Abstract
Systemic inflammatory responses to severe trauma and surgical illnesses may be partly responsible for numerous complications, including sepsis, multiple organ failure and unregulated hypermetabolism leading to protein-calorie malnutrition. The integrity of the gastrointestinal tract appears to be an important factor in the pathogenesis of the systemic inflammatory response and sepsis. Resuscitation and nutrition support strategies for preserving gut mucosal integrity have therefore been strongly promoted. This review summarizes the scientific rationale for emphasizing enteral nutritional support of surgical patients, discusses some important limitations of enteral feeding and argues for a flexible approach to nutrition support for these complex patients.
Abstract
Les réactions inflammatoires systémiques aux traumatismes graves et les maladies chirurgicales peuvent être en partie la cause de nombreuses complications, y compris la septicémie, la défaillance d'organes multiples et l'hypermétabolisme non régularisé qui entraÎne une malnutrition protéique et calorique. L'intégrité du tractus gastro-intestinal semble constituer un facteur important de la pathogénèse de la réaction inflammatoire systémique et de la septicémie. C'est pourquoi on a préconisé vivement des stratégies de réanimation et de soutien nutritionnel pour préserver l'intégrité de la muqueuse intestinale. L'étude résume les raisons scientifiques qui justifient d'insister sur le soutien nutritionnel entéral chez les patients qui ont subi une intervention chirurgicale, analyse certaines limites importantes de l'alimentation par voie entérale et préconise une approche flexible du soutien nutritionnel chez ces cas complexes.
Nutrition support, besides offsetting the potentially devastating effects of malnutrition that result from stress-induced hypercatabolism, also affects the pathogenesis of a systemic inflammatory response in severe surgical or traumatic illness. Our increasing understanding of the relation between nutrition support (specifically, enteral feeding) and the immunologic and barrier functions of the bowel have radically altered patient care in this area and have stimulated clinical and basic scientific research into the mechanisms involved. In this review, we discuss the enteric immune system in general, focussing on the potential mechanistic relationships between enteral nutrition (EN) and mucosal immunity, and we review the clinical importance of findings reported in landmark publications on nutrition support in critical illness.1,2,3 Finally, we detail the practical limitations of enteral feeding and make recommendations for a balanced approach to nutrition support.
Metabolic and inflammatory responses to trauma and surgical illness
Surgical intervention results in the local release of inflammatory cytokines such as tumour necrosis factor (TNF), interleukin-1 (IL-1) and interleukin-6 (IL-6), and in systemic release of such counter-regulatory hormones as adrenocorticotrophic hormone (ACTH), antidiuretic hormone (ADH), catecholamines and cortisol (Fig. 1). When inflammatory responses overwhelm the local milieu of the injury and systemic levels of cytokines become excessive, the local wounding paradigm begins to blend with the multiorgan system failure paradigm. Cytokines along with systemic hormones induce hypercatabolism, which is characterized by protein breakdown within skeletal muscle, accelerated breakdown of branched-chain amino acids and increased release of glutamine and alanine into the systemic amino-acid pool. Glutamine is critical as an energy source for enterocytes, immune cells and rapidly growing tissues.4 Within this system, IL-6 levels, which correlate directly with the production of hepatic acute-phase protein and inversely with the production by the liver of constitutive proteins such as albumin and transferrin,5 appear to be a useful indicator of the overall stress response. The extent of the systemic inflammatory response is determined by the extent of the inciting wound and its immunologic and nutritional context.
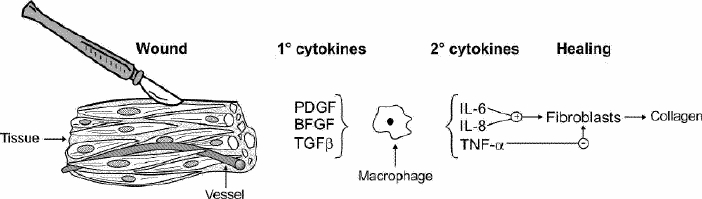
FIG. 1. Schematic diagram of the cytokine signals associated with wound healing. PDGF = platelet-derived growth factor; BFGF = basic fibroblast growth factor; TGFβ = transforming growth factor-β; TNF-α = tumour necrosis factor-α.
In patients with a multisystem stressor, such as major trauma, prolonged operative exposure or burns, there may be a period of shock with reduced end-organ perfusion. Reperfusion may compound the effects of the original injury by causing a massive systemic release of cytokines from the bowel and sometimes the lung (Fig. 2). Changes in enteral flora coupled with increased intestinal permeability result in a predictable flooding of the liver with endotoxin and possibly translocated bacteria, which may amplify the cytokine response, promote sepsis or multiorgan failure and potentiate hypercatabolism and protein–calorie malnutrition.
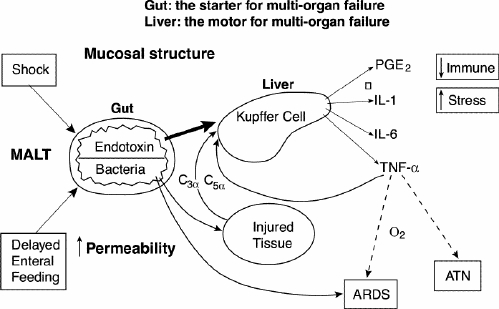
FIG. 2. Cytokine signalling associated with multiorgan failure. (Redrawn from Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma — reduced septic morbidity. J Trauma 1989; 29: 916-23.) MALT = mucosal-associated lymphoid tissue, ARDS = acute respiratory distress syndrome, ATN = acute tubular necrosis.
Enteral nutrition and mucosal immunity
The enteric immune system: nonspecific mechanisms
The intestinal immune system must deal with a large volume of antigenic material as part of normal feeding, must allow continuous and relatively rapid absorption of the nutritional component of feedings with appropriate exclusion of pathogenic organisms and must allow the development of “tolerance” to common food- associated antigens. The sequential barriers of the enteric immune system that mediate these functions are shown in Fig. 3. The first barrier is the nonspecific mucus (containing the endogenously secreted enzymes lactoferrin and lysozyme), which is probably under-appreciated in terms of its importance. Mucus is made by goblet cells which are present throughout the gastrointestinal tract.6 Lactoferrin, secreted by the pancreas, binds iron, preventing its utilization in critical bacterial metabolic steps; lysozyme, secreted by Paneth cells, breaks down bacterial cell walls. Both lactoferrin and lysozyme are effective, nonspecific inhibitors of microbial growth.7,8 Finally, although difficult to monitor, the population of resident microflora at the mucosal surface likely has significant impact on local permeability and intestinal well-being.9
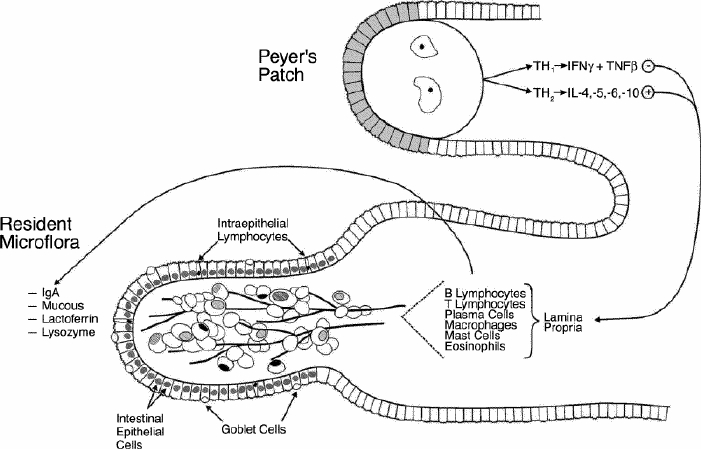
FIG. 3. General schematic diagram of the enteric immune system. The system can be divided into 3 components: nonspecific mucosal immunity; affector limb (primarily Peyer's patches) and effector limb; and epithelial enterocytes and B and T cells with lamina propria producing secretory immunoglobulin A (IgA).
The next barrier of nonspecific defence is the tight junctions between intestinal epithelial cells. This barrier is dynamic in its permeability and is energy dependent.10 The predominant metabolic fuel for the enterocyte tight junctions is glutamine, whose supply in turn depends partly on local nutrient absorption and metabolism.4 Although the exact mechanisms are not well understood, EN seems important in maintaining each of the components of the nonspecific enteral immune barrier.8
Active immune mechanisms and the effect of enteral nutrition
The lymphoid elements within the gut (so-called gut-associated lymphoid tissue, or GALT) form a subset of the larger system of mucosal-associated lymphoid tissue (MALT), which also includes respiratory and mammary tissue. Within this framework, cells sensitized to develop immunity within a single mucosal surface circulate via the systemic circulation, and will “home” to other mucosal sites throughout the body, so that all mucosal surfaces share immune competence against previously recognized antigens.8 This system works through affector and effector regions. Affector or “inductive” sites are concentrated in the Peyer's patches within the intestine. The surface layer of the Peyer's patch is composed of microfold or M cells, which sample the many foreign antigens within the gut. These processed antigens are then presented to the dendritic cells within the central nodal region of the Peyer's patch. These cells disperse through the systemic circulation but return almost exclusively to other MALT sites. This very specific “homing” occurs through selective binding of lymphocyte surface adhesion molecules such as L-selectin, and a MALT-specific modified mucosal addressin cell adhesion molecule (MAdCAM-1) on the postvenous endothelium. Thus, lymphocytes that are sensitized within the Peyer's patches migrate by way of the mesenteric lymphatics to the thoracic duct and throughout the body.
These sensitized B and T cells form the bulk of the immunocompetent population within the intestinal lamina propria. Normally, the CD4: CD8 ratio in the lamina ranges from 2:1 to 3:1. The T-helper (Th) cell populations are unique and highly activated, with high levels of IL-2 receptor. Within this population, the CD4 cytokine production profile is critical; Th1 cells produce IL-2, interferon-γ and TNF-β, and Th2 cells produce IL-4, IL-5, IL-6 and IL-10.11 The Th2 cytokine profile stimulates B cells to produce immunoglobulin A (IgA), but the Th1 cytokine profile does not, and interferon-γ specifically inhibits secretory IgA (sIgA) production. Thus, the local cytokine milieu within the lamina propria is critical for the ongoing production of sIgA and the control of potential pathogens.
This system is profoundly influenced by the route of nutrition support. Animals maintained on total parenteral nutrition (TPN) show a significant drop in IL-4 and IL-10 levels, but the interferon-γ concentration remains unchanged. Further, in animals maintained on TPN, the ratio of CD4:CD8 lymphocytes drops to 1:1.12 Overall, these effects result in a significant decrease in intestinal and respiratory sIgA levels.13,14 In these animals, the cytokine profile can be normalized by providing a complex chow diet for longer than 4 days.12,15 The use of elemental diet results in some improvement in the cytokine profile but is intermediate between the low levels of IL-4 and IL-10 with TPN and the high levels with normal chow feeding.15 TPN feedings significantly reduce the ability of subjects to survive septic stress.3,16
Enteral nutrition and extraintestinal immunity
The previously described effects of TPN on gut-associated lymphoid tissue have been shown to affect the ability of an individual to neutralize infections in other MALT-associated sites. Kudsk and associates14 showed in a mouse model of animals maintained with intragastric TPN, intravenous TPN or a complex enteral diet that animals maintained with TPN lost the ability to clear the influenza virus. When animals with established immunity to an influenza viral strain were re-challenged, 50% of the animals maintained on TPN had continued viral shedding, demonstrating a loss of the previous immunocompetent IgA protective mechanism. The animals cleared their secretions of virus within 5 days of re-establishing enteral feedings.14 Interestingly, animals maintained with intragastric feedings of TPN solution have an intermediate level of immune suppression.12 In a more virulent bacterial model of Pseudomonas infection, chow-fed and enterally fed animals maintained normal immune competence, whereas animals maintained with intravenous TPN showed no benefit from previous immunization.15
Pharmacologic maintenance of enteral immunity
Hanna and Kudsk,17 Johnson and Kudsk18 and others19 have championed the use of various pharmacologic manipulations to maintain enteral immunity. Studies have shown that supplementation of TPN with 2% glutamine maintains normal cell populations within the Peyer's patches, specifically reversing the previously described alterations in the CD4:CD8 ratio, and Th2 cytokine production.19 Similar findings have been noted by other workers using oral glutamine to enhance MALT effector function.20
Interestingly, Kudsk's group21,22 has also noted that pharmacologic stimulation of the enteric neuronal system with gastrin-releasing peptide (GRP) analogue (bombesin) can maintain normal enteric immune function. Animals supported with TPN, but with supplemental administration of bombesin, have maintained normal immune competence. The recently described intestinal trophic peptide glucagon-like peptide 2 (GLP-2), whose release is also triggered by GRP,23 has also been shown to prevent TPN-induced hypoplasia.24 Continued clarification of interactions between the enteric immune and neuronal systems will have exciting implications for interventions designed to preserve the critical immune function of the gut.
Summary of mechanistic relationships between enteral feeding and immunity
The studies reviewed have demonstrated the significant relationships between EN and enteral immunity. Starvation itself results in a variable degree of mucosal atrophy, with some increase in intestinal permeability. However, ongoing nutrition support with TPN results in significant changes in the lymphocyte population and cytokine profile, and subsequent secretory IgA production within the bowel. This leads to an increased susceptibility to respiratory and likely intestinal infection. Enteral administration of a complex diet may reverse this situation and may have the potential to modify the inflammatory response to trauma and surgical illness. There is a potential for the supplemental use of glutamine or pharmacologic stimulation of the enteric neuronal system as experimental therapy.
Clinical correlations
Total parenteral nutrition in the critically ill patient
TPN has been widely used to offset the potentially severe impact of malnutrition and hypercatabolism in surgical patients. But evidence to support this practice has been conflicting. In their meta-analysis, Heyland and associates25 identified 26 randomized trials containing 2211 patients that compared the use of TPN with standard care consisting of oral feedings plus intravenous dextrose. In the cumulative results of these trials, TPN had no effect on mortality (risk ratio [RR] 1.03; 95% confidence interval [CI] 0.81–1.31). The use of TPN in malnourished patients may have provided some protection against infectious complications, but surprisingly, this was the only group showing any benefits.
Enteral nutrition: outcomes in trauma, critical care and general surgery
In 1989, Moore and colleagues2 reported the results of a randomized trial of EN versus TPN in 59 trauma patients undergoing emergency laparotomy. In the EN group they noted a blunted acute-phase response, with improved constitutive liver synthetic function (production of albumin, transferrin and retinol-binding protein). Significant infections developed in 17% of the EN patients compared with 37% of the TPN patients. Of these, major septic complications occurred in only 3% in the EN group, compared with 20% in the TPN group. These results were supported by another landmark study by Kudsk and colleagues3 published in 1992, which randomized 98 patients with blunt or penetrating trauma to receive either EN or TPN. This report noted that patients with an Injury Severity Score greater than 20 and an abdominal trauma index greater than 24 showed the most benefit from EN.
A meta-analysis by Braunschweig and associates26 considered 27 studies involving 1828 patients. These aggregated studies confirmed a significantly lower risk of infection with EN (RR 0.64; CI 0.54–0.76) than with TPN. A third group, receiving standard care (maintenance intravenous therapy only) also had a lower risk of infection than those receiving TPN. However, in patients who were malnourished, mortality and risk of infection were higher in the standard care group than in the TPN group. The overall best results were noted in patients receiving EN.
In their meta-analysis, Lewis and associates27 used data from 11 randomized trials involving 837 general surgery patients and concluded that early enteral feeding reduced infection rates (RR 0.72, CI 0.54–0.98), hospital length of stay and mortality. Interestingly, anastomotic dehiscence rates were also diminished (RR 0.53, CI 0.26–1.08). Although the analysis was limited by the methodologic quality of the component trials, its findings pertaining to intestinal anastomoses appear to be supported by other studies. Braga and associates28 noted improved gut oxygen tension in enterally fed patients who underwent resection for cancer. Experimental studies have shown that early postoperative feeding actually increases intestinal anastomotic strength, especially when there is coexisting sepsis. Enteral feedings had a significant attenuating effect on TNF-α production, which correlated with improved healing at the anastomosis.29
Clinical studies of pancreatitis also appear to support the notion that EN is safe. Windsor and colleagues30 documented better APACHE and systemic inflammatory response scores in EN patients compared with TPN controls. Although Powell and colleagues31 did not observe a change in levels of inflammatory mediators or intestinal permeability in EN patients in a similar randomized trial of 27 patients, the control group in their study consisted of fasting patients rather than TPN patients. McClave and colleagues32 randomized 30 patients to EN or TPN and observed that EN patients met 71% of their estimated caloric requirements. Endpoint differences, apart from cost, were not statistically significant. Kalfarentzos and associates,33 in a randomized trial involving 38 patients with pancreatitis, compared the effects of semi-elemental nasoenteric feeding and TPN. Interestingly, they found that nitrogen balance was similar between groups but that patients fed enterally had fewer septic and total complications.
The effects of immunonutrition (enteral feeding supplemented with compounds thought to have immune-enhancing properties: glutamine, arginine, omega-3 fatty acids, nucleotides) compared to standard EN were evaluated in a meta-analysis of 22 randomized trials with a total of 2419 surgical patients and patients in intensive care.34 Overall, immunonutrition was associated with an increased risk of mortality (RR 1.10, CI 0.93–1.31) but a lower risk of infection (RR 0.66, CI 0.54 – 0.80). Subgroup analysis demonstrated that commercial formulas of high arginine content were associated with a significant reduction in infectious complications and a trend to a lower mortality in comparison with other immune-enhancing diets. Surgical patients on the immune modulating formulas showed a significant reduction in infectious complications, but this effect was not seen in critically ill patients, perhaps because of the interaction of these formulas with an already immunostimulated environment. The authors concluded that immunonutrition may decrease infectious complications but is not associated with a significant survival advantage. Surgical patients, however, demonstrated some benefit. This last observation emphasizes the importance of understanding the effects of the surgical and multiorgan failure paradigms as outlined in Fig. 1 and Fig. 2.
Limitations of enteral nutrition
Although we have presented some persuasive basic and clinical data on the benefits of EN compared with TPN, the choice between these modalities may, at times, be unclear. The adequacy of nutrition support with EN is often questionable. A recent quality improvement and related review at our institution, revealed that patients in the multidisciplinary intensive-care unit (ICU) received only 56% of their goal caloric requirements via EN during the course of their stay; energy intake improved to 83% of goal, however, after the implementation of a rigorous nutrition support protocol. Similar figures have been reported by other investigators who have noted that physicians underprescribe EN (on average 65.6% of goal) and that of this only about 78% is actually delivered. It is no surprise then, that the majority of enterally fed patients in the ICU actually lose weight.35 Underfeeding has primarily been attributed to gut dysfunction and elective stoppage of feeds.36 Fortunately, it appears that the creation of nutrition support protocols guiding initiation, advancement and stoppage criteria for EN, improves delivery.36,37
Intolerance to EN appears to have clinical significance. In a prospective cohort study of 153 patients, Mentec and colleagues38 observed that feeding intolerance, indicated by high gastric residual volumes, occurred in 46% of patients. Use of sedation and administration of catecholamines were significant predictors of intolerance. Intolerance of EN may have been a marker of severe illness: intolerant subjects received less nutrition, had higher rates of vomiting and pneumonia, had increased length of hospital stay and increased adjusted death rates.
Enteral feeding is frequently associated with access and infusion- related complications. In most ICUs, feeding is initiated nasogastrically or orogastrically. Nasogastric tubes are associated with sinusitis and may thereby predispose patients to pneumonia. Before EN infusion by either of these routes, tube position must be confirmed radiographically or by aspiration of gastric contents to avoid the risk of tube aspiration. Feeding via these routes may be complicated by tube dislodgement (45%) and clogging (12%).39 Percutaneous endoscopic gastrostomy, the preferred definitive access modality, has resulted in minor and major complications in 13% and 18% of patients, respectively.40,41 Post-pyloric or jejunal placement of feeding tubes may bypass gastric ileus and promote increased tube feed delivery but has not definitively been shown to prevent aspiration-related pulmonary complications.42 The North American Summit on Aspiration in the Critically Ill Patient published a consensus statement that included recommendations for minimizing aspiration risk, such as elevating the head of the bed more than 30°–45° and regular assessment of tolerance and tube placement.43
Diarrhea is a common problem causing cessation of tube feeding and occurs in up to 68% of patients in the ICU.44 Although frequently attributed to the osmotic effect of tube feeding, it may be multifactorial (antimicrobial therapy, bacterial overgrowth secondary to acid suppression, or fat or carbohydrate malabsorption). The initial approach to this problem is often to adjust the feeding regimen (rate, volume, osmolality, feeding solution) in a systematic fashion. Continuous feeding may be preferable to bolus feeding. Some studies have suggested a beneficial effect of soluble fibre and pectin supplementation of tube feeds,45 the use of semi-elemental feeding46 and the preventive effect of Saccharomyces boulardii 47 solutions on the frequency and severity of diarrhea related to tube feeding. When infectious causes of diarrhea are excluded, persistent feeding-associated diarrhea can be treated cautiously with anti-diarrheal agents. Of note is that antibiotic-associated diarrhea occurs in 5%–20% of patients receiving antibiotics.48 An aggressive approach to diagnosis and treatment is required to avoid its progression to a more fulminant condition. The Infectious Diseases Society of America advocates enzyme immunoassay or cytotoxin assay to diagnose the presence of Clostridium difficile in patients suspected of antibiotic-associated diarrhea. Metronidazole, 500 mg orally 3 times a day or 250 mg 4 times a day, for 10 days, is the preferred therapy.
Bowel obstruction, secondary to aggressive EN supplemented with fibre, has been reported. This unusual complication must be suspected in patients who are unable to tolerate enteral feeding. High-grade small-bowel obstruction should routinely be excluded when patients are intolerant of EN.49 When splanchnic perfusion is severely compromised, feeding, which may increase intestinal metabolic demand, poses a theoretical risk of intestinal ischemia. Non-occlusive bowel necrosis, which has an estimated incidence of less than 0.5% of patients in the ICU, may initially present with highly nonspecific findings in patients who had previously been tolerating EN. The most common findings in a series of 13 cases were tachycardia, fever, abnormal leukocyte count and abdominal distension. Survival depends on early recognition and definitive surgical therapy, and in that series was 56%.50
A role for total parenteral nutrition
TPN has been de-emphasized recently because of its association with perioperative septic complications. However, it does provide a reliable means to deliver protein and energy substrates and to correct electrolyte and vitamin deficiencies. It has been shown to confer a survival advantage in malnourished ICU and surgical patients over standard care (intravenous fluids and oral diet as tolerated). Much of the negative opinion surrounding TPN is related to its association with high rates of septic complications. Although we have presented an immunologic rationale for this observation, TPN-induced hyperglycemia may have also contributed to the sepsis rates observed in previous studies; aggressive control of hyperglycemia may diminish TPN-related complications. In fact, intensive insulin therapy (maintaining blood glucose levels between 80 and 110 g/L) was found in 1 trial to reduce mortality, primarily from multiorgan failure. This effect was most pronounced in patients admitted for longer than 5 days. Bloodstream infections were also noted to be substantially lower when this strategy was employed.51
Conclusions
Basic science and clinical evidence strongly support the use of EN in critically ill trauma and surgical patients. EN is relatively inexpensive, allows for more efficient use of exogenous substrates, promotes gut immunity and prevents septic complications, and is an effective therapy of hypermetabolism-induced protein – calorie malnutrition.
However, EN may have shortcomings, including underfeeding, perceived intolerance, aspiration, access-related complications and diarrhea, to name a few. Clearly standardized approaches to feeding and avoidance of complications (i.e., liberal use of prokinetic agents to improve tolerance or frequent assessment of mental status and elevation of the head of the bed to reduce aspiration risk) could increase the success of this feeding strategy. Furthermore, sensible nutritional protocols must offer TPN as an alternative or complementary strategy where EN is contraindicated or inadequate to maintain nutritional status. Future basic scientific and clinical insights will likely continue to refine the indications for different nutritional therapies and add exciting modalities to our evolving protocols.
Acknowledgments
We thank the Alberta Children's Hospital Research Foundation for laboratory support and Gail Wright-Wilson for secretarial support.
Competing interests: None declared.
Correspondence to: Dr. S. Morad Hameed, Departments of Surgery and Critical Care, Foothills Medical Centre, 1403–29 St. NW, Calgary AB T2N 2T9; fax 403 283-9994; morad.hameed@calgaryhealthregion.ca
Accepted for publication Sept. 26, 2003.
References
- 1.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma: a prospective, randomized study. J Trauma 1986;26:874-81. [DOI] [PubMed]
- 2.Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma — reduced septic morbidity. J Trauma 1989;29:916-23. [DOI] [PubMed]
- 3.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 1992;215:503-13. [DOI] [PMC free article] [PubMed]
- 4.Hall JC, Heel K, McCauley R. Glutamine. Br J Surg 1996;83:305-12. [DOI] [PubMed]
- 5.Kudsk KA. Catabolic states and immune dysfunction: relation to gastrointestinal feeding [review]. Nestle Nutr Workshop Ser Clin Perform Programme 2000;3:157-69. [DOI] [PubMed]
- 6.Neutra MR, Forstner JF. Gastrointestinal mucus: synthesis, secretion, and function. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 2nd ed. New York: Raven Press; 1987. p. 975-1010.
- 7.Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature 2002;417:552-5. [DOI] [PubMed]
- 8.Zarzaur BL, Kudsk KA. The mucosa- associated lymphoid tissue structure, function, and derangements [review]. Shock 2001;15:411-20. [DOI] [PubMed]
- 9.Schneider SM, Le Gall P, Girard-Pipau F, Piche T, Pompei A, Nano JL, et al. Total artificial nutrition is associated with major changes in the fecal flora. Eur J Nutr 2000;39:248-55. [DOI] [PubMed]
- 10.Pappenheimer JR. Paracellular intestinal absorption of glucose, creatinine, and mannitol in normal animals: relation to body size. Am J Physiol 1990;259:G290-9. [DOI] [PubMed]
- 11.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma 1995;39:44-52. [DOI] [PubMed]
- 12.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg 1999;229:662-7. [DOI] [PMC free article] [PubMed]
- 13.Janu P, Li J, Reneger KB, Kudsk KA. Recovery of gut-associated lymphoid tissue and upper respiratory tract immunity after parenteral nutrition. Ann Surg 1997;225:707-17. [DOI] [PMC free article] [PubMed]
- 14.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg 1996;223:629-38. [DOI] [PMC free article] [PubMed]
- 15.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut- associated lymphoid tissue and mucosal immunity. Arch Surg 1997;132:1303-9. [DOI] [PubMed]
- 16.Kudsk KA, Stone JM, Carpenter G, Sheldon GF. Enteral and parenteral feeding influences mortality after hemoglobin–E. coli peritonitis in normal rats. J Trauma 1983;23:605-9. [DOI] [PubMed]
- 17.Hanna MK, Kudsk KA. Nutritional and pharmacological enhancement of gut- associated lymphoid tissue. Can J Gastroenterol 2000;14:145D-151D. [DOI] [PubMed]
- 18.Johnson CD, Kudsk KA. Nutrition and intestinal mucosal immunity. Clin Nutr 1999;18:337-44. [DOI] [PubMed]
- 19.Burke DJ, Alverdy JC, Aoys E, Moss GS. Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg 1989;124:1396-9. [DOI] [PubMed]
- 20.Manhart N, Vierlinger K, Spittler A, Bergmeister H, Sautner T, Roth E. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer's patches in endotoxemic mice. Ann Surg 2001;234:92-7. [DOI] [PMC free article] [PubMed]
- 21.DeWitt RC, Wu Y, Renegar KB, King BK, Li J, Kudsk KA. Bombesin recovers gut-associated lymphoid tissue and preserves immunity to bacterial pneumonia in mice receiving total parenteral nutrition. Ann Surg 2000;231:1-8. [DOI] [PMC free article] [PubMed]
- 22.Fukatsu K, Lundberg AH, Kudsk KA, Hanna MK, Johnson CD, Wu Y, et al. Modulation of organ ICAM-1 expression during IV-TPN with glutamine and bombesin. Shock 2001;15:24-8. [DOI] [PubMed]
- 23.Chance WT, Sheriff S, Foley-Nelson T, Thomas I, Balasubramaniam A. Maintaining gut integrity during parenteral nutrition of tumor-bearing rats: effects of glucagon-like peptide 2. Nutr Cancer 2000;37:215-22. [DOI] [PubMed]
- 24.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient- induced glucagon-like peptid-1 secretion. Endocrinology 1999;140:1687-94. [DOI] [PubMed]
- 25.Heyland DK, MacDonald S, Keefe L, Drover JW. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA 1998;80:2013-9. [DOI] [PubMed]
- 26.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr 2001;74:534-42. [DOI] [PubMed]
- 27.Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ 2001;323:773-6. [DOI] [PMC free article] [PubMed]
- 28.Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med 2001;29:242-8. [DOI] [PubMed]
- 29.Khalili TM, Navarro RA, Middleton Y, Margulies DR. Early postoperative enteral feeding increases anastomotic strength in a peritonitis model. Am J Surg 2001;182:621-4. [DOI] [PubMed]
- 30.Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut 1998;42:431-5. [DOI] [PMC free article] [PubMed]
- 31.Powell JJ, Murchison JT, Fearon KC, Ross JA, Siriwardena AK. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. Br J Surg 2000;87:1375-81. [DOI] [PubMed]
- 32.McClave SA, Greene LM, Snider HL, Makk LJ, Cheadle WG, Owens NA, et al. Comparison of the safety of early enteral vs parenteral nutrition in mild acute pancreatitis. JPEN J Parenter Enteral Nutr 1997;21:14-20. [DOI] [PubMed]
- 33.Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg 1997;84:1665-9. [PubMed]
- 34.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001;286:944-53. [DOI] [PubMed]
- 35.McClave SA, Sexton LK, Spain DA, Adams JL, Owens NA, Sullins MB, et al. Enteral tube feeding in the intensive care unit: factors impeding adequate delivery. Crit Care Med 1999;27:1252-6. [DOI] [PubMed]
- 36.Adam S, Batson S. A study of problems associated with the delivery of enteral feed in critically ill patients in five ICUs in the UK. Intensive Care Med 1997;23:261-6. [DOI] [PubMed]
- 37.Spain DA, McClave SA, Sexton LK, Adams JL, Blanford BS, Sullins ME, et al. Infusion protocol improves delivery of enteral tube feeding in the critical care unit. JPEN J Parenter Enteral Nutr 1999;23:288-92. [DOI] [PubMed]
- 38.Mentec H, Dupont H, Bocchetti M, Cani P, Ponche F, Bleichner G. Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit Care Med 2001;29:1955-61. [DOI] [PubMed]
- 39.Pancorbo-Hidalgo PL, Garcia-Fernandez FP, Ramirez-Perez C. Complications associated with enteral nutrition by nasogastric tube in an internal medicine unit. J Clin Nurs 2001;10:482-90. [DOI] [PubMed]
- 40.Eleftheriadis E, Kotzampassi K. Percutaneous endoscopic gastrostomy after abdominal surgery. Surg Endosc 2001;15:213-6. [DOI] [PubMed]
- 41.Schurink CA, Tuynman H, Scholten P, Arjaans W, Klinkenberg-Knol EC, Meuwissen SG, et al. Percutaneous endoscopic gastrostomy: complications and suggestions to avoid them. Eur J Gastroenterol Hepatol 2001;13:819-23. [DOI] [PubMed]
- 42.Kortbeek JB, Haigh PI, Doig C. Duodenal versus gastric feeding in ventilated blunt trauma patients: a randomized controlled trial. J Trauma 1999;46:992-6. [DOI] [PubMed]
- 43.McClave SA, DeMeo MT, DeLegge MH, DiSario JA, Heyland DK, Maloney JP, et al. North American Summit on Aspiration in the Critically Ill Patient: consensus statement [review]. JPEN J Parenter Enteral Nutr 2002;26(6 Suppl):S80-5. [DOI] [PubMed]
- 44.Dobb GJ, Towler SC. Diarrhoea during enteral feeding in the critically ill: a comparison of feeds with and without fibre. Intensive Care Med 1990;16:252-5. [DOI] [PubMed]
- 45.Schultz AA, Ashby-hughes B, Taylor R, Gillis DE, Wilkins M. Effects of pectin on diarrhea in critically ill tube-fed patients receiving antibiotics. Am J Crit Care 2000;9:403-11. [PubMed]
- 46.Meredith JW, Ditesheim JA, Zaloga GP. Visceral protein levels in trauma patients are greater with peptide diet than with intact protein diet. J Trauma 1990;30:825-9. [DOI] [PubMed]
- 47.Bleichner G, Blehant H, Mentec H, Moyse D. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients: a multi-centre, randomized, double-blind placebo-controlled trial. Intensive Care Med 1997;23:517-23. [DOI] [PubMed]
- 48.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002;346:334-7. [DOI] [PubMed]
- 49.Scaife CL, Saffle JR, Morris SE. Intestinal obstruction secondary to enteral feedings in burn trauma patients. J Trauma 1999;47:859-63. [DOI] [PubMed]
- 50.Marvin RG, McKinley BA, McQuiggan M, Cocanour CS, Moore FA. Non-occlusive bowel necrosis occurring in critically ill trauma patients receiving enteral nutrition manifests no reliable clinical signs for early detection. Am J Surg 2000;179:7-12. [DOI] [PubMed]
- 51.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001;345:1359-67. [DOI] [PubMed]


