Abstract
Root elongation, hematoxylin staining, and changes in the ultrastructure of root-tip cells of an Al-tolerant maize variety (Zea mays L. C 525 M) exposed to nutrient solutions with 20 μm Al (2.1 μm Al3+ activity) for 0, 4, and 24 h were investigated in relation to the subcellular distribution of Al using scanning transmission electron microscopy and energy-dispersive x-ray microanalysis on samples fixed by different methods. Inhibition of root-elongation rates, hematoxylin staining, cell wall thickening, and disturbance of the distribution of pyroantimoniate-stainable cations, mainly Ca, was observed only after 4 and not after 24 h of exposure to Al. The occurrence of these transient, toxic Al effects on root elongation and in cell walls was accompanied by the presence of solid Al-P deposits in the walls. Whereas no Al was detectable in cell walls after 24 h, an increase of vacuolar Al was observed after 4 h of exposure. After 24 h, a higher amount of electron-dense deposits containing Al and P or Si was observed in the vacuoles. These results indicate that in this tropical maize variety, tolerance mechanisms that cause a change in apoplastic Al must be active. Our data support the hypothesis that in Al-tolerant plants, Al can rapidly cross the plasma membrane; these data clearly contradict the former conclusions that Al mainly accumulates in the apoplast and enters the symplast only after severe cell damage has occurred.
It is largely recognized that root tips are the primary site of Al-induced injury in plants (Ryan et al., 1993). The accumulation of Al in root tips has been found to be significantly correlated with root-growth inhibition in maize (Zea mays L.) varieties differing in Al tolerance (Llugany, 1994; Llugany et al., 1994). In Al-sensitive maize plants an inhibition of root elongation has been observed after only 30 min of exposure to Al (Llugany et al., 1995). Such a short response time, in addition to the common belief (Kochian, 1995) that Al accumulates mainly in the apoplast and crosses the plasma membrane slowly, has led to the hypothesis that Al-induced inhibition of root elongation may be caused by toxicity mechanisms that occur in the apoplast (Rengel, 1990, 1996; Horst, 1995) and that there is no need for Al to enter the symplast to cause primary toxicity effects (Rengel, 1992). However, investigations using the highly Al-sensitive technique of secondary ion MS have shown that significant Al concentrations accumulate in the symplast of root-tip cells of soybean plants after only 30 min of exposure to Al (Lazof et al., 1994, 1996). Recent experiments on giant algae (Chara corallina) cells, where cell walls were separated from the cells by microsurgery, have also shown that Al uptake across the plasmalemma may be linear and occurs without delay (Rengel and Reid, 1997). These investigations support the view that symplastic phytotoxicity mechanisms may also be responsible for Al-induced inhibition of root elongation after short exposure times (Kochian, 1995).
More information on the subcellular distribution of Al in root tips would help to establish both the relative importance of apoplastic and symplastic sites in the Al-toxicity syndrome and the role of Al compartmentation in Al resistance or tolerance. Unfortunately, ultrastructural investigations under environmentally realistic growth conditions that relate the subcellular localization of Al in root tips to root growth in Al-tolerant varieties are scarce (Delhaize et al., 1993). Major difficulties for such an approach are the low sensitivity of electron probe x-ray microanalysis for Al determination (Lazof et al., 1994, 1997) and the poor visual distinction of subcellular structures in freeze-dried samples, in combination with the extremely low Al tissue concentrations, which have been shown to cause inhibition of root elongation (Lazof et al., 1994, 1996).
Using a highly sensitive monitoring technique for root growth, we have previously shown that 20 μm Al (2.1 μm Al3+ activity) causes a significant decrease in the relative root-elongation rate in the Al-tolerant maize var C 525 M after 112 min of exposure, whereas after 24 h the relative elongation rate did not differ from that of the controls (Llugany et al., 1995). In this paper we report results on the changes in the subcellular distribution of Al in root tips during the initial root-growth response (0–24 h) of var C 525 M exposed to 20 μm Al (2.1 μm Al3+ activity). Hematoxylin staining, ultrastructural observations, and EDXMA were performed on root tips after 0, 4, and 24 h of exposure of plants to control or Al-containing nutrient solutions to detect a possible relationship between changes in subcellular Al compartmentation and ultrastructural alterations, which may explain why, after a transient inhibition, the root-elongation rate recovers during the initial 24 h of exposure to Al. EDXMA with scanning TEM on glutaraldehyde-fixed, PA-stained, and freeze-substituted samples were performed. Although these techniques only allow a semiquantitative estimation of mineral contents, the better visual resolution obtained results in more reliable data on the subcellular localization than EDXMA with SEM on freeze-dried or frozen-hydrated bulk specimens (Van Steveninck and Van Steveninck, 1991).
MATERIALS AND METHODS
Plant Material and Growth Conditions
Al-tolerant maize (Zea mays L. var C 525 M, Embrapa, Siete Lagoas, Brazil) seeds were germinated in the dark at 25°C on filter paper moistened with 1 mm CaSO4. After 96 h, uniform seedlings with a radicle length of 13.7 ± 0.9 cm were transferred to plastic beakers (14-L capacity; 24 plants per beaker) filled with continuously aerated nutrient solution (pH 4.3) of the following composition (in μm): 500 Ca(NO3)2, 395 K2SO4, 5 KH2PO4, 100 MgSO4, 200 NH4NO3, 0.06 (NH4)6Mo7O24, 5 MnSO4, 0.38 ZnSO4, 0.16 CuSO4, 16 H3BO3, and 10 FeEDTA. After 72 h, the plants were transferred to treatment solutions of the same composition and volume per plant. One-half of the plants received solution supplemented with 20 μm Al as AlCl3. The pH of the control nutrient solutions remained constant throughout the experiment (4.31 ± 0.01). In Al-supplemented solutions pH values were 4.34 ± 0.02 and 4.13 ± 0.02 after 4 and 24 h, respectively. According to the GEOCHEM speciation program (Parker et al., 1987), the activity of free Al3+ in the treatment solution was 2.1 μm and all Al was in soluble form. The concentrations of monomeric Al in the solution, analyzed by the short-term pyrocatechol method (Kerven et al., 1989), was 13 μm.
The seedlings were grown in an environmentally controlled growth chamber under the following conditions: 16 h of light/8 h of darkness, day/night temperature 26°C/20°C, RH 70%, and PPFD 190 μmol m−2 s−1.
Root Growth and Hematoxylin Staining
Seedling seminal root length (n ≥ 24 per treatment and time sample) was measured with a ruler before the transfer of the plants to nutrient solution, after the 72-h pretreatment (0-h treatment), and after the 4- and 24-h treatments with solutions containing 0 (control) or 20 μm Al. Hematoxylin staining of whole roots was performed on 10 plants per treatment and time sample (Polle et al., 1978).
Sample Fixation for Electron Microscopy and EDXMA
For EM studies, after a short (10 s) rinse with distilled water, the tips (0–2 mm and the following 2–5 mm) from primary roots were excised from control and Al-treated seedlings after 0, 4, and 24 h of exposure to nutrient solutions. The samples were immediately fixed by the different methods described below.
Some samples were fixed in 2.5% (w/v) glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.2), but were not postfixed with osmium. The fixed material was dehydrated in a graded alcohol series and embedded in Spurr's resin (Spurr, 1969). Some of the non-osmified, thin, longitudinal tip sections (near root halves) were stained with saturated aqueous uranyl acetate, followed by Reynolds lead citrate (Reynolds, 1963). Stained and unstained longitudinal serial sections, between 0 and 1.5 mm from apex, were studied by electron microscopy (model H-7000, Hitachi, Tokyo, Japan), and the elemental distribution in cell walls and vacuoles was determined by EDXMA on unstained, dry-cut sections.
Other sampled tips were treated with PA to retain easily diffusible cations (Mentré and Escaig, 1988; Mentré and Halpern, 1988). The composition of the fixation mixture was 4% PA, 2% paraformaldehyde, and 1% phenol (pH 7.8). After the treatment the specimens were rinsed with distilled water, then dehydrated in graded alcohol and embedded in Spurr's resin. Longitudinal tip sections were studied by light microscopy (Optiphot, Nikon) and electron microscopy. The elemental composition of PA precipitates was identified by EDXMA.
A third group of root tips was processed by freeze-substitution, as previously described (Harvey, 1982; Vázquez et al., 1992). The samples were cryofixed in pentane cooled with liquid nitrogen. The freeze-substitution with acetone precooled with liquid nitrogen was allowed to proceed for 1 week at −80°C in a deep freezer. The specimens were gradually warmed to room temperature for 24 h and then infiltrated with Spurr's resin. Transverse sections at approximately 0.5 and 3 mm from the apex were stained for light and electron microscopy (photographs not shown) as for the glutaraldehyde-fixed sections described above. Corresponding unstained, dry-cut sections were used for EDXMA.
EDXMA
Dry-cut sections of approximately 1.5 μm were mounted onto gold grids. The microanalytical determinations were performed on an electron microscope (model H-800, Hitachi) operated at 100 kV in the scanning TEM mode using an energy-dispersive detector (Kevex, Valencia, CA) and a Delta class 4460 analyzer (Kevex). The counts were made over a 100-s period and spectra were recorded. Gaussian deconvolution was applied to the results and, after background correction, the data were expressed as the counts to second ratio. A variable number of samples was used for each treatment and fixation method. For glutaraldehyde-fixed samples or those treated with PA, n values were as follows: control plants, n ≥ 4; Al-treated plants, n ≥ 9. The n values for freeze-substituted samples were 9 and 15 for control and Al-treated plants, respectively. No Al signals were detectable by EDXMA in electron-translucent cell areas and all data shown are from electron-dense deposits. As usual in EDXMA studies, the sd values of the results were high; therefore, the ranges were given in addition to mean values ± sd. Blank resin was analyzed to check for contaminants.
RESULTS
Maize seedlings exposed to Al for 4 h exhibited decreased root-elongation rates, whereas after 24 h the rates had recovered to the control values of plants before the start of the Al treatment (Table I).
Table I.
Root-elongation rates of maize seedlings
| Exposure Time | Control | Al |
|---|---|---|
| h | mm/h | |
| 0 | 1.73 ± 0.03 | – |
| 4 | 1.65 ± 0.05 | 1.22a± 0.09 |
| 24 | 1.66 ± 0.05 | 1.70 ± 0.04 |
Plants were exposed to nutrient solutions containing 0 (control) or 20 μm Al for different times. The growth rate of the 0-h time sample was determined during the 72-h pretreatment. Values are means ± se (n ≥ 24).
Significantly different at P < 0.05 (analysis of variance followed by Tukey's honestly significant difference [HSD] mean-separation test).
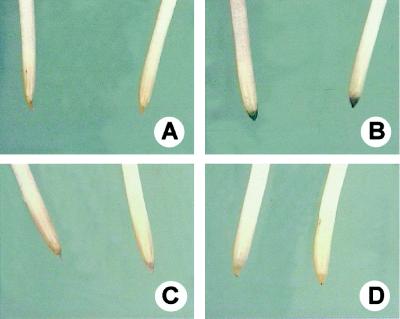
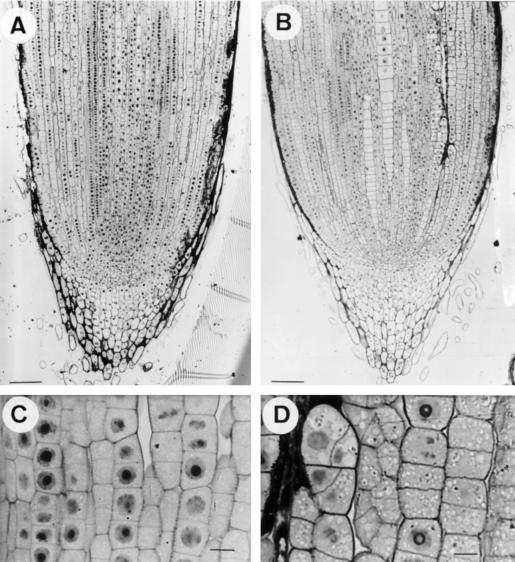
Seminal roots of seedlings stained with hematoxylin are shown in Fig. 1. After 4 h of exposure to Al-containing nutrient solution, root tips exhibited intense staining (Fig. 1B), whereas after 24 h of Al exposure, no staining could be observed (Fig. 1D) and the plants did not differ from controls (Fig. 1, A and C).
Figure 1.
Seminal roots from maize plants stained with hematoxylin. A and C, Control (0 μm Al) plants after 4 and 24 h, respectively. B and D, Plants treated with 20 μm Al containing nutrient solution for 4 and 24 h, respectively.
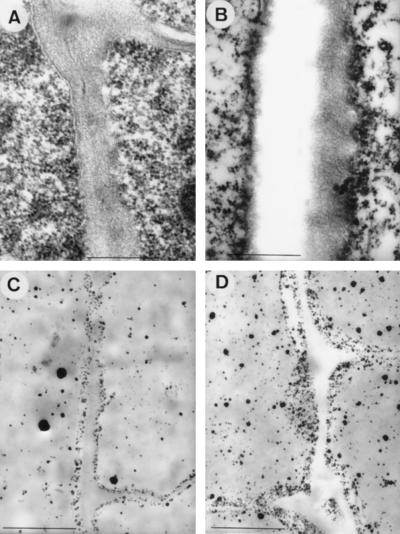
Ultrastructural alterations in cell walls of root-tip (1.5-mm) cells were observed after 4 h of exposure to Al (Fig. 2). A thickening of tangential cell walls occurred in the internal (third–sixth) cortex cells (Fig. 2, B and D). Sections from glutaraldehyde-fixed specimens revealed cell walls with electron-translucent areas and electron-dense zones near the plasmalemma (Fig. 2B). In the corresponding sections that had been stained with PA for visualizing otherwise soluble cations, a significantly higher accumulation of PA-stained deposits was found at the internal site of these cell walls in Al-treated plants (Fig. 2D) than in controls (Fig. 2C). Al-treated plants also exhibited a higher amount of PA-stained deposits inside of the cortex cells than control plants.
Figure 2.
TEM images from longitudinal sections of root tips of maize plants exposed for 4 h to control (0 μm Al) (A and C) or 20 μm Al (B and D) in nutrient solution. A and B, Non-osmified, glutaraldehyde-fixed samples. C and D, PA-stained samples. Note thickening of cell walls (B) and higher amount of PA precipitates at the internal site of cell walls (D) in samples from Al-treated plants. Scale bars represent 0.5 μm in A and B, and 1.0 μm in C and D.
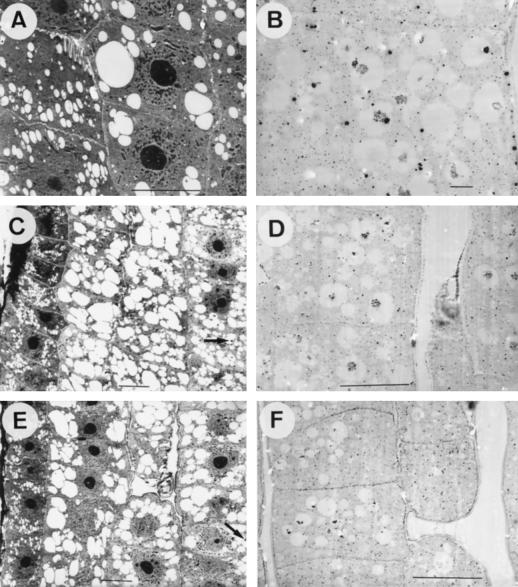
The cortex cells exhibited numerous small vacuoles. In glutaraldehyde-fixed samples from plants prior to the transfer to treatment solutions (0 h) (Fig. 3A) and from plants exposed to control (Fig. 3C) or Al-containing (Fig. 3E) nutrient solution for 4 h, the small vacuoles were electron-lucent and only small, peripherical, electron-dense deposits were detected in some vacuoles of internal cortical cells (mainly the third to the sixth) of each section (Fig. 3, C and E, arrows). In the corresponding PA-stained sections abundant electron-opaque precipitates in the central zone of the vacuoles were found (Fig. 3, B, D, and F).
Figure 3.
TEM images from longitudinal sections of root tips of maize plants exposed for 0 (A and B) or 4 h (C–F) to control (0 μm Al) (A–D) or 20 μm Al (E and F) containing nutrient solution. A, C, and E, Glutaraldehyde-fixed samples. B, D, and F, PA-stained samples. Note the abundance of electron-translucent vacuoles with only some peripheric electron-dense deposits (C and E, arrows) in conventionally fixed samples and the abundance of electron-dense precipitates in the central part of vacuoles from PA-stained samples (B, D, and F). All scale bars represent 10 μm, except B, where bar is 1 μm.
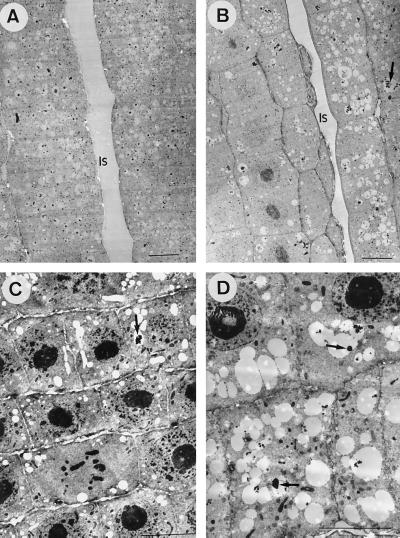
After 24 h of exposure to Al-containing nutrient solutions, thickened cell walls similar to those observed after 4 h of exposure were not detected (Fig. 4, B and D). Root-tip vacuoles of plants exposed to Al for 24 h (Fig. 4D) exhibited a considerably higher amount of electron-opaque aggregates than those from the 4-h Al treatment (Fig. 3E). After 24 h, the vacuolar deposits in Al-treated plants (Fig. 4D) were also more abundant than in the corresponding control samples (Fig. 4C).
Figure 4.
TEM images from longitudinal sections of root tips of maize plants exposed for 24 h to control (0 μm Al) (A and C) or 20 μm Al in nutrient solution (B and D). A and B, PA-stained samples. C and D, Non-osmified, glutaraldehyde-fixed samples. Note the abundance of electron-dense vacuolar deposits (arrows) in conventionally fixed samples from plants exposed to Al (D) in comparison with the scarce presence of deposits in controls (C). All scale bars represent 10 μm. Is, Intercellular space.
After 24 h of exposure to Al, but not after 4 h, abnormal, irregular divisions of internal cortex cells were detected in longitudinal sections of PA-stained samples (Fig. 5, B and D), whereas in the samples of control plants all cell divisions occurred in regular planes (Fig. 5, A and C).
Figure 5.
Light-microscopy images from longitudinal sections (PA stained) of root tips of maize plants exposed for 24 h to control (0 μm Al) (A and C) or 20 μm Al in nutrient solution (B and D). Controls (A and C) show well-organized cortical cell lines with almost horizontal cell-division planes. Al-exposed plants (B and D) exhibit irregular cell-division planes in internal cortical cells. A and B, Scale bars represent 100 μm; C and D, scale bars represent 10 μm.
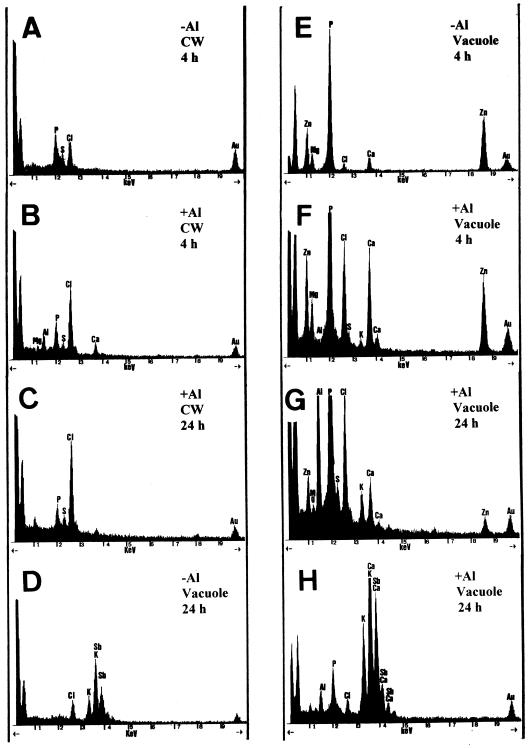
Tables II and III show relative contents (in counts per second) of Al and selected mineral nutrients found by EDXMA in the electron-dense deposits of cell walls and vacuoles of control and Al-treated plants after 0, 4, and 24 h of exposure. In glutaraldehyde-fixed samples small amounts of Al were detected in electron-dense deposits in the walls of the roots treated with 20 μm Al after only 4 h of exposure. P, S, Ca, K, and Zn were also found in these deposits (Table II; Fig. 6B). However, Al was not detectable in electron-dense areas of the walls after 24 h of exposure to Al either in glutaraldehyde-fixed material or in samples prepared by freeze-substitution or PA staining (Tables II and IV). Al was not detected in the cell walls of controls at any sampling time (Table II; Fig. 6A).
Table II.
Relative contents of Al and selected mineral nutrients in cell walls of maize root tips (0–1.5 mm)
| Exposure Time | Al Treatment | Relative Contents
|
|||||
|---|---|---|---|---|---|---|---|
| Al | P | S | K | Ca | Zn | ||
| h | μm | counts s−1 | |||||
| 0 | 0 | NDa | 17 ± 2 | 12 ± 2 | 0.4 ± 0.0 | 1.2 ± 0.5 | 1.2 ± 0.6 |
| 4 | 0 | ND | 22 ± 4 | 11 ± 1 | ND | 1.3 ± 0.1 | ND |
| 4 | 20 | 2.8 ± 1.8 | 12 ± 7 | 5.4 ± 2.4 | 3.2 ± 1.1 | 1.3 ± 0.5 | 0.7 ± 0.2 |
| 24 | 0 | ND | 18 ± 3 | 8.1 ± 2.9 | ND | 3.7 ± 0.1 | ND |
| 24 | 20 | ND | 7.1 ± 4.2 | 9.2 ± 4.2 | ND | ND | ND |
| 24b | 20 | ND | 13 ± 6 | 7.5 ± 3.8 | 23 ± 8 | 2.5 ± 1.0 | ND |
| 24c | 20 | ND | 16 ± 10 | 11 ± 6 | 105 ± 52 | 6.0 ± 2.8 | ND |
Plants were exposed for different times to nutrient solutions containing 0 (control) or 20 μm Al. If not indicated otherwise, the values are from glutaraldehyde-fixed samples. Values are given as means ± sd; n ≥ 4 for control and n ≥ 9 for Al-treated plants.
ND, Not detected.
Samples prepared by freeze-substitution.
Samples prepared by pyroantimoniate staining.
Table III.
Relative contents of Al and selected mineral nutrients in vacuoles of root-tip (0–1.5 mm) cells of maize
| Exposure Time | Treatment | Relative Contents
|
|||||
|---|---|---|---|---|---|---|---|
| Al | P | S | Mg | Ca | Zn | ||
| h | μm Al | counts s−1 | |||||
| 0 | 0 | NDa | 141 ± 90 | ND | 16 ± 4 | 22 ± 15 | 70 ± 51 |
| (43–223) | (14–19) | (4.2–31) | (17–119) | ||||
| 4 | 0 | ND | 88 ± 29 | ND | 11 ± 3 | 7.6 ± 3.4 | 44 ± 19 |
| (65–130) | (6.9–14) | (5.0–13) | (28–71) | ||||
| 4 | 20 | 2.4 ± 1.5 | 90 ± 65 | 5.3 ± 2.7 | 8.0 ± 6.0 | 26 ± 13 | 37 ± 35 |
| (0.7–4.1) | (23–197) | (2.8–8.8) | (5.2–19) | (20–39) | (7.2–106) | ||
| 24 | 0 | 3.9 ± 3.1 | 98 ± 33 | ND | ND | 4.3 ± 2.3 | 28 ±8 |
| (ND–7.4) | (61–122) | (1.7–6.4) | (23–37) | ||||
| 24 | 20 | 44 ± 35 | 205 ± 103 | 6.2 ± 2.0 | 9.2 ± 5.5 | 6.6 ± 2.7 | 21 ± 15 |
| (2.7–88) | (116–347) | (3.2–8.6) | (5.6–17) | (4.7–8.5) | (6.2–39) | ||
Plants were exposed for different times to nutrient solutions containing 0 (control) or 20 μm Al. All data are from conventionally fixed samples. Values are given as ranges (parentheses) and means ± sd; n ≥ 4 for control and n ≥ 9 for Al-treated plants.
ND, Not detected.
Figure 6.
Representative EDXMA spectra from electron-dense deposits in cell walls (A–C) and vacuoles (D–H) of root-tip (0–1.5 mm) cells after 4-h (A, B, E, and F) and 24-h treatments (C, D, G, and H). A, D, and E are from control plants and B, C, F, G, and H are from Al-treated plants. All spectra are from glutaraldehyde-fixed samples, except D and H, which are from PA-stained samples. All spectra are printed at 1000 counts. Au is from the grid.
Table IV.
Relative contents of Al and selected mineral nutrients in cell walls and vacuoles of maize root-tip cells (transverse sections 3 mm from apex)
| Treatment | Relative Contents
|
||||||
|---|---|---|---|---|---|---|---|
| Al | Si | P | S | K | Ca | Mg | |
| μm Al | counts s−1 | ||||||
| Cell Wall | |||||||
| 0 | NDa | 8.0 ± 2.0 (6.6–13) | 19 ± 12 (7.8–26) | 14 ± 3 (ND–19) | 45 ± 22 (14–79) | 5.4 ± 1.5 (3.3–8.2) | ND |
| 20 | ND | 9.9 ± 5.0 (5.2–14) | 29 ± 10 (12–50) | 15 ± 2 (13–19) | 55 ± 21 (15–82) | 6.6 ± 2.8 (3.0–12) | 1.0 ± 0.4 (ND–1.2) |
| Vacuole | |||||||
| 0 | 24 ± 16 (ND–52) | 12 ± 1 (ND–13) | 61 ± 32 (ND–120) | 11 ± 4 (ND–16) | 74 ± 43 (13–148) | 9.9 ± 8.8 (4.4–27) | 2.5 ± 0.2 (ND–1.2) |
| 20b | 6.9 ± 1.8 (ND–9.6) | 7.5 ± 1.9 (ND–9.7) | 68 ± 65 (23–130) | 13 ± 4.6 (6.3–24) | 52 ± 19 (27–77) | 4.1 ± 1.8 (1.1–7.1) | 6.5 ± 5.0 (ND–9.6) |
| 20c | 119 ± 43 (46–173) | 193 ± 55 (120–229) | 10 ± 1 (ND–11) | 32 ± 9 (21–44) | 73 ± 18 (46–97) | 11 ± 3 (8.6–15) | 6.4 ± 3.4 (0.6–12) |
Plants were exposed for 24 h to nutrient solutions with 0 (control) or 20 μm Al. All data are from freeze-substituted samples. Values are given as ranges (parentheses) and means ± sd; n ≥ 9 for control and n ≥ 15 for Al-treated plants.
ND, Not detected.
Element composition of P-rich vacuolar deposits.
Element composition of Si-rich vacuolar deposits.
Small amounts of Al were detected in electron-dense precipitates found in the vacuoles of internal cortex cells of 1.5-mm tips of Al-treated plants after the 4-h Al treatment (Table III; Fig. 6F). P, Zn, Ca, Mg, and S were also present in these deposits. The few electron-dense deposits in the vacuoles of control plants after 0 and 4 h of exposure contained neither Al nor S, but exhibited counts for P, Mg, Ca, and Zn (Table III; Fig. 6E), that were similar to those of Al-treated plants (Fig. 6F). After 24 h of exposure to Al, higher Al counts were found in the vacuoles (Table III; Fig. 6, G and H). After 24 h in control solutions, small amounts of Al were also detected in some of the few vacuolar deposits of controls (Table III). These deposits contained P, Zn, Ca, S, and Mg.
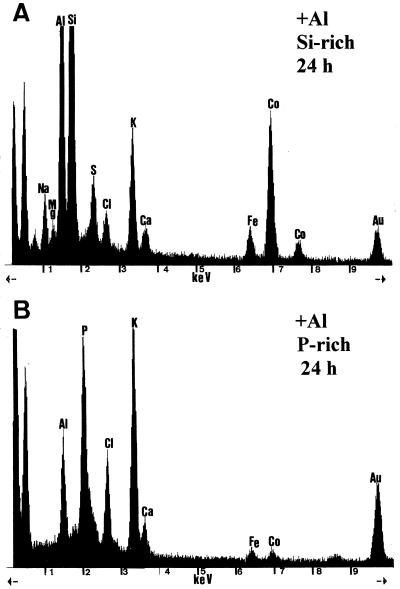
Additional measurements on freeze-substituted samples were performed in the more differentiated cells at 3 mm from the tip after 24 h of treatment (Table IV). Al was undetectable in electron-dense cell wall zones in both control and Al-treated plants (Table IV). In these cell wall zones, similar counts for P, S, K, and Ca were found in controls and Al-treated plants. Counts for Si in walls were within the values of those in blank resin, where 4.8 to 11 counts s−1 Si were found. Traces of Mg were only detected in some of the Al-treated samples. In a few of the scarce, electron-dense deposits of the vacuoles of controls, Al was detected (Table IV). In all deposits where the Al signal was significant, P, K, and Ca were found, whereas signals for Mg and S were only occasionally detected. Two types of electron-dense deposits were found in the vacuoles of cortex cells at approximately 3 mm from the tip in plants exposed to Al for 24 h (Table IV; Fig. 7, A and B). Deposits with a relatively high P content, either without or with low Al amounts (Table IV; Fig. 7B), and Si-rich deposits that contained high Al amounts in addition to S, K, Ca, and Mg. These Si- and Al-rich deposits exhibited either low P counts or did not contain P (Table IV; Fig. 7A).
Figure 7.
Representative EDXMA spectra from electron-dense deposits in the vacuoles of root-tip cortex cells (3 mm) of plants exposed to Al for 24 h. A, Al-containing deposit with high Si content. B, Al-containing deposit with P, but without Si. All samples were prepared by freeze-substitution. All spectra are printed at 1000 counts; Fe and Co are instrument contaminants; and Au is from the grid.
DISCUSSION
The tropical maize variety used in this study, C 525 M, has been found to be Al tolerant in both short-term (hours–days) nutrient solution studies (Llugany et al., 1995; Poschenrieder et al., 1995; Calba and Jaillard, 1997; Horst et al., 1997) and long-term (weeks) field experiments on acid soils (M.F. Guimaraes, C.H. The, C. Welcker, Final Report CE-project TS 3-CT 92–0071, unpublished). Nonetheless, after short-term exposure to nutrient solution with a low Al3+ activity (2.1 μm), a decrease of root elongation has already been observed in a previous investigation (Llugany et al., 1995). However, as in the present study (Table I), this inhibition was only transient, and after 24 h of exposure, the elongation rates recovered to those of controls. As shown by the analysis of nutrient solutions after the experiment, the recovery of the root-elongation rate was neither due to an increase of pH in the nutrient solution nor to depletion of Al during the experiment.
Ultrastructural observations performed in this study also show that the Al-tolerant maize var C 525 M is highly responsive to low Al concentrations after 4 h of exposure, but is poorly affected after 24 h. Cell wall thickening and disturbance of apoplastic and symplastic PA-stainable cations occurred only during the initial period of the Al treatment (4 h), but not after longer (24 h) exposure times. The swelling of cell walls is an early Al-toxicity symptom (Eleftheriou et al., 1993), that seems to be related to a displacement of Ca from the walls (Demarty et al., 1984). In our study, alterations of the cation content in the cell walls of cortex cells of plants exposed for 4 h to Al could be observed in PA-stained samples. PA staining has been shown to immobilize easily diffusible cations such as Ca and Na (Mentre and Escaig, 1988; Mentré and Halpern, 1988). After 4 h of exposure, considerably higher amounts of PA precipitates were found at the internal site of cell walls and inside of the cytoplasm of cortex cells of Al-treated plants than in the controls. EDXMA showed that the PA deposits had a high K and Ca content. This result suggests that after short-term (4 h) exposure to Al, the cation homeostasis in the apoplast was severely disturbed. The transient character of Al-induced alterations of both cell wall ultrastructure and cation homeostasis in our experimental plants was chronologically related to the change in root-elongation rate.
There also was a clear coincidence in time between the Al-induced inhibition of root-elongation rates and the detection of Al in the apoplast of root tips by two different techniques: hematoxylin staining and EDXMA. Hematoxylin is considered to stain Al-P deposits in root tips that have been damaged by Al exposure (Ownby, 1993). In Al-tolerant wheat a decrease of hematoxylin staining intensity after 6 to 24 h of Al exposure has been observed (Rincón and Gonzales, 1992). However, to our knowledge, this is the first time that such a change in apoplastic Al in an Al-tolerant variety has been confirmed by EDXMA. This change was related in time to a change in root-elongation rates and ultrastructure.
The facts that Al caused damage in the apoplast and cation homeostasis, decreasing root elongation only during the first hours of exposure, and that the recovery of the plants occurred in coincidence with a change in apoplastic Al, suggest that in maize var C 525 M, Al-tolerance or -resistance mechanisms must have been activated that induced a change in the speciation of apoplastic Al, making it undetectable for hematoxylin and EDXMA. At present, there is considerable experimental evidence indicating that increased exudation of organic acids from root tips may play an important role in Al detoxification in Al-tolerant maize and wheat (Pellet et al., 1995, 1997; Jorge and Arruda, 1997). Ownby (1993) has shown that root tips of an Al-sensitive wheat variety that stain intensely with hematoxylin exhibited no coloration when the roots were rinsed with citrate before the staining procedure. We are currently investigating whether exudation of organic acids was the cause for the observed changes in apoplastic Al in the maize variety used in this study. Our results on apoplastic Al, ultrastructural alterations in the apoplast, and root-elongation rates support the hypothesis that apoplastic Al can be toxic to plants (Rengel, 1992; Horst 1995). However, because of the fact that after only 4 h of exposure Al was detectable in root-tip vacuoles, we cannot exclude that symplastic Al can also be responsible for the toxic effects observed after 4 h of exposure.
Our EDXMA data showing a significant increase of vacuolar Al as soon as 4 h after the start of Al supply and only a transient occurrence of insoluble Al deposits in the apoplast of root tips are in clear contrast to those from several other studies. Marienfeld and Stelzer (1993) and Marienfeld et al. (1995) have observed a high accumulation of Al in root-tip cell walls and could not find Al inside cells unless plants were exposed to Al for longer times. Lazof et al. (1997) indicated that in only 6 out of 17 electron probe x-ray microanalysis studies was Al detected inside of plant cells, whereas in all studies Al was found mainly in cell walls. This apparent contradiction with our results may be explained, at least in part, by clear differences in the nutrient solutions employed, the exposure time, and the fact that most of these studies were performed with Al-sensitive plants exposed to considerably higher Al concentrations.
That Al may enter root cells after short-term exposure to Al has previously been demonstrated in investigations using secondary ion MS (Lazof et al., 1994, 1996) or fluorescence microscopy on morin-stained root tips (Tice et al., 1992). However, this is the first time to our knowledge that Al has been shown to be present in root-tip vacuoles of an Al-tolerant variety after a few hours of exposure. Tice et al. (1992) also found most of the Al inside of the cells of root tips from Al-tolerant wheat. In contrast, they could not detect Al in vacuoles. However, it is not likely that morin would stain insoluble Al complexes in vacuoles.
The Al-rich vacuolar deposits, found in root-tip cells between 0 and 1.5 mm from the apex of our experimental plants contained P, Ca, Zn, and Mg (Table II; Fig. 6H), and their mineral composition was similar to that reported for phytate (Mikus et al., 1992). Phytate or polyphosphate deposits in the vacuoles may be involved in vacuolar storage of Al in a way similar to that reported for Zn (Van Steveninck et al., 1987). Compartmentational analysis of 32P elution in root cortical cells of intact roots of Lolium perenne exposed to nontoxic Al concentrations also suggests that considerable amounts of a condensed Pi form may complex Al in root-cortex vacuoles (Macklon and Sim, 1992).
According to our results, in the expanding cells at 3 mm from the apex a second storage form for considerable Al amounts occurs in this Al-tolerant maize variety. In this more mature root zone most of the vacuolar Al of Al-treated plants was associated with high Si amounts (Table III; Fig. 7A). The Al that was detectable occasionally in the corresponding cells of control plants and that probably derived from the seeds that had been formed on maternal plants growing on acid soils in Brazil was exclusively found in P-rich deposits with a mineral composition similar to phytate (Table III). There are several earlier reports showing that the ameliorative effect of Si is not only due to Al-Si interactions at the substrate level, but that Al-Si interactions inside plants may play an important role in Al tolerance (Barceló et al., 1993; Hodson and Evans, 1995; Corrales et al., 1997). It is tentative to speculate that a preferential storage of Al in the form of Si-rich deposits in the vacuoles of expanding cortex cells after 24 h of exposure to Al would reduce the toxic effects of Al and may contribute to the enhancement of root elongation after an initial transient growth reduction.
The relationship between vacuolar storage of Al in root-tip cells and Al tolerance, however, remains unclear. Ernst (1998) states that compartmentation into the vacuole is the principle of all hypotheses explaining intracellular metal tolerance in plants. Genotype differences in intracellular tolerance, however, cannot be explained per se by the presence of metal deposits in vacuoles, but are probably related to both the capacity of plants to form metal complexes that would be less toxic to cell components during the transport to the vacuole and the velocity of metal transport across the tonoplast.
In spite of the fact that the accumulation of Al increased in the small vacuoles of root-tip cells, a toxic effect on the plane of cell division occurred after 24 h of exposure to Al (Fig. 4D). This result suggests that under our experimental conditions, not all Al was efficiently detoxified by chelation and vacuolar storage, and that there was sufficient Al remaining to interfere directly or indirectly, perhaps by an interaction with phosphatidylinositol bisphosphate (Kochian and Jones, 1997), with the direction of the cytoskeletal-directed plane of cell division.
In conclusion, the fact that after 4 h of exposure to Al, significant Al was detected in root-tip cell compartments, cell walls, and vacuoles does not allow us to clarify the problem of the primary site of Al toxicity: the apoplast or symplast. However, to our knowledge, this is the first experiment performed with an Al-tolerant maize variety that provides analytical data supporting a relation in time between the lowering of insoluble, apoplastic Al, increased Al accumulation in root-tip vacuoles, and a decrease of Al-toxicity symptoms in the apoplast. Moreover, our results provide evidence for the view that even in Al-tolerant maize Al enters rapidly into the cells.
ACKNOWLEDGMENT
The supply of seeds of var C 525 M from Embrapa is gratefully acknowledged.
Abbreviations:
- EDXMA
energy-dispersive x-ray microanalysis
- PA
pyroantimoniate
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
Footnotes
This work was supported by the Research Council of the European Union (contract nos. TS*CT922-0071 and ERBIC188CT-0063) and by the Spanish National Research Council (contract no. DGICYT PB97-0163-C02-01).
LITERATURE CITED
- Barceló J, Guevara P, Poschenrieder CH. Silicon amelioration of aluminium toxicity in teosinte (Zea mays L. ssp. Mexicana) Plant Soil. 1993;54:249–255. [Google Scholar]
- Calba H, Jaillard B. Effect of aluminium on ion uptake and H+ release by maize. New Phytol. 1997;137:607–616. [Google Scholar]
- Corrales I, Poschenrieder CH, Barceló J. Influence of silicon pretreatment on aluminium toxicity in maize roots. Plant Soil. 1997;190:203–209. [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.). I. Uptake and distribution of aluminum in root apices. Plant Physiol. 1993;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarty M, Morvan C, Thellier M. Calcium and the cell wall. Plant Cell Environ. 1984;7:449–456. [Google Scholar]
- Eleftheriou EP, Moustakas M, Fragiskos N. Aluminate-induced changes in morphology and ultrastructure of Thinopyrum roots. J Exp Bot. 1993;44:427–436. [Google Scholar]
- Ernst WHO (1998) Effects of heavy metals in plants at the cellular and organismic level. In G Schüürmann, B Markert, eds, Ecotoxicology. Wiley & Sons, New York, pp 587–620
- Harvey DMR. Freeze substitution. J Microsc. 1982;127:209–221. doi: 10.1111/j.1365-2818.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Hodson MJ, Evans DE. Aluminium/silicon interactions in higher plants. J Exp Bot. 1995;46:161–171. doi: 10.1093/jxb/eraa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ. The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernähr Bodenkd. 1995;158:419–428. [Google Scholar]
- Horst WJ, Püschel AK, Schmohl N. Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize. Plant Soil. 1997;192:23–30. [Google Scholar]
- Jorge RA, Arruda P. Aluminum-induced organic acids exudation by roots of an aluminum-tolerant tropical maize. Phytochemistry. 1997;45:675–681. [Google Scholar]
- Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kokot S. Aluminium determination in soil solution. II. Short-term colorimetric procedures for the measurement of inorganic aluminium in the presence of organic acid ligands. Aust J Soil Res. 1989;27:91–102. [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Kochian LV, Jones DL (1997) Aluminum toxicity and resistance in plants. In RA Yokel, MS Golub, eds, Research Issues in Aluminum Toxicity. Taylor and Francis Publishers, Washington, DC, pp 69–89
- Lazof DB, Goldsmith JG, Linton RW. The in situ analysis of intracellular aluminium in plants. In: Behnke HD, Lüttge U, Esser K, Kadereit JW, Runge M, editors. Progress in Botany, Vol 58. Berlin: Springer Verlag; 1997. pp. 112–149. [Google Scholar]
- Lazof DB, Goldsmith JG, Rufty TW, Linton RW. Rapid uptake of aluminum into cells of intact soybean root tips. A microanalytical study using secondary ion mass spectrometry. Plant Physiol. 1994;106:1107–1114. doi: 10.1104/pp.106.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof DB, Goldsmith JG, Rufty TW, Linton RW. The early entry of Al into cells of intact soybean roots. A comparison of three developmental root regions using secondary ion mass spectrometry imaging. Plant Physiol. 1996;112:1289–1300. doi: 10.1104/pp.112.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llugany M (1994) Respuestas diferenciales de cultivares de Zea mays L. a la toxicidad por aluminio. PhD thesis, Universidad Autónoma de Barcelona, Bellaterra, Spain
- Llugany M, Massot N, Wissemeier A, Poschenrieder CH, Barceló J. Differences in aluminium tolerance between maize varieties as assesssed by callose formation and root elongation. Z Pflanzenernähr Bodenkd. 1994;157:447–451. [Google Scholar]
- Llugany M, Poschenrieder CH, Barceló J. Monitoring of aluminium-induced inhibition of root elongation in four maize cultivars differing in tolerance to aluminium and proton toxicity. Physiol Plant. 1995;93:265–271. [Google Scholar]
- Macklon AES, Sim A. Modifying effects of a non-toxic level of aluminium on phosphate fluxes and compartmentation in root cortex cells of intact ryegrass seedlings. J Exp Bot. 1992;43:1483–1490. [Google Scholar]
- Marienfeld S, Lehmannn H, Stelzer R. Ultrastructural investigations and EDX-analyses of Al-treated oat (Avena sativa) roots. Plant Soil. 1995;171:167–173. [Google Scholar]
- Marienfeld S, Stelzer R. X-ray microanalyses in Al-treated Avena sativa plants. J Plant Physiol. 1993;141:569–573. [Google Scholar]
- Mentré P, Escaig F. Localization of cations by pyroantimoniate. I. Influence of fixation on distribution of calcium and sodium: an approach by analytical ion microscopy. J Histochem Cytochem. 1988;36:49–54. doi: 10.1177/36.1.3121721. [DOI] [PubMed] [Google Scholar]
- Mentré P, Halpern S. Localization of cations by pyroantimoniate. II. Electron probe microanalysis of calcium and sodium in skeletal muscle of mouse. J Histochem Cytochem. 1988;36:55–64. doi: 10.1177/36.1.3335770. [DOI] [PubMed] [Google Scholar]
- Mikus M, Bobák M, Lux A. Structure of protein bodies and elemental composition of phytin from dry germ of maize (Zea mays L.) Bot Acta. 1992;105:26–33. [Google Scholar]
- Ownby JD. Mechanisms of reaction of hematoxylin with aluminium-treated wheat roots. Physiol Plant. 1993;87:371–380. [Google Scholar]
- Parker DR, Zelazny LW, Kinraide TB. Improvements to the program GEOCHEM. Soil Sci Soc Am J. 1987;51:488–491. [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminium tolerance mechanism in maize (Zea mays L) Planta. 1995;196:788–95. [Google Scholar]
- Pellet DM, Papernik LA, Jones DL, Darrah PR, Grunes DL, Kochian LV. Involvement of multiple aluminium exclusion mechanisms in aluminium tolerance in wheat. Plant Soil. 1997;192:63–68. [Google Scholar]
- Polle E, Konzak CF, Kittrick JA. Visual detection of aluminium tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Sci. 1978;18:823–827. [Google Scholar]
- Poschenrieder CH, Llugany M, Barceló J. Short-term effects of pH and aluminium on mineral nutrition in maize varieties differing in proton and aluminium tolerance. J Plant Nutr. 1995;18:1495–1507. [Google Scholar]
- Rengel Z. Competitive Al3+ inhibition of net Mg2+ uptake by intact Lolium multiflorum roots. II. Plant age effects. Plant Physiol. 1990;93:1261–1267. doi: 10.1104/pp.93.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z. Role of calcium in aluminium toxicity. New Phytol. 1992;121:499–513. doi: 10.1046/j.1469-8137.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- Rengel Z. Uptake of aluminium by plant cells. New Phytol. 1996;134:389–406. [Google Scholar]
- Rengel Z, Reid RJ. Uptake of Al across the plasma membrane of plant cells. Plant Soil. 1997;192:31–35. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208–210. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M, Gonzales RA. Aluminum partitioning in intact roots of aluminum-tolerant and aluminum-sensitive wheat (Triticum aestivum L.) cultivars. Plant Physiol. 1992;99:1021–1028. doi: 10.1104/pp.99.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastr Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tice KR, Parker DR, DeMason DA. Operationally defined apoplastic and symplastic aluminium fractions in root tips of aluminum-intoxicated wheat. Plant Physiol. 1992;100:309–318. doi: 10.1104/pp.100.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steveninck RFM, Van Steveninck ME, Fernando DR, Horst WJ, Marschner H. Deposition of zinc phytate in globular bodies in roots of Deschampsia caespitosa ecotypes: a detoxification mechanism? J Plant Physiol. 1987;131:247–257. [Google Scholar]
- Van Steveninck RFM, Van Steveninck ME. Microanalysis. In: Hall JL, Hawes C, editors. Electron Microscopy of Plant Cells. London: Academic Press; 1991. pp. 415–455. [Google Scholar]
- Vázquez MD, Barceló J, Poschenrieder CH, Mádico J, Hatton P, Baker AJM, Cope GH. Localization of zinc and cadmium in Thlaspi caerulescens (Brassicaceae), a metallophyte that can hyperaccumulate both metals. J Plant Physiol. 1992;140:350–355. [Google Scholar]