greater than 1 or a positive RD indicates that the outcome favours narrower excision. Differences in outcomes were considered statistically significant if the 95% CI does not include 1 for RR or 0 for RD. In the case of a statistically significant risk reduction or increase, the number needed to treat (or harm) was calculated. Two separate meta-analyses of mortality, any recurrence and local recurrence were performed using the original trials in one analysis and the follow-up studies in the second analysis. Mortality, any recurrence and local recurrence were grouped over a range of follow-up time because of different follow-up in each trial.
Data synthesis
Description of studies
Details of the included studies are shown in Table 1.12,13,14,15,16,17 Three studies totalling 1867 patients met the inclusion criteria. Two studies were international multicentre studies with participating centres in Europe, North and South America, and Russia.12,13 The third study was a multicentre study completed in Sweden.14 Three studies, all follow-up studies to the original 3, also met the criteria; the study by Cohn-Cedermark and associates17 added patients to the original trial, which increased the total number to 2087. Three studies were excluded because they were repeat reports of the original studies.18,19,20
Table 1
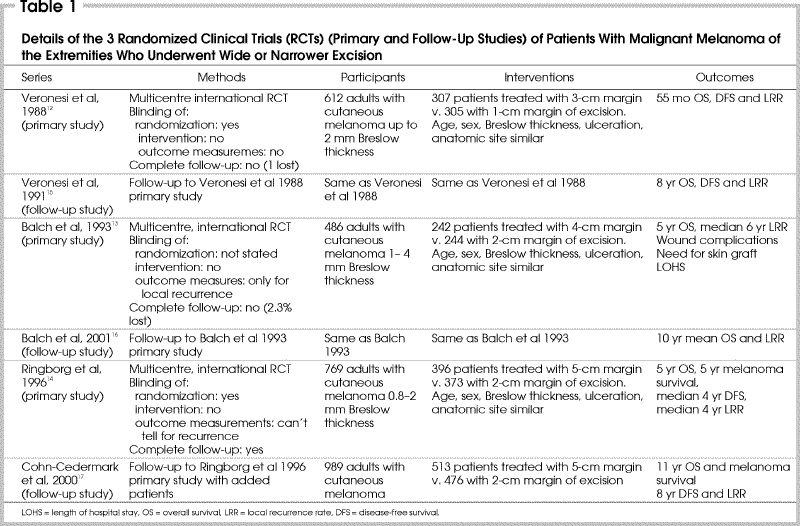
The trial of Veronesi and colleagues12 enrolled patients with melanomas up to 2 mm in Breslow thickness. Patients were randomized to wide excision of 3 cm or to 1-cm excision. After the skin incision was made, the flaps were undermined radially for at least 1 cm and continued to fascia. The 3 outcome measures were overall survival, disease-free survival and local recurrences. Local recurrence was defined as a first recurrence within the scar or within 1 cm from the scar.15 Mean duration of follow-up was 55 months. In the follow-up study had a mean duration of follow-up of 8 years.15
In the trial reported by Balch and associates,13 patients were enrolled if the melanoma was between 1 and 4 mm thick according to Breslow's classification. Patients were randomized to wide excision of 4 cm or 2 cm. Excisions incorporated the skin at the stipulated margin and underlying subcutaneous tissues to fascia; inclusion of fascia in the specimen was optional. This trial also had a second randomization to an elective lymph-node dissection or observation of the regional nodal basin. The outcome of local recurrence was defined as a biopsy-proven first recurrence within 2 cm of the scar; if the local recurrence occurred after the appearance of distant metastases, for example, then a local recurrence was not counted. Overall survival was another outcome measure. Median survival was 72 months. In the follow-up study the mean survival was 10 years.16
In their trial, Ringborg and colleagues14 enrolled 769 patients with melanoma ranging from 0.8 to 2.0 mm in Breslow thickness. Patients were randomized to wide excision of 5 cm or 2 cm. The excision technique was similar to that of Balch and associates.13 Outcome measures included overall survival, disease-free survival and local recurrence rate. Local recurrence was defined as recurrence within the scar or skin graft. Median follow-up was 5.8 years for survival, and 4 years for recurrences. The follow-up study included additional patients to a total of 989, and median follow-up of 11 years for survival and 8 years for recurrences.17
Study quality
In general, the methodologic quality of the 3 trials was good, recognizing that in surgical trials it is more difficult to blind the treating clinicians and physicians responsible for measuring the outcome. Therefore, because all studies were surgical, each lacked at least 1 important methodologic criterion that would possibly produce bias: they all lacked blinding of the intervention. In all trials it was difficult to determine whether individual validity criteria were met because often these aspects were not stated explicitly in the methods section. Particularly with respect to outcome measurements other than the dichotomous outcome of “alive” or “dead,” in these surgical trials the outcome of disease-free survival or local recurrence was often assessed by clinicians who may not have been blinded to treatment allocation. It is possible, therefore, that the clinicians responsible for detecting recurrences could introduce bias by measuring the outcome in a different manner, depending on their preference, if they knew the patient's treatment allocation.
The trial sponsored by Veronesi and colleagues12 provided explicit details on randomization: the process was concealed. Only 1 patient was lost to follow-up from the wide excision arm. Analysis was not using the intention-to-treat principle, because those who received the wrong treatment (8 patients from the narrow excision group and 7 from the wide excision group) were excluded.
The study of Balch and associates13 did not report whether allocation was concealed. We can assume that the intervention was known by participating surgeons and other clinicians. The outcome that was identified by a panel blinded to treatment was local recurrence: the panel of pathologists identified biopsy-proven recurrences, which would help to avoid bias for this outcome. Not stated is whether disease-free survival was assessed in a blinded fashion. Finally, although follow-up was not complete, only 2.3% were lost to follow-up. The actual number of patients lost in each arm was not stated.
In the Ringborg trial14 the methodology of randomization was outlined more clearly in the updated trial.17 A telephone system was used for randomization at central cancer centres, and presumably allocation would be blinded to enrolling physicians. Regarding outcomes of local recurrence and any recurrence, the authors did not state about blinding using adjudication panels, so it can be assumed that treatment allocation might have been deduced by the outcome assessors. Follow-up was complete.
Mortality
All 3 initial studies reported on mortality at 4 to 6 years' follow-up (Fig. 1). Balch and associates13 showed an RR of death of 0.79 in favour of wide excision, but the 95% CIs ranged from 0.54 to 1.15, not statistically significant. The Veronesi12 and Ringborg14 trials showed a slight advantage of a narrower excision, with RRs of 1.02 and 1.19, respectively, but with 95% CIs ranging from 0.71 to 1.45 and 0.52 to 2.72, respectively. With all studies combined, the RR of death with wide excision compared to a narrower excision was not significantly decreased (RR = 0.93, 95% CI 0.73–1.19; RD = –0.01; 95% CI –0.04 to 0.02) (Fig. 1).
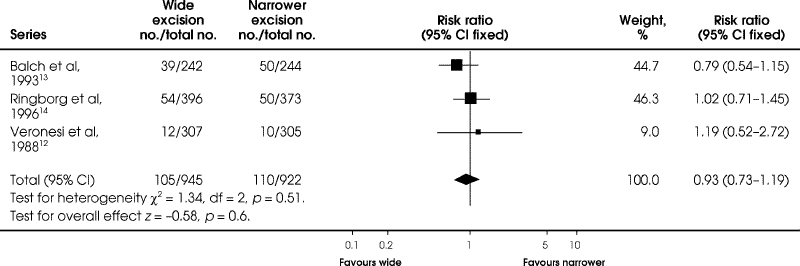
FIG. 1. Comparison of mortality at 4 to 6 years between wide and narrower excision in the 3 original trials of melanoma of the extremities.
Mortality at 8 to 11 years' follow-up was not significantly different between wide excision and a narrower excision (RR = 0.95, 95% CI 0.81–1.12; RD = –0.01; 95% CI –0.05 to 0.02) (Fig. 2).
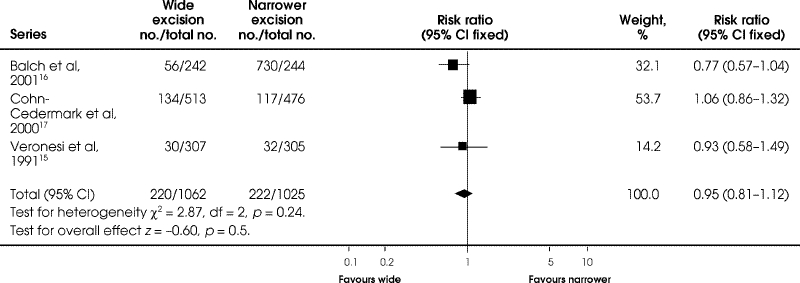
FIG. 2. Comparison of mortality at 8 to 11 years between wide and narrower excision in the 3 follow-up trials of melanoma of the extremities.
Any recurrence
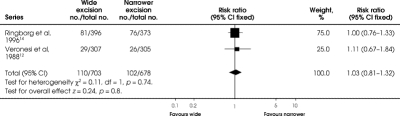
The trials of Veronesi and colleagues12 and Ringborg and colleagues14 reported any recurrence that occurred between 55 and 69.6 months (Fig. 3). Neither trial showed a significant difference between wide excision and a narrower excision. Also, the 2 studies together show no difference between wide versus narrower excision (RR 1.03, 95% CI 0.81–1.32; RD = 0.00, 95% CI –0.03 to 0.04) for any recurrence (Fig. 3).
FIG. 3. Comparison of any recurrence at 55 to 70 months between wide and narrower excision in 2 original trials of malignentmelanoma of the hands and feet. The trial reported by Balch and associates 13 did not report this outcome.
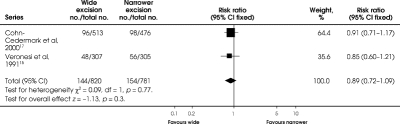
At longer follow-up of 8 years, no difference (RR = 0.89, 95% CI 0.72, 1.09; RD = –0.02; 95% CI –0.06, 0.02) remained between the 2 interventions from the follow-up studies (Fig. 4).
FIG. 4. Comparison of any recurrence at 8 years between wide and narrower excision in 2 follow-up trials of melignant melanoma of the extremities. The trial reported by Balch and associates 13 did not report this outcome.
Local recurrence rates
All 3 initial studies reported local recurrence rates; none showed differences in local recurrence rates between wide and narrower excisions at 48 to 72 months' follow-up (Fig. 5). The Veronesi12 and Balch13 trials reported local recurrences only as the first recurrence, whereas the Ringborg trial14 reported total local recurrences. Combining the 3 studies revealed no difference in local recurrence between wide and narrower excisions (RR = 0.98, 95% CI 0.38–2.52; RD = 0.00, 95% CI –0.01, 0.01) (Fig. 5).
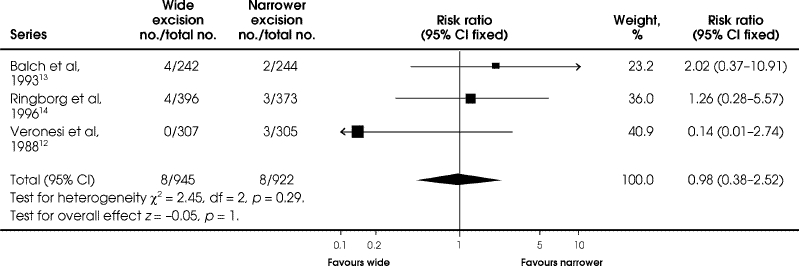
FIG. 5. Comparison of local recurrence at 48 to 72 months between wide and narrower excision in the 3 original trials of patients with malignant of the extremities.
At 8 to 10 years, the local recurrence rates remained similar between interventions in the 3 follow-up studies (Fig. 6). Relative risk for local recurrence with the 3 follow-up studies combined was 0.90 (95% CI 0.41–2.00), and the RD was 0 (95% CI –0.01 to 0.01).
FIG. 6. Comparison of local recurrence at 8 to 10 years between wide and narrower excision in the 3 follow-up trials of patients with melanoma of the extremities.
Wound complications
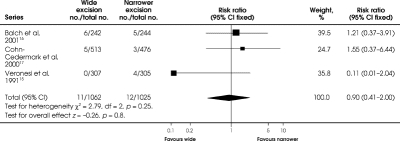
Only Balch and associates13 reported wound complications. Postoperative wound infection favoured wide excision (RR = 0.85, 95% CI 0.39–1.87; RD = –0.01, 95% CI –0.05 to 0.03) as did wound dehiscence (RR = 0.92, 95% CI 0.40–2.12; RD = 0, 95% CI –0.04 to 0.03), but these RRs and RDs were not statistically significant.
Need for skin grafting
Once again, only Balch and associates13 reported on the need for skin grafting. There was a significant reduction in the need for skin grafting with narrow excision compared with wide excision (RR = 4.15 95% CI 2.83–6.07; RD = 0.35, 95% CI 0.27–0.42; p < 0.001). The number needed to harm from wide excision is 3, with 95% CI ranging from 2.38 to 3.7; for every 3 patients who undergo wide excision, 1 patient will require a skin graft who would not have needed it if a narrower excision had been performed.
Discussion
This meta-analysis showed no benefit for a wide excision over a narrower excision in patients with melanoma of the trunk or extremities. The data indicate that an excision margin of no greater than 2 cm is adequate for treatment of primary melanoma, and that overall survival, disease-free survival and local recurrence rates are not adversely affected by this margin of excision.
The trials differed in eligibility criteria, particularly the criterion of Breslow thickness. The margins that were compared and the outcome measures differed. For these reasons, many questions remain unanswered, particularly the optimal narrower margin and whether different subsets of Breslow thickness can be treated with different excision margins. For instance, the surgical excision performed by Veronesi and colleagues12 differed from that of the other 2 studies: flaps were raised in all directions, and at the fascial level, the subcutaneous tissue in the narrow 1-cm excision specimen would have been similar to the 2-cm excision in the other trials. Local recurrences were defined differently in terms of distance from the scar and in terms of initial recurrence. Nevertheless, the events were very rare, and the excision margin had little impact on the propensity for local recurrence.
From the results of this meta-analysis, it is difficult to recommend the minimum excision margin required. The minimum margin studied was 1 cm; however, more data were available on a 2-cm margin with 2 studies combined. The 2-cm excision margin has never been compared directly to a 1-cm margin in a randomized trial.
It has been proposed that a 1-cm margin of excision be performed for melanomas less than 1 mm thick, reserving wider excisions for tumours 1 mm or thicker.21,22 There are many descriptive studies supporting this policy, showing that the local recurrence rates are acceptable and similar to that of wider excisions. However, it should be noted that this meta-analysis does not strictly support this policy because the 3 major RCTs contain data on fewer than 185 patients with melanomas less than 1 mm thick who had a 1-cm excision. Therefore, a 2-cm margin should be the goal.
Only the Balch trial13 reported on wound complications and the need for skin grafting. Total wound complications did not differ significantly between the wide and narrow excision. The need for skin grafting, not surprisingly, was higher in the wide excision group. However, only about 10% of patients required skin grafting with a 4-cm excision. Unfortunately, skin grafting requires more intense nursing and wound care than an excision that is closed primarily. With a number needed to harm of 3 with wide excision, a narrower excision readily helps to avoid the morbidity of a skin graft.
Conclusions
A surgical excision margin of no more than 2 cm around a melanoma of the trunk or extremities is adequate; mortality and recurrence rates should not be adversely affected compared to a wider margin. Surgical margins should be no less than 1 cm around the primary melanoma. A 1-cm margin has never been tested against a 2-cm margin in any randomized trial, but more data are available for a 2-cm excision as the minimum surgical margin for optimal overall survival, disease-free survival and local recurrence.
A trial that randomized patients with cutaneous melanoma up to 4 mm thickness to a 1-cm versus 2-cm surgical excision would answer the question of what is the optimal narrow excision. However, with such low event rates, and the fact that 90% of wounds probably could be closed primarily even with a 2-cm margin, the answer may be more academic than clinically useful. With such little impact of surgical margin on prognosis, in contrast to the great influence of tumour thickness, more effort should be expended on primary and secondary prevention so that early detection will decrease the proportion of patients presenting with thicker melanomas.
Acknowledgments
This manuscript was prepared during HAD 5308H, a course offered by the Department of Health Administration, University of Toronto, Toronto, Ont., and completed by the first author as a requirement for the Masters of Science in Clinical Epidemiology, University of Toronto. We thank Dr. Arne Ohlsson, the HAD 5308H course instructor, for his valuable critiques and insight throughout the preparation of this manuscript.
Competing interests: None declared.
Correspondence to: Dr. Philip I. Haigh, Department of Surgical Oncology, Kaiser Permanente Los Angeles Medical Center, 4760 Sunset Blvd., Los Angeles CA 90027; fax 323 783-8747; philip.i.haigh@kp.org
Accepted for publication Feb. 11, 2003.
References
- 1.Handley WS. The pathology of melanotic growths in relation to their operative treatment. Lancet 1907;1:927.
- 2.Cochran AJ. Studies of the melanocytes of the epidermis adjacent to tumors. J Invest Dermatol 1971;57:38-43. [DOI] [PubMed]
- 3.Wong CK. A study of the melanocytes in the normal skin surrounding malignant melanoma. Dermatologica 1970;141: 215-25. [DOI] [PubMed]
- 4.Peterson NC, Bodenham DC, Lloyd OC. Malignant melanomas of the skin: a study of the origin, development, etiology, spread, treatment, and prognosis. Br J Plast Surg 1962;15:49. [DOI] [PubMed]
- 5.Elberg JJ, Poulsen J, Ladefoged C. The influence of resection margin on prognosis in clinical stage I malignant melanoma of the lower leg. Scand J Plast Reconstr Surg 1989;23:59-63. [DOI] [PubMed]
- 6.Goldman LI, Byrd R. Narrow resection margins for patients with low-risk melanoma. Am J Surg 1988;155:242-4. [DOI] [PubMed]
- 7.Elias EG, Didolkar MS, Goel IP, Formeister JF, Valenzuela LA, Pickren JL, et al. A clinicopathologic study of prognostic factors in cutaneous malignant melanoma. Surg Gynecol Obstet 1977;144:327-34. [PubMed]
- 8.Olsen G. The malignant melanoma of the skin: new theories based on a study of 500 cases. Acta Chir Scand 1966;365:1-222. [PubMed]
- 9.Clarke M, Oxman AD, editors. Cochrane reviewers' handbook 4.1.5 [updated April 2002]. In: The Cochrane Library, issue 2, 2002. Oxford: Update Software. [updated quarterly].
- 10.Guyatt GH, Sackett DL, Cook DJ. Users' guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 1993;270:2598-601. [DOI] [PubMed]
- 11.Guyatt GH, Sackett DL, Cook DJ. Users' guides to the medical literature. II. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA 1994;271:59-63. [DOI] [PubMed]
- 12.Veronesi U, Cascinelli N, Adamus J, Balch C, Bandiera D, Barchuk A, et al. Thin stage I primary cutaneous malignant melanoma. Comparison of excision with margins of 1 or 3 cm. N Engl J Med 1988;318:1159-62. [DOI] [PubMed]
- 13.Balch CM, Urist MM, Karakousis CP, Smith TJ, Temple WJ, Drzewiecki K, et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1–4 mm). Ann Surg 1993;218:262-9. [DOI] [PMC free article] [PubMed]
- 14.Ringborg U, Andersson R, Eldh J, Glaumann B, Hafstrom L, Jacobsson S, et al. Resection margins of 2 versus 5 cm for cutaneous malignant melanoma with a tumor thickness of 0.8 to 2.0 mm. A randomized study by the Swedish Melanoma Study Group. Cancer 1996;77:1809-14. [DOI] [PubMed]
- 15.Veronesi U, Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg 1991;126:438-41. [DOI] [PubMed]
- 16.Balch CM, Soong SJ, Smith T, Ross MI, Urist MM, Karakousis CP, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann Surg Oncol 2001;8:101-8. [DOI] [PubMed]
- 17.Cohn-Cedermark G, Rutqvist LE, Andersson R, Breivald M, Ingvar C, Johansson H, et al. Long term results of a randomized study by the Swedish Melanoma Study Group on 2-cm versus 5-cm resection margins for patients with cutaneous melanoma with a tumor thickness of 0.8–2.0 mm. Cancer 2000;89:1495-501. [PubMed]
- 18.Demidov LV, Barchuk AS. [Surgical treatment of superficial melanoma of the skin]. Voprosy Onkol 1998;44:149-54. [PubMed]
- 19.Karakousis CP, Balch CM, Urist MM, Ross MM, Smith TJ, Bartolucci AA. Local recurrence in malignant melanoma: long-term results of the multi-institutional randomized surgical trial. Ann Surg Oncol 1996;3:446-52. [DOI] [PubMed]
- 20.Rogers GS. Narrow versus wide margins in malignant melanoma. J Dermatol Surg Oncol 1989;15:33-4. [DOI] [PubMed]
- 21.Karakousis CP. Surgical treatment of malignant melanoma. Surg Clin North Am 1996;76:1299-312. [DOI] [PubMed]
- 22.Ng AK, Jones WO, Shaw JH. Analysis of local recurrence and optimizing excision margins for cutaneous melanoma. Br J Surg 2001;88:137-42. [DOI] [PubMed]