Abstract
Introduction
The Canadian Task Force on Preventive Health Care (CTF-PHC) recently revised its screening recommendations for colorectal cancer (CRC). We wished to assess the effect of this change on the screening beliefs and clinical practice of primary care physicians.
Methods
We surveyed 160 primary-care physicians, quasi-randomly sampled, in June–July 2001 and again in April–July 2002, 9 months after publication of the guidelines. Descriptive statistics and McNemar χ2 analyses were carried out on data from physicians who responded to both surveys.
Results
Of the those sampled, 47% responded to both surveys. After the publication of the CTF-PHC guidelines, the proportion reporting that they recommend CRC screening to their patients at average risk increased from 43% to 60% (p = 0.02). Before publication of the revised guidelines 48% stated that the CTF-PHC did not support screening, compared with 24% afterward (p = 0.01). CTF-PHC guidelines were acknowledged by 30% to be a source of CRC screening information. Around 9 months post-publication, 24% of the physicians stated their awareness of the revised screening guidelines. The most commonly cited reasons for not recommending CRC screening to average-risk patients were that the evidence is inconclusive and that CTF-PHC guidelines do not support screening.
Conclusions
After publication of the revised CTF-PHC guidelines more primary-care physicians reported that they recommend CRC screening to their average-risk patients. The belief that the evidence is inconclusive nevertheless remains a considerable barrier to implementation. To increase the use of screening for CRC, additional strategies are required.
Abstract
Introduction
Le Groupe d'étude canadien sur les soins de santé préventifs (GEC-SSP) a révisé récemment ses recommandations sur le dépistage du cancer colorectal (CCR). Nous voulions évaluer l'effet de ce changement sur les hypothèses relatives au dépistage et sur la pratique clinique des médecins de première ligne.
Méthodes
Nous avons sondé 160 médecins de première ligne, choisis presque au hasard, avant le 21 juillet et de nouveau d'avril à juillet 2002, neuf mois après la publication des lignes directrices. On a établi des statistiques descriptives et procédé à des analyses McNemar χ2 de données provenant des médecins ayant répondu aux deux sondages.
Résultats
Parmi les médecins sondés, 47 % ont répondu aux deux questionnaires. Après la publication des lignes directrices du GEC-SSP, la proportion des répondants qui ont signalé recommander le dépistage du CCR à leurs patients à risque moyen est passée de 43 % à 60 % (p = 0,02). Avant la publication des lignes directrices révisées, 48 % avaient déclaré que le GEC-SSP n'appuyait pas le dépistage, comparativement à 24 % après la publication (p = 0,01). Les lignes directrices du GEC-SSP ont été reconnues par 30 % comme une source d'information sur le dépistage du CCR. Environ neuf mois après la publication, 24 % des médecins ont déclaré connaÎtre les lignes directrices révisées sur le dépistage. Comme raisons évoquées le plus couramment pour ne pas recommander le dépistage du CCR aux patients à risque moyen, on a affirmé que les données probantes ne sont pas concluantes et que les lignes directrices du GEC-SSP n'appuient pas le dépistage.
Conclusions
Après la publication des lignes directrices révisées du GEC-SSP, plus de médecins de premier recours ont dit recommander le dépistage du CCR à leurs patients à risque moyen. La croyance selon laquelle les données probantes ne sont pas concluantes demeure néanmoins un obstacle important à la mise en œuvre des lignes directrices. D'autres stratégies s'imposent afin d'étendre le dépistage du CCR.
Colorectal cancer (CRC) is the third most common cancer and the second most frequent cause of cancer-related deaths in Canada.1 In 1994 the Canadian Task Force on the Periodic Health Examination (since renamed the Canadian Task Force on Preventive Health Care, or CTF-PHC) stated that evidence for CRC screening of asymptomatic individuals 40 years of age or older was insufficient to support the inclusion or exclusion of fecal occult blood testing (FOBT), sigmoidoscopy or colonoscopy.2 Since then, evidence from randomized controlled trials and case– control studies has shown that screening can reduce the incidence of CRC and prevent CRC- related deaths among those at average risk.3,4,5,6,7 As a result, in 2001 CTF-PHC published amended recommendations for CRC screening. The revised guidelines state that there is “good evidence to include multiphasic screening with [FOBT] and fair evidence to include flexible sigmoidoscopy in the periodic health examination for average-risk individuals at least 50 years of age.”8 For colonoscopy, the recommendations remain unchanged from 1994, as the guidelines hold that evidence is still insufficient to include or exclude this manœuvre as an initial screening modality.
Established in 1976, CTF-PHC is a scientific panel developing evidence-based clinical practice guidelines for preventive interventions.9 Although much effort and many resources have been put into guideline development, effective implementation remains a formidable challenge. Despite their purpose, insufficiencies in general awareness, agreement, adoption and adherence have given clinical practice guidelines less influence than intended over the clinical behaviour of primary care physicians.10 It is unknown whether this holds true in Canada for the recent CRC screening recommendations. Our objectives were to document the self-reported application of current CRC screening recommendations by Canadian primary care physicians, awareness of the revised CTF-PHC CRC screening guidelines and influence on primary care practices after the publication.
Methods
Before the publication of CTF-PHC's clinical practice guidelines11,12 and about 9 months after, 160 primary-care physicians were surveyed. Sampling was quasi-randomized by selecting the first primary-care physician on every fourth page of the 2001 Canadian Medical Directory.13 Data were included only from primary care physicians currently in practice in Canada who responded to both questionnaires.
Questionnaires in both surveys were mailed with postage-paid return envelopes. The pre-publication questionnaires were sent in June and July of 2001. Two weeks after the first mail-out, nonrespondents were telephoned to confirm their availability and mailing address, and the initial survey mailed to them a second time. The follow-up questionnaires were sent in April through July of 2002.
The questionnaires were developed for this study. The first contained 3 sections, on beliefs (5 statements about the benefits of, evidence for and guidelines about CRC screening, indicated on a 5-point Likert scale: strongly agree to strongly disagree), current practice (questions about participants' current recommendations for CRC screening), and demographic and practice characteristics. The follow-up questionnaire also included a fourth section asking about their awareness of CTF-PHC's recently revised CRC screening guidelines.
Responses were entered into 1997 Microsoft Access software and analyzed with SAS V8. Associations between the physicians' beliefs, demographics and practice characteristics were assessed with analysis of variance (ANOVA). McNemar χ2 analyses were conducted to identify changes in the self-reported behaviour and beliefs of the physicians from the first to the second questionnaire.
Results
From the initial 160 potential subjects, 31 (19%) were excluded: 16 were not primary care physicians; 13 were not in practice (retired, taking a sabbatical or on maternity leave); and 2 responded to the initial questionnaire after publication of the revised guidelines. Of the remaining 129 physicians, 76 (59%) responded to the first and 60 (47%) to both questionnaires; thus, the responses of 60 primary care physicians were included in the analysis. Table 1 shows their demographics and practice characteristics.
Table 1
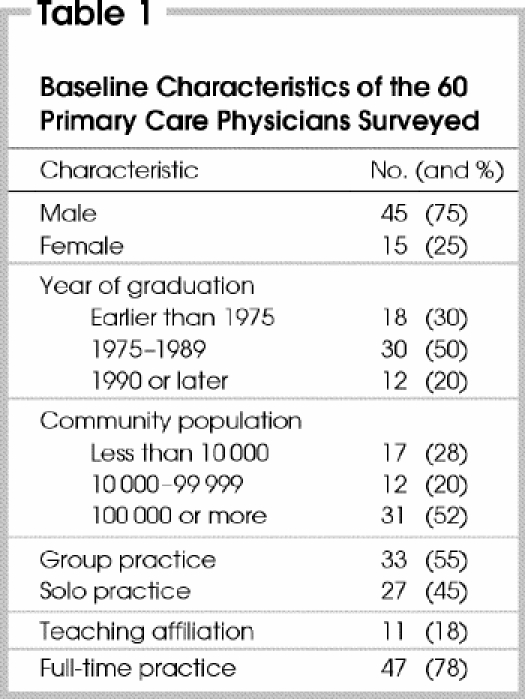
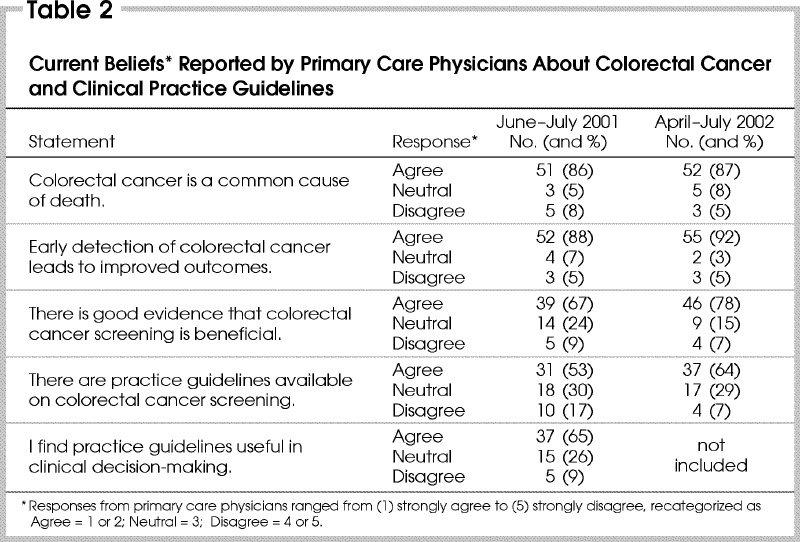
About 80% of respondents believed that CRC is a common cause of death and that early detection of CRC leads to improved outcomes (Table 2). A smaller proportion felt that there is good evidence supporting screening and that clinical practice guidelines are available. These opinions did not vary according to year of graduation, academic affiliation or type of practice.
Table 2
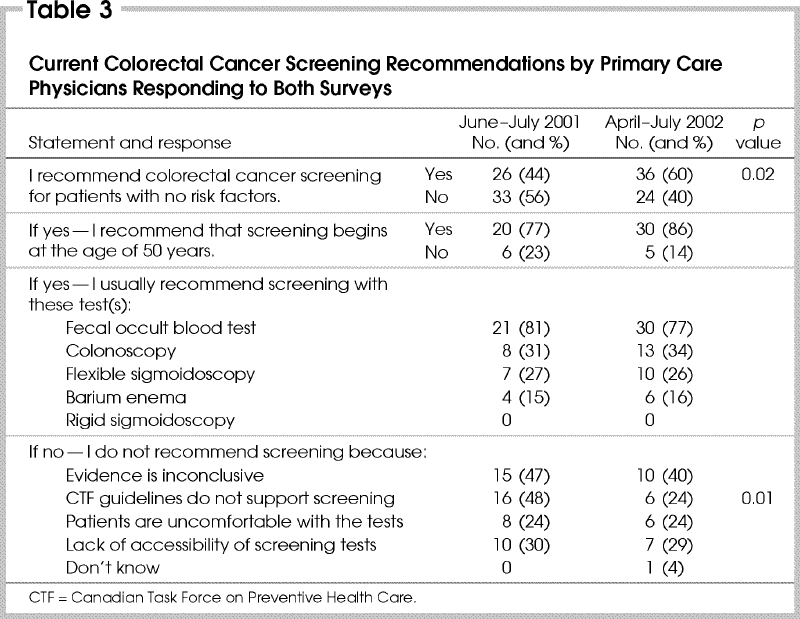
From the initial questionnaire, 44% reported that they recommend CRC screening to their average-risk patients, compared with 60% from the follow-up questionnaire (p = 0.02). Of those who recommend screening, over 75% prescribe initial screening with FOBT in patients at least 50 years of age ( Table 3). In the first survey the reason most commonly cited for not recommending CRC screening to their average-risk patients was because CTF-PHC guidelines at the time did not support screening (48%). This decreased to 24% after the publication of the revisions (p = 0.01). Other reasons included inconclusive evidence (40%), the lack of accessibility of tests (30%) and patient discomfort (24%).
Table 3
The most commonly reported sources of information about CRC screening were continuing medical education events (73%), journal articles (68%) and colleagues (52%). The CTF-PHC guidelines were less frequently reported as a source (30%). After publication, 24% of respondents were aware of the revised CTF-PHC CRC screening guidelines (Table 4); these individuals were distributed across the demographic and practice categories. Of the 14 respondents who were aware, 11 had read the summary or full text of the guidelines, and 9 reported that the guidelines had influenced their CRC screening practices in general terms. Nevertheless, when the responses from the first and second questionnaire were compared only 3 physicians (4%) indicated that they had changed their practices because of the revised guidelines.
Table 4
Discussion
The results of this study suggest that within 1 year of the publication of the revised CTF-PHC guidelines for CRC screening, there was a significant increase in self-reported routine CRC screening recommendations by this sample of Canadian primary-care physicians. Understanding how this change may have been influenced by the publication of the recent guidelines is more complex. Even though 76% of respondents were unaware of the publication of the revised CRC screening guidelines, after the revised guidelines were published there was nevertheless a statistically significant 24% reduction in the number of physicians citing CTF-PHC recommendations as a reason for not recommending routine screening. Despite the 16% increase in self-reported CRC screening recommendation after the publication of the guidelines, 40% of the respondents still reported that they did not routinely recommend CRC screening to individuals 50 years of age or older. The physicians' clinical behaviour was self-reported and may be more indicative of the physicians' overall belief of the relative screening benefits versus the risks for an age-appropriate general population rather than accurately reflecting actual practice.14 As well, the period from publication of the guidelines to the follow-up questionnaires was short and may not have been long enough to expect a change in clinical behaviour — although conversely, with time there might be a drop-off in awareness of the benefits of screening.
This study had a small sample size and a relatively low response rate. The response rate was influenced by our inclusion of only those who responded to both questionnaires. However, the distribution of demographics, geographic and practice characteristics of the 60 physicians included in the analysis (Table 1) are representative of Canada's primary-care physicians.15 Responses to the initial questionnaire from the 16 physicians who did not respond to the follow-up questionnaire were similar to those of the respondents included in the analysis.
Our results demonstrate that this sample of primary care physicians believe in the benefits of early detection and prevention of CRC, but with less agreement about the availability and usefulness of clinical practice guidelines to guide decision-making. Concerns about a lack of usefulness of CRC screening guidelines may be related to numerous factors. Before publication of the revised CTF-PHC screening guidelines, Canadian primary-care physicians reported that they perceived the guidelines for FOBT to be controversial, unclear or conflicting.16 Physicians' CRC screening recommendations may also be influenced by their belief that there is a lack of public acceptance of the available tests17 plus their own ambivalence about the value of the available screening modalities, especially FOBT.8 Some physicians see guidelines as threatening their clinical independence, or may be influenced by local social norms.10,18,19 Three main factors that have been found to determine the acceptability of preventive guidelines for primary care physicians are the approval of peers and local experts, and an absence of controversy.18,19
CTF-PHC guidelines are not consistently implemented for other preventive health-care recommendations as well.20,21,22 In a systematic review of implementation strategies, Davis and Taylor-Vaisey10 described 2 stages of implementation for clinical practice guidelines. The first involves primary dissemination by means of publication, to increase awareness, improve knowledge and predispose individuals to a change in behaviour. However, it is necessary to move beyond the first to the second stage, which requires active implementation that enables and reinforces change in the clinical setting. A recent systematic review23 assessing interventions directed at primary care physicians to improve the delivery of preventive care concluded that there is no solid basis for assuming that any individual or package of interventions is most effective, although tailoring them to address barriers identified in a particular setting is important.
Conclusions
Although more physicians reported that they recommend CRC screening to their average-risk patients after the publication of the CTF-PHC guidelines, the belief that the evidence is inconclusive remains a serious barrier to the implementation of CRC screening. It is important for general surgeons to familiarize themselves with the quality of current evidence supporting the various modalities for CRC screening: as local experts, they can play a credible role in increasing levels of awareness and acceptance of routine CRC screening that is appropriate to their locality, among primary care physicians as well as the general population.
Acknowledgments
Dr. Asano is a research fellow of the National Cancer Institute of Canada supported with funds provided by the Canadian Cancer Society.
Podium Presentation at the Canadian Surgical Forum, London, Ont., Sept. 20, 2002
Competing interests: None declared.
Correspondence to: Dr. Robin S. McLeod, Mount Sinai Hospital suite 449, 600 University Ave., Toronto ON M5G 1X5; fax 416 586-8644; rmcleod@mtsinai.on.ca
Accepted for publication Dec. 3, 2003
References
- 1.National Cancer Institute of Canada. Canadian Cancer Statistics 2001. Toronto: NCIC; 2001.
- 2.Solomon MJ, McLeod RS. Periodic health examination, 1994 update: 2. Screening strategies for colorectal cancer. CMAJ 1994;150:1961-70. [PMC free article] [PubMed]
- 3.Mandel JS, Bond JH, Church TR. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993;328:1365-71. [DOI] [PubMed]
- 4.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with fæcal-occult-blood test. Lancet 1996;348:1467-71. [DOI] [PubMed]
- 5.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of fæcal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472-7. [DOI] [PubMed]
- 6.Kewenter J, Bjork S, Haglind E, Smith L, Svanvik J, Ahren C. Screening and rescreening for colorectal cancer. A controlled trial of fecal occult blood testing in 27 700 subjects. Cancer 1988;62:645-51. [DOI] [PubMed]
- 7.Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst 1992;84:1572-5. [DOI] [PubMed]
- 8.McLeod R, with the Canadian Task Force on Preventive Health Care. Screening strategies for colorectal cancer: systematic review and recommendations. London ON: Canadian Task Force; 2001 Feb. CTF-PHC Tech Rept 01-2.
- 9.Evidence-based clinical prevention. In: Canadian Task Force on Preventive Health Care [Web site of the CTF-PHC]. Available: www.ctfphc.org (accessed 2003 Nov 11).
- 10.Davis DA, Taylor-Vaisey A. Translating guidelines into practice: a systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ 1997;157:408-16. [PMC free article] [PubMed]
- 11.McLeod RS, with the Canadian Task Force on Preventive Health Care. Screening strategies for colorectal cancer: a systematic review of the evidence. Can J Gastroenterol 2001;15:647-60. [DOI] [PubMed]
- 12.McLeod RS. Colorectal cancer screening: recommendation statement from the Canadian Task Force on Preventive Health Care. Can Fam Physician 2001;47:1811-5. [PMC free article] [PubMed]
- 13. Canadian medical directory. 47th ed. Toronto: Southam Information Products Group; 2001.
- 14.Montano DE, Phillips WR. Cancer screening by primary care physicians: a comparison of rates obtained from physician self-report, patient survey, and chart audit. Am J Public Health 1995;85:795-800. [DOI] [PMC free article] [PubMed]
- 15.The College of Family Physicians of Canada. The CFPC national family physician survey: summary report. Mississauga ON: The College; 1998. Available: www.cfpc.ca/local/files/Research/janussummary.pdf (accessed 2003 Nov 11).
- 16.Tudiver F, Brown J, Medved W, Herbert C, Ritvo P, Guibert R. Making decisions about cancer screening when the guidelines are unclear or conflicting. J Fam Practice 2001;50:682-7. [PubMed]
- 17.Cooper GS, Yuan Z, Veri L, Rimm AA, Stange KC. Colorectal carcinoma screening attitudes and practices among primary care physicians in counties at extremes of either high or low cancer case-fatality. Cancer 1999;86:1669-74. [DOI] [PubMed]
- 18.Beaulieu MD, Hudon E, Roberge D, Pineault R, Forte D, Legare J. Practice guidelines for clinical prevention: Do patients, physicians and experts share common ground? CMAJ 1999;161:519-23. [PMC free article] [PubMed]
- 19.Ferrier BM, Woodward CA, Cohen M, Williams AP. Clinical practice guidelines: new- to-practice family physicians' attitudes. Can Fam Physician 1996;42:463-8. [PMC free article] [PubMed]
- 20.Freedman A, Pimlott N, Naglie G. Do family physicians comply with recommendations of the Canadian Task Force on Preventive Health Care? Can Fam Physician 2000;46:350-7. [PMC free article] [PubMed]
- 21.Hutchinson B, Woodward CA, Norman GR, Abelson J, Brown JA. Provision of preventive care to unannounced standardized patients. CMAJ 1998;158:185-93. [PMC free article] [PubMed]
- 22.Smith HE, Herbert CP. Preventive practice among primary care physicians in British Columbia: relation to recommendations of the Canadian Task Force on the Periodic Health Examination. CMAJ 1993;149:1795-800. [PMC free article] [PubMed]
- 23.Hulscher MEJL, Wensing M, van der Weijden T, Grol RP. Interventions to implement prevention in primary care [Cochrane review]. In: The Cochrane Library; Issue 1, 2004. Oxford: Update Software.



