Abstract
In natural habitats Marsilea quadrifolia L. produces different types of leaves above and below the water level. In aseptic cultures growth conditions can be manipulated so that leaves of the submerged type are produced continuously. Under such conditions the application of either blue light or an optimal concentration of abscisic acid (ABA) induced the development of aerial-type leaves. When fluridone, an inhibitor of ABA biosynthesis, was added to the culture medium it did not prevent blue light induction of aerial leaf development. During blue light treatment the endogenous ABA level in M. quadrifolia leaves remained unchanged. However, after the plants were transferred to an enriched medium, the ABA level gradually increased, corresponding to a transition in development from the submerged type of leaves to aerial leaves. These results indicate that the blue light signal is not mediated by ABA. Therefore, in the regulation of heterophyllous determination, discrete pathways exist in response to environmental signals.
Many aquatic plants produce distinct types of leaves in the parts of the shoot above and below the water level (Allsopp, 1965; Sculthorpe, 1967). The submerged leaves are linear or dissected, with undifferentiated mesophyll and few stomata in the epidermis (Gaudet, 1964; Schmidt and Millington, 1968; Deschamp and Cooke, 1985; Young et al., 1987). In contrast, the aerial leaves are entire and broad, having differentiated mesophyll and stomata in both the upper and lower epidermis (Gaudet, 1964; Schmidt and Millington, 1968; Deschamp and Cooke, 1985; Young et al., 1987). During the life cycle of a plant this type of heterophylly is reversible in response to environmental changes (Allsopp, 1965; Sculthorpe, 1967). In addition to the position relative to the water surface, other environmental factors have been examined individually. In general, long photoperiods, high fluence rates, high temperatures, an aerobic atmosphere, or osmotic stress favor the development of aerial leaves (for reviews, see Allsopp, 1965; Trewavas and Jones, 1991). However, the effects of environmental changes are dosage dependent and there is interplay between these signals (McCully and Dale, 1961; Bostrack and Millington, 1962; Gaudet, 1965; Schmidt and Millington, 1968; Bodkin et al., 1980). Therefore, the leaf form is the final result of interactions among environmental stimuli and manifests the greatest adaptive relevance.
From a series of experiments with Marsilea drummondii in aseptic cultures, Allsopp (1965) concluded that various factors triggering the heterophyllous switch contributed to the “hydration state” of the cell, i.e. culture conditions may have an effect on heterophylly by changing the cellular solute concentration. Indeed, when ABA, the phytohormone known to mediate the signal of osmotic stress, was applied at an optimal range of concentrations to the culture medium of Marsilea quadrifolia, it was able to substitute for the various environmental changes and induce the aerial type morphology (Liu, 1984). In addition to M. quadrifolia, this has also been shown in Potamogeton nodosus (Anderson, 1978), Limnophila indica (Mohan Ram and Rao, 1982), Callitriche heterophylla (Deschamp and Cooke, 1983), Ranunculus flabellaris (Young and Horton, 1985), Hippuris vulgaris (Kane and Albert, 1987a), and Proserpinaca palustris (Kane and Albert, 1987b). The question immediately arises whether ABA is the endogenous physiological agent for inducing heterophyllous switch. In H. vulgaris there is a good correlation between the increase in endogenous ABA content and the induction of aerial leaf development by osmotic stress (Goliber and Feldman, 1989), supporting the hypothesis that, upon reaching the water surface, desiccation of the shoot tip causes an increase in endogenous ABA, which triggers aerial leaf development (Anderson, 1978).
In nature aerial-type leaves are also produced below the water surface in Hippuris ssp. (McCully and Dale, 1961; Bodkin et al., 1980). Moreover, many growth conditions used in the laboratory to induce the formation of aerial leaves on submerged shoots do not seem to involve the desiccation of the responsive tissues (Allsopp, 1955; Jones, 1955; McCully and Dale, 1961; Bostrack and Millington, 1962; Gaudet, 1963, 1965; Schmidt and Millington, 1968; Bodkin et al., 1980). In nature the rhizome of M. quadrifolia is either rooted in the mud or traverses the water, and so the only portions of the plant that are exposed to dry air are the upper part of the petiole and the blades of the aerial leaf. If the accumulation of ABA is due to desiccation of these parts of the plant, then there has to be an earlier signal causing the petiole to elongate beyond the water level (Liu, 1984). Therefore, factors other than desiccation must evoke an equally effective mechanism to induce morphogenesis. The question, then, is whether these factors are also mediated by ABA.
In H. vulgaris, the effect of high fluence on aerial leaf formation has been correlated with a detectable level of ABA (Goliber, 1989). It has been, therefore, suggested that high fluence increases photosynthetic activity and hence the cell solute content, thereby presenting a form of osmotic stress to the cell (Goliber, 1989). This explanation is appealing because it not only suggests that the light signal uses ABA as a second messenger to turn on downstream responses, but it implies that photomorphogenesis is a result of elevated photosynthesis. Blue light is a potent photomorphogen (Kaufman, 1993; Deng, 1994; Short and Briggs, 1994; Ahmad and Cashmore, 1996) and has been shown to induce aerial form development in Marsilea vestita (Gaudet, 1965); therefore, we investigated its effects. We present data showing that the blue light signal is not mediated by ABA and that photomorphogenesis and photosynthesis are uncoupled.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Treatments
Aseptic cultures of Marsilea quadrifolia L. were established according to the method of Liu (1984). Plants were cultured in a liquid basal medium (Laetsch, 1967) supplemented with 3% Suc and propagated by cutting the rhizomes into two-node segments to induce the formation of new shoots from the axillary buds. Clones of the same plant were used in the experiments reported here. Cultures were kept in a growth chamber set at 25°C with a 16-h photoperiod, and illumination was provided by fluorescent tubes (FL40D-EX, Mitsubishi, Tokyo, Japan) emitting a near-sunlight spectrum to reach 40 μmol m−2 s−1 at the culture level. Treatments were applied to the newly developed shoots 7 d after subculture. Other than the treatments indicated in the text, the culture conditions were kept the same.
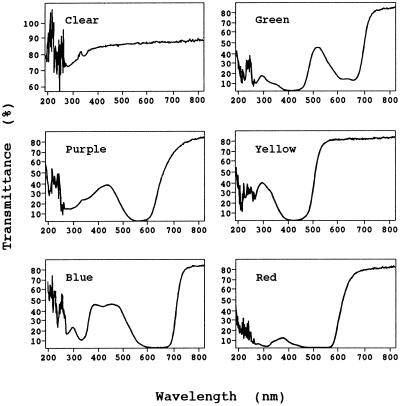
ABA or fluridone was added from a 1000× stock solution to the culture medium to reach the indicated final concentrations. Light sources of various colors were provided by filtering the output of the fluorescent tubes through cellophane sheets purchased from a local supplier. The transmission spectra of the cellophane filters were measured and recorded with a diode array spectrophotometer (model 8452A, Hewlett-Packard; Fig. 1). The fluence rate under each light was adjusted to the same as that of the control. The length of the petiole was measured with a ruler on plants removed from the culture flask. Six to 10 plants were used in each treatment and the experiments were repeated three times. The resulting leaf morphology and the patterns of changes in petiole length were consistent in the repeated experiments. The measurements of petiole length varied up to 20% between the experiments. Table II shows data from one experiment.
Figure 1.
Transmission spectra of cellophane filters providing the light sources of different colors for testing the effect on heterophylly. The transmittance was measured and recorded with a diode array spectrophotometer.
Table II.
The progressive effects of various treatments on petiole elongation in M. quadrifolia
| Leafa | Control | Fluridoneb | Fluridone + Blue Light | ABAb | Blue Light | Blue Light + ABA |
|---|---|---|---|---|---|---|
| mm | ||||||
| 1 | 7.8 ± 0.8 | 5.7 ± 1.7 | 20.1 ± 2.2 | 21.3 ± 3.5 | 27.4 ± 9.1 | 20.2 ± 4.3 |
| 2 | 8.4 ± 0.5 | 2.6 ± 0.9 | 21.0 ± 4.2 | 23.6 ± 5.9 | 61.0 ± 14.0 | 35.2 ± 8.3 |
| 3 | 8.8 ± 0.8 | 1.7 ± 0.4 | 11.0 ± 7.4 | 32.0 ± 4.9 | 93.2 ± 14.1 | 60.2 ± 10.1 |
| 4 | 9.8 ± 0.8 | 1.0 ± 0.1 | 6.4 ± 2.3 | 34.2 ± 8.9 | 100.0 ± 10.0 | 76.0 ± 5.7 |
| 5 | 9.6 ± 1.4 | 2.9 ± 1.6 | 47.0 ± 7.1 | 86.0 ± 6.6 | ||
| 6 | 10.0 ± 1.0 | 1.5 ± 0.1 | ||||
Results are shown as petiole length (average ± sd) recorded after 3 weeks of treatment. Aside from the indicated treatments, other culture conditions were kept the same.
The leaves are listed in the order produced during the treatment, 1 being the first leaf, etc. In addition to petiole length, the number of leaves produced in each treatment varied during the experimental period, indicating that the treatments also affect the initiation and emergence of new leaves.
Fluridone and ABA were applied at 1 μm.
Alternatively, plants were cultured in liquid MS medium (Murashige and Skoog, 1962) supplemented with 3% Suc. The osmotic potentials of the culture media were measured with a vapor pressure osmometer (model 5100C, Wescor, Logan, UT). To obtain plant materials grown in the field, cultured plants were transferred to a plot (1 × 2 m) in the experimental field of the Institute of Botany, Academia Sinica, and the water level was kept approximately 1 cm in depth.
Measurement of ABA Content
Endogenous ABA was extracted from M. quadrifolia leaves with 80% methanol according to the method of Walker-Simmons (1987) and quantified with an ELISA using monoclonal antibodies against ABA (Idetek, San Bruno, CA). As an internal standard for monitoring recovery, 0.2 pmol (10 μCi) of dl-cis,trans-[U-3H]ABA (Amersham) was added to the extract from each gram of fresh tissue. In a total of 50 samples the recovery was 74% ± 12%.
RESULTS
Blue Light Induction of Heterophyllous Switch
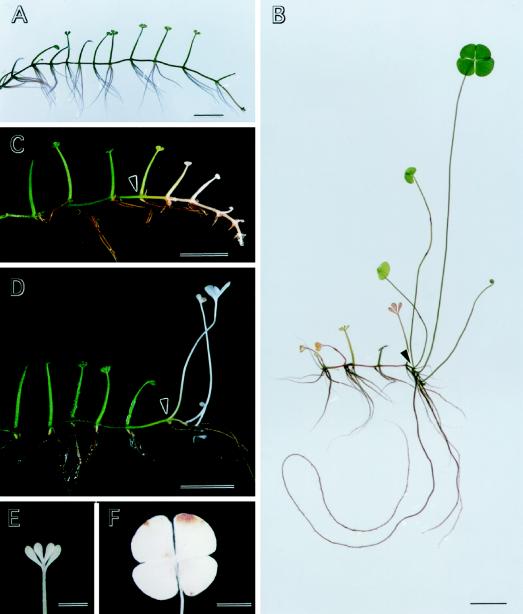
The aerial leaf of M. quadrifolia resembles a four-leaf clover, with quadrifid lamina expanded at an angle to the petiole (Fig. 2, B, D, and F), whereas the submerged leaf has divided, oblanceolate leaflets, expanded in the plane of the petiole (Fig. 2, A–E). Under our culture conditions, in basal medium and a 16-h photoperiod at 25°C, M. quadrifolia plants continued to produce the submerged type of leaves (Fig. 2A). When the plants were irradiated with blue light, while other culture parameters remained the same, aerial leaves were induced (Fig. 2B). We determined the action spectra for heterophyllous transition by irradiating the plants with various lights at the same fluence rate. When other culture conditions were kept unchanged, aerial leaf development was inducible by blue or purple, but not by red, yellow, or green light (Table I; see Fig. 1 for the spectra of the light sources).
Figure 2.
The effects of blue light and fluridone on heterophyllous switch in M. quadrifolia. Plants were cultured aseptically in a basal medium (Laetsch, 1967) supplemented with 3% Suc and were kept at 25°C with 16-h photoperiod. A, Control plant grown under the stated conditions for 3 weeks. B, Plant cultured under control conditions for 7 d (as the shoot apex reached the position indicated by the arrowhead) and then transferred to blue light and grown for another 2 weeks. Note the production of aerial leaflet morphology, the elongation of petioles and roots, the formation of lateral roots, and the shortening of the internodes in the part of plant developed under blue light (to the right of the arrowhead). C, Plant grown under control conditions for 7 d (as in A and B) initially and then with the addition of 1 μm fluridone to the culture medium for 2 weeks. The part of the plant to the right of the arrowhead was produced in the presence of 1 μm fluridone. Note the reduction in the coloration of shoot tissues and in the size of the organs. D, Plant as in C except blue light was applied simultaneously with fluridone. Note the formation of aerial characteristics under blue light in the presence of 1 μm fluridone. E, Leaflet morphology of a submerged-type leaf produced in the presence of 1 μm fluridone as described in C. F, Leaflet morphology of an aerial leaf produced under blue light in the presence of 1 μm fluridone as described in D. Scale bars = 1 cm (A–D), 2 mm (E), and 4 mm (F).
Table I.
The effect of light quality on the induction of heterophyllous switch in M. quadrifolia
| Light Source | Heterophyllous Switch | Final Leaf Type |
|---|---|---|
| Controla | No | Submerged |
| Purple | Yes | Aerial |
| Blue | Yes | Aerial |
| Green | No | Submerged |
| Yellow | No | Submerged |
| Red | No | Submerged |
Plants were transferred to the indicated light sources 7 d after subculture. The final leaf type was recorded 2 weeks later. Growth conditions other than the light regime were kept the same. The fluence rate was adjusted to 40 μmol m−2 s−1 at the culture level under each light source.
In the control experiment the plants were illuminated through a clear cellophane filter.
In responding to the inductive irradiation, the developmental switch occurred only in that part of the plant newly produced during the treatment; the tissues already formed were unaffected (Fig. 2B). The switch occurred in various organs. In addition to the formation of the characteristic aerial lamina, the morphological changes induced by blue light included elongation of petioles and roots, lateral root formation, and shortening of internodes (Fig. 2B). These responses to blue light are similar, if not identical, to those induced by ABA (Liu, 1984).
ABA Effects
Since the effect of ABA is dosage dependent (Liu, 1984), we tested a range of ABA concentrations (10 nm to 100 μm) under our culture conditions. ABA at higher than 0.5 μm is effective in inducing aerial leaf development. At high concentrations (10, 50, and 100 μm), the leaves were smaller and growth was gradually retarded (data not shown). These results are similar to those reported by Liu (1984). We therefore routinely used 1 μm ABA as the optimal concentration in further experiments.
Blue Light Induction of Heterophyllous Switch in the Presence of Fluridone
Fluridone blocks the conversion of phytoene to phytofluene (Vaisberg and Schiff, 1976; Bartels and Watson, 1978; Fong and Schiff, 1979), which is a primary step in the carotenoid pathway leading to the synthesis of a number of compounds including carotenoids, chlorophylls, and ABA. Fluridone has also been shown to inhibit the accumulation of endogenous ABA (Moore and Smith, 1984; Moore et al., 1985; Gamble and Mullet, 1986). If the blue light signal is mediated by ABA and causes the biosynthesis or accumulation of ABA, then fluridone treatment would be expected to block the blue light induction of the heterophyllous transition. Under our growth conditions with regular illumination, after 1 μm fluridone was added to the culture medium, a distinctive reduction in the coloration of the newly produced tissues was observed, indicating an inhibition of carotenoid and chlorophyll synthesis (Fig. 2, C and E). However, when the cultures were irradiated with blue light in the presence of 1 μm fluridone, despite the bleaching, the new leaves still developed into the aerial form (Fig. 2, D and F). There was a general growth inhibition by 1 μm fluridone, with a gradual decrease in the size of the new leaves (Fig. 2C; Table II). Higher concentrations of fluridone (10 and 100 μm) increasingly inhibited growth but did not prevent the induction of aerial leaf development by blue light (not shown). These data imply that either ABA is not synthesized in the carotenoid pathway in M. quadrifolia or blue light action does not require de novo synthesis of ABA.
We used petiole length as an index to compare the growth of leaves in various treatments. As shown in Table II, blue light and 1 μm ABA, either applied individually or together, caused a progressive increase in the petiole length of new leaves produced during the 3-week treatment. On the contrary, 1 μm fluridone caused a progressive decrease in petiole length whether the plant was grown under blue light or regular light. With 1 μm fluridone and blue light, the first leaves produced on the plants were comparable to those on plants treated with 1 μm ABA alone, blue light alone, or both (Table II). This indicates that petiole elongation, a significant feature of aerial leaf development stimulated by blue light, was initially unaffected by the inhibitory effect of fluridone. In the given culture conditions, the leaves produced later were longer in plants irradiated with blue light than in those treated with 1 μm ABA under regular light, whereas the petiole length in plants grown in 1 μm ABA under blue light fell in between (Table II). These data suggest that, in the control of heterophylly, blue light acts in a pathway distinct from but interacting with that of ABA. Consequently, the key issue is whether blue light causes an increase in the endogenous ABA level.
Endogenous ABA Levels
We then measured the endogenous ABA content in M. quadrifolia leaves grown under various conditions. As shown in Table III, ABA levels were similar in the submerged leaves grown in culture and in the aerial leaves whether induced by blue light in culture or grown in a semidry field. Therefore, during blue light treatment, the plants did not contain elevated levels of ABA.
Table III.
Endogenous ABA levels in the leaves of M. quadrifolia produced under various conditions
| Growth Condition | Leaf Form | ABA Content |
|---|---|---|
| pmol g−1 fresh wt | ||
| Culturea | ||
| Control | Submerged | 220.9 ± 11.8 |
| Blue Light | Aerial | 250.0 ± 28.5 |
| Field | Aerial | 266.4 ± 105.7 |
Aerial leaves grown in the field or induced by blue light in culture contain ABA levels similar to submerged leaves produced in culture. ABA contents are the averages ± sd of three samples. Leaves were pooled from different plants to give 1 to 2 g fresh weight of tissue per sample. ABA contents were detected with ELISA using monoclonal antibodies against ABA.
Plants were cultured in the basal medium (Laetsch, 1967). The leaves were harvested from plants grown in culture for 7 weeks.
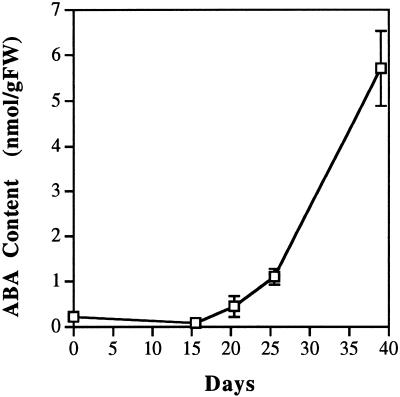
As a contrast MS medium (Murashige and Skoog, 1962) was also used to induce aerial form development. This medium was chosen mainly because it is commonly used in tissue culture. When the components were compared, MS medium contained higher concentrations of several major and minor mineral salts than the basal medium (comparisons not shown). The osmotic potential in MS medium was 194 mmol kg−1, which is 1.5-fold that in the basal medium (131 mmol kg−1). After being transferred to MS medium, M. quadrifolia plants continued to produce the submerged leaves for the first 2 weeks. Starting in the 3rd week with the formation of a series of transitional characteristics, the leaves gradually changed from the submerged form to the aerial form (data not shown). The endogenous ABA measurements in these plants showed increases in parallel with the morphogenetic transition in the MS medium (Fig. 3). These results indicate that the effect of this enriched medium on heterophyllous switch is gradual and is correlated with the accumulation of endogenous ABA.
Figure 3.
Relationship between the endogenous ABA level in leaves of M. quadrifolia and the duration that the plants were cultured in MS medium. Leaves from several plants were pooled to give 1 to 2 g fresh weight (gFW) of tissue per sample. Data points represent the averages and sd of samples measured in three independent experiments. The sd in the samples for d 0 and 16 are less than 0.05 nmol/g fresh weight. ABA contents were determined with ELISA using monoclonal antibodies against ABA.
DISCUSSION
In M. quadrifolia blue light induction of aerial form characteristics does not appear to require de novo synthesis of ABA and does not result in the accumulation of ABA. We therefore conclude that ABA does not mediate the blue light signal in inducing the heterophyllous switch. This is in contrast to the effect of an enriched medium, in which the developmental transition is correlated with the gradual increase in the endogenous ABA level. These data indicate that environmental stimuli are transduced via multiple signaling pathways that converge on the master switch of heterophylly. Some but not all of the environmental signals are mediated by ABA.
A close examination of the aerial-form characteristics in M. quadrifolia suggests that the adaptive value of the developmental switch is related to photosynthetic capacity. While growing with a rhizome in a liquid environment, the limiting factor for survival is the availability of light rather than water. The elongation of the petiole, along with the increase in leaf size and the change in leaflet shape that maximize the leaf surface area (see Fig. 2), greatly enhances the ability of the plant to capture light. Aerial form development thus appears to be a response to a favorable environment that provides light in abundance. Our results suggest that either blue light or ABA simulates a favorable environment. The well-established facts that blue light is a potent photomorphogen and that its energy can be used in photosynthesis prompt us to suggest that blue light changes the route of development via a prescribed signaling pathway involving the fine tuning of photosynthetic capacity. ABA, on the other hand, is known to be associated with the response to drought stress and has been shown to down-regulate photosynthetic genes (for review, see Zeevaart and Creelman, 1988; Skriver and Mundy, 1990; Chandler and Robertson, 1994; Weatherwax et al., 1996). When aquatic plants reach the surface of water, they experience a drought stress that is likely mediated by ABA. However, reaching the surface of the water means a combination of drought stress and the availability of sunlight. Therefore, the ABA signal may be decoded by the plant as being both and hence is followed by aerial form development.
In the presence of fluridone, an inhibitor of the carotenoid biosynthesis pathway, the photosynthetic pigments appear to be missing in the newly developed tissues of M. quadrifolia. However, these tissues still respond to blue light induction by changing their route of development. This clearly shows the uncoupling of photomorphogenesis and photosynthetic capacity. Although the development of the aerial characteristics has a significant consequence in increasing photosynthetic capability, the latter appears not to be a prerequisite for such a development. This phenomenon provides a system for investigating the cross-talk between the signaling pathways responding to various environmental factors affecting both morphogenesis and photosynthetic activities.
Quantification of growth by the measurement of petiole length shown in Table II indicates the interplay among the various factors: blue light, ABA, and fluridone. Although we calibrated the fluence level of blue light and tested a range of concentrations of fluridone and ABA, the data suggest a difference in the effectiveness of each factor. For example, the blue light level we used appears to be more effective than the ABA level in inducing petiole elongation. Moreover, the application of both blue light and ABA at the given levels results in an averaging rather than an additive effect. The nature of the dosage or effectiveness and the interaction of these factors remain to be defined at the cellular and molecular levels. The data presented here indicate that the morphogenetic determination involved in heterophyllous transition in M. quadrifolia is not only qualitative but also quantitative.
ACKNOWLEDGMENTS
We thank Drs. Sham Goyal, Judy Jernstedt, and Wen-Yuan Kao for the measurements of osmotic potential; Ms. Yi-Chieh Chang and Mr. Hsueh-Jen Liao for technical assistance; and the Institute of Botany, Academia Sinica, for the use of the experimental field. We are grateful to Drs. M.M. Green, Roger Hangarter, and Tuan-hua David Ho, as well as two anonymous reviewers, for comments concerning the manuscript.
Abbreviation:
- MS
Murashige and Skoog
Footnotes
This work was supported by grants to B.-L.L. from Academia Sinica and the National Science Council (no. NSC85-2311-B-001-091), Republic of China.
LITERATURE CITED
- Ahmad M, Cashmore AR. Seeing blue: the discovery of cryptochrome. Plant Mol Biol. 1996;30:851–861. doi: 10.1007/BF00020798. [DOI] [PubMed] [Google Scholar]
- Allsopp A. Experimental and analytical studies of pteridophytes. XXVII. Investigations on Marsilea. 5. Cultural conditions and morphogenesis with special reference to the origin of land and water forms. Ann Bot. 1955;19:247–264. [Google Scholar]
- Allsopp A. Land and water forms: physiological aspects. Handb Pflanzenphysiol. 1965;15:1236–1255. [Google Scholar]
- Anderson LWJ. Abscisic acid induces formation of floating leaves in the heterophyllous aquatic angiosperm Potamogeton nodosus. Science. 1978;201:1135–1138. doi: 10.1126/science.201.4361.1135. [DOI] [PubMed] [Google Scholar]
- Bartels PG, Watson CW. Inhibition of carotenoid synthesis by fluridone and nonflurazon. Weed Sci. 1978;26:198–203. [Google Scholar]
- Bodkin PC, Spence DHN, Weeks DC. Photoreversible control of heterophylly in Hippuris vulgaris L. New Phytol. 1980;84:533–542. [Google Scholar]
- Bostrack JM, Millington WF. On the determination of leaf form in an aquatic heterophyllous species of Ranunculus. Bull Torrey Bot Club. 1962;89:1–20. [Google Scholar]
- Chandler PM, Robertson M. Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:113–141. [Google Scholar]
- Deng X-W. Fresh view of light signal transduction in plants. Cell. 1994;76:423–426. doi: 10.1016/0092-8674(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Deschamp PA, Cooke TJ. Leaf dimorphism in aquatic angiosperms: significance of turgor pressure and cell expansion. Science. 1983;219:505–507. doi: 10.1126/science.219.4584.505. [DOI] [PubMed] [Google Scholar]
- Deschamp PA, Cooke TJ. Leaf dimorphism in the aquatic angiosperm Callitriche heterophylla. Am J Bot. 1985;72:1377–1387. [Google Scholar]
- Fong F, Schiff JA. Blue-light-induced absorbance changes associated with carotenoids in Euglena. Planta. 1979;146:119–127. doi: 10.1007/BF00388221. [DOI] [PubMed] [Google Scholar]
- Gamble PE, Mullet JE. Inhibition of carotenoid accumulation and abscisic acid biosynthesis in fluridone-treated dark-grown barley. Eur J Biochem. 1986;160:117–121. doi: 10.1111/j.1432-1033.1986.tb09947.x. [DOI] [PubMed] [Google Scholar]
- Gaudet JJ. Marsilea vestita: conversion of the water form to the land form by darkness and by far-red light. Science. 1963;140:975–976. doi: 10.1126/science.140.3570.975. [DOI] [PubMed] [Google Scholar]
- Gaudet JJ. Morphology of Marsilea vestita. II. Morphology of the adult land and submerged leaves. Am J Bot. 1964;51:495–502. [Google Scholar]
- Gaudet JJ. The effect of various environmental factors on the leaf form of the aquatic fern Marsilea vestita. Physiol Plant. 1965;18:674–686. [Google Scholar]
- Goliber TE. Endogenous ABA content correlates with photon fluence rate and induced leaf morphology in Hippuris vulgaris. Plant Physiol. 1989;89:732–734. doi: 10.1104/pp.89.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goliber TE, Feldman LJ. Osmotic stress, endogenous abscisic acid, and the control of leaf morphology in Hippuris vulgaris L. Plant Cell Environ. 1989;12:163–171. doi: 10.1111/j.1365-3040.1989.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Jones H. Further studies on heterophylly in Callitriche intermedia: leaf development and experimental induction of ovate leaves. Ann Bot. 1955;19:369–389. [Google Scholar]
- Kane ME, Albert LS. Abscisic acid induces aerial leaf morphology and vasculature in submerged Hippuris vulgaris L. Aquat Bot. 1987a;28:81–88. [Google Scholar]
- Kane ME, Albert LS. Integrative regulation of leaf morphogenesis by gibberellic acid and abscisic acids in the aquatic angiosperm Proserpinaca palustris L. Aquat Bot. 1987b;28:89–96. [Google Scholar]
- Kaufman LS. Transduction of blue-light signals. Plant Physiol. 1993;102:333–337. doi: 10.1104/pp.102.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch WM (1967) Ferns. In FH Wilt, NK Wessells, eds, Methods in Developmental Biology. Crowell, New York, pp 319–328
- Liu B-LL. Abscisic acid induces land form characteristics in Marsilea quadrifolia L. Am J Bot. 1984;71:638–644. [Google Scholar]
- McCully ME, Dale HM. Heterophylly in Hippuris, a problem in identification. Can J Bot. 1961;39:1099–1116. [Google Scholar]
- Mohan Ram HY, Rao S. In vitro induction of aerial leaves and of precocious flowering in submerged shoots of Limnophila indica by abscisic acid. Planta. 1982;155:521–523. doi: 10.1007/BF01607577. [DOI] [PubMed] [Google Scholar]
- Moore R, Smith JD. Growth, graviresponsiveness and abscisic-acid content of Zea mays seedlings treated with fluridone. Planta. 1984;164:342–344. [PubMed] [Google Scholar]
- Moore R, Smith JD, Fong F. Gravitropism in abscisic-acid deficient seedlings of Zea mays. Am J Bot. 1985;72:1311–1313. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Schmidt BL, Millington WF. Regulation of leaf shape in Proserpinaca palustris. Bull Torrey Bot Club. 1968;95:264–286. [Google Scholar]
- Sculthorpe CD. The Biology of Aquatic Vascular Plants. London: Arnold; 1967. [Google Scholar]
- Short TW, Briggs WR. The transduction of blue light signals in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:143–172. [Google Scholar]
- Skriver K, Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990;2:503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas AJ, Jones HG (1991) An assessment of the role of ABA in plant development. In WJ Davies, HG Jones, eds, Abscisic Acid: Physiology and Biochemistry. BIOS Scientific Publishers, Oxford, UK, pp 169–188
- Vaisberg AJ, Schiff JA. Events surrounding the early development of Euglena chloroplasts. 7. Inhibition of carotenoid biosynthesis by the herbicide SAN 9789 (4-chloro5-(methylamino)-2-(α,α,α,-trifluoro-m-tolyl)-3(2H)pyridazinone) and its developmental consequences. Plant Physiol. 1976;57:260–269. doi: 10.1104/pp.57.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons MK. ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol. 1987;84:61–66. doi: 10.1104/pp.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherwax SC, Ong MS, Degenhardt J, Bray EA, Tobin EM. The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiol. 1996;111:363–370. doi: 10.1104/pp.111.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JP, Dengler NG, Horton RF. Heterophylly in Ranunculus flabellaris: the effect of abscisic acid on leaf anatomy. Ann Bot. 1987;69:117–125. [Google Scholar]
- Young JP, Horton RF. Heterophylly in Ranunculus flabellaris Raf.: the effect of abscisic acid. Ann Bot. 1985;55:899–902. [Google Scholar]
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–473. [Google Scholar]