Abstract
Total mesorectal excision (TME) is a precise dissection of the rectum and all pararectal lymph nodes within an oncologic package: the mesorectal envelope. This article is a brief description of the technical aspects of the dissection, illustrated by cadaver dissection. Here the TME dissection is organized into 6 steps to facilitate learning: (1) left retroperitoneum; (2) superior rectal and inferior mesenteric vessels; (3) upper mesorectum; (4/5) right and left mid-mesorectum; and (6) distal mesorectum and anorectal junction. The relationship of the autonomic nerves, blood vessels and adjacent organ structures are described at each step. The 6 steps are recommended for learning and performing TME dissection in cadavers and patients.
Abstract
L'excision mésorectale totale (EMT) est une dissection du rectum et de tous les ganglions lymphatiques pararectaux dans un ensemble oncologique : l'enveloppe mésorectale. Cet article décrit brièvement les aspects techniques de la dissection, illustrés par une dissection de cadavre. La dissection EMT est subdivisée en 6 étapes de façon à faciliter l'apprentissage : (1) rétropéritoine gauche; (2) vaisseaux rectaux supérieurs et mésentériques inférieurs; (3) mésorectum supérieur; (4/5) mésorectum intermédiaire droit et gauche; (6) mésorectum distal et jonction anorectale. On décrit à chaque étape le lien entre les nerfs autonomes, les vaisseaux sanguins et les structures des organes adjacents. On recommande la démarche en 6 étapes pour apprendre la dissection EMT et la pratiquer sur des cadavres et des patients.
Total mesorectal excision (TME) is becoming accepted universally as the preferred technique for surgical excision of rectal cancer. TME results in the lowest rates of local recurrence, especially when combined with preoperative radiation treatment.1,2,3,4 A second benefit of TME is increased sparing of the anal sphincter, with a concomitant decrease in permanent stomas from abdominoperineal resection (APR).5 The third benefit is that TME is a nerve-sparing dissection, less likely to lead to bladder and sexual dysfunction.6,7 This article is a brief discussion of the technical aspects of TME, illustrated by photographs of cadaver dissection and a surgical TME specimen.
TME is “specimen-oriented” surgery: completeness of the mesorectal excision predicts local recurrence and survival.4,8,9 A simple analogy may be excision of a sebaceous cyst: recurrence is more likely if the cyst wall is incompletely excised or the cyst is perforated. The key technical points of TME all contribute to the excision of the entire rectum with blood vessels and surrounding lymph nodes within a visceral fascial envelope that is still intact. Careful and fastidious attention to preserving the integrity of the mesorectal fascial envelope, associated with a clear circumferential (radial) margin, is the essence of minimizing pelvic recurrence. Included in TME is en bloc resection of lymph nodes along the superior rectal and inferior mesenteric arteries. All pararectal lymph nodes are removed in this dissection.
The proximal resection bowel margin ought to be at least 5 cm — often longer — and is usually determined by choosing healthy sigmoid or descending colon for a low anastomosis. The distal resection margin is past the distal edge of the mesorectum; it is identified by incising Waldeyer's fascia, which reflects from the levators at the pelvic floor and covers the anorectal junction. When the anorectal junction is uncovered, the distal edge of the mesorectum can be identified, and then the bared distal rectal muscular tube and puborectalis sling.
TME increases sphincter-sparing resection and decreases permanent stoma rates from APR. Because TME results in dissection to the anorectal junction, tumours of the distal rectum not invading the anal sphincter can be resected while sparing the anal sphincter. The bared distal rectal tube at the anorectal junction is further dissected in the upper intersphincteric plane in order to achieve a 1-cm distal resection margin. Reconstruction at this level necessitates making a coloanal anastomosis.
TME is a nerve-sparing dissection. The pelvic autonomic nerves are identified and preserved (Fig. 1). The sympathetic splanchnic nerves pass fibres distally via preaortic nerve branches; preaortic nerves then run in fat within the bifurcation of the aorta to form the superior hypogastric plexus found near the sacral promontory. The superior hypogastric plexus gives rise to the right and left hypogastric nerves, which are found along the posterolateral pelvic brim just below the course of the ureters. The hypogastric nerves lie in the areolar tissue plane between the visceral mesorectal fascia and the endopelvic parietal fascia. The plane between visceral and endopelvic fasciae is very thin at the level of the pelvic brim such that the hypogastric nerves seem adherent to the visceral mesorectal fascia. The endopelvic parietal fascia covers presacral vessels, internal iliac blood vessels and lymphatics, posterolateral endopelvic muscles (piriformis) and the levators (iliococcygeus, pubococcygeus and puborectalis). The parasympathetic nervi erigentes are anterior branches of the S2–4 nerve roots; the nervi erigentes are covered by endopelvic parietal fascia and meet the hypogastric nerves in the right and left inferior hypogastric plexus. Rectal branches of the inferior hypogastric plexus traverse the plane between the endopelvic parietal fascia and the visceral mesorectal fascia as the “lateral stalks” of the rectum. At this location again there is minimal areolar tissue between visceral and parietal pelvic fascia, making dissection of an intact mesorectal fascial envelope laterally and anteriorly in the mid- and distal pelvis difficult. Terminal branches of the middle hemorrhoidal vessels accompany the rectal nerve branches in the lateral stalks. The blood vessels of the lateral stalks are mostly small but occasionally large enough to require ligation. Genitourinary branches of the inferior hypogastric plexus pass beneath the anterolateral endopelvic parietal fascia to the bladder and either the prostate and seminal vesicles or the vagina and uterus.
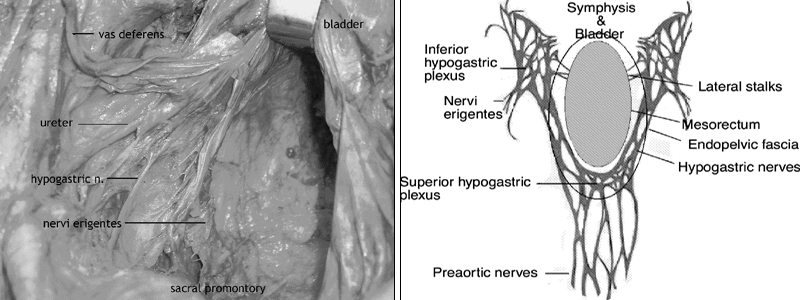
FIG. 1. Left, cadaver dissection: the left-lateral pelvic sidewall showing, from above to below, the vas deferens, ureter, hypogastric nerve and nervi erigentes. At the top of the picture the retractor is holding the seminal vesicles and bladder. Right, a schematic of autonomic nerve anatomy in the pelvis, in relation to the mesorectum and endopelvic fascia.
With this article I propose TME as a dissection of 6 steps. The steps organize a sequence of techniques to remove the rectum, from a proximal vascular division to a distal resection at the anorectal junction. The plan facilitates instruction by considering anatomic details at the location of each step. Photographs of a cadaver dissection illustrate the surgical procedure.
Step 1
The left retroperitoneum is dissected by incising the retroperitoneal reflection of Toldt and mobilizing the sigmoid colon, left colon and rectosigmoid junction. The splenic flexure is taken down if the distal descending or proximal sigmoid colon is to be used for the low anastomosis. (If free of multiple diverticula and stenosis, the distal sigmoid colon can be used instead.) Identified and preserved are the gonadal vessels and ureter as the mesocolon is mobilized medially from the retroperitoneum (Fig. 2). The iliac vessels are left undisturbed. The plane beneath the fascia covering the superior rectal and inferior mesenteric vessels (mesosigmoid) is dissected, leaving intact the preaortic nerves and the superior hypogastric plexus lying on the retroperitoneum covering the distal aorta and between the common iliac vessels just above the sacral promontory. Fascia covering the superior rectal and inferior mesenteric vessels (mesosigmoid) is continuous with the visceral mesorectal fascial envelope and is resected en bloc with the mesorectum.
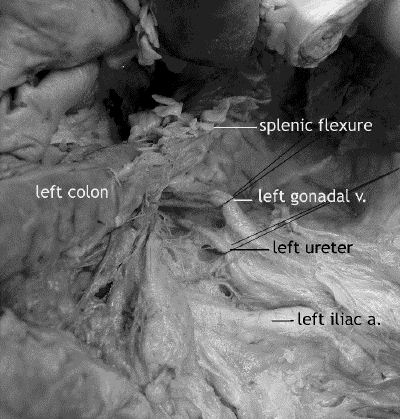
FIG. 2. Cadaver dissection: the left retroperitoneum showing the testicular artery and ureter. The left colon has been reflected from the retroperitoneum. The splenic flexure is at the top of the picture.
Step 2
After dissecting the left retroperitoneum, the second step is to complete the dissection of the superior rectal and inferior mesenteric vessels by incising the retroperitoneum to the right of these vessels (Fig. 3). Dissection of the superior rectal and inferior mesenteric vessels can also be done from right to left with the incision of the retroperitoneum to the right of these vessels followed by that to the left. The right-to-left approach is more often used during laparoscopic dissection.
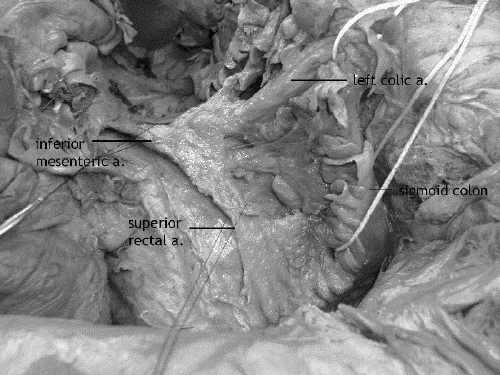
FIG. 3. Cadaver dissection: the inferior mesenteric artery and branches. The splenic flexure of the colon is at the top of the picture.
In the absence of palpable proximal mesenteric lymph nodes, the inferior mesenteric vessels are divided just below the left colic branch if the distal sigmoid is to be used for the low anastomosis, or above the left colic branch if the distal descending or proximal sigmoid colon is to be used. A proximal resection margin is chosen in suitable healthy sigmoid or descending colon; the marginal artery and vein are dissected and divided at the proximal resection margin. Sigmoid vessels are divided at midpoint to leave the lymph nodes surrounding the superior rectal and inferior mesenteric vessels for the en bloc resection along with the mesorectal specimen, an act that also preserves blood flow along the marginal vessels to the proximal resection margin. Once the adequacy of this blood supply is assured, the bowel is divided between 2 clamps or with a linear cutting stapler.
Step 3
The third step, dissection of the upper mesorectum, begins with identification of the right and left hypogastric nerves along the posterolateral pelvic brim (Fig. 4), followed by posterior dissection down to the pelvic floor.
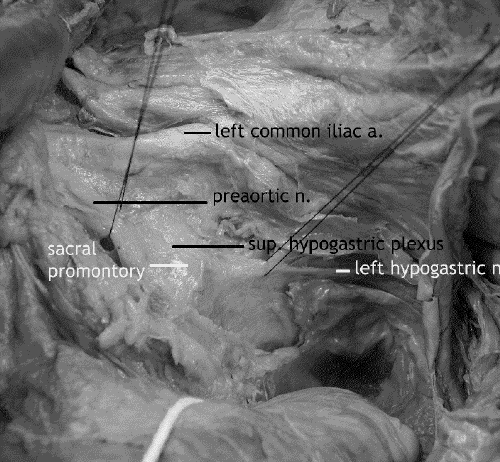
FIG. 4. Cadaver dissection: the preaortic superior hypogastric nerve plexus and left hypogastric nerve. The aortic bifurcation is at the top of the picture.
Once identified, the posterior and lateral mesorectal fascia are preserved by division of the flimsy areolar tissue in the plane between the visceral mesorectal fascia and the parietal endopelvic fascia. The hypogastric nerves adhere to the mesorectal fascia and may be injured if care is not taken to identify them at this point. The upper posterolateral mesorectal fascia must be dissected carefully to preserve and free the right and left hypogastric nerves from the mesorectal specimen. The endopelvic fascia is left intact, covering presacral vessels below the sacral promontory in the median plane and laterally covering internal iliac vessels and endopelvic muscles, piriformis.
At the level of S2–4 there may be a condensation of the areolar tissue called the rectosacral fascia, which will be incised to mobilize the posterior mesorectum from the posterolateral endopelvic parietal fascia. If the median endopelvic fascia is kept intact, the presacral periosteal fascia on the sacrum will not be seen and bleeding from presacral and internal iliac blood vessels is avoided.
The nervi erigentes are not viewed during this dissection as they are covered by the posterolateral endopelvic parietal fascia (Fig. 5). However, location of the nervi erigentes behind the endopelvic fascia may be indicated by a gentle upward (anterior) tugging of the hypogastric nerves, giving the impression of vertical pillars laterally on the pelvic sidewall from the sacrum to the junction of the hypogastric nerves and inferior hypogastric plexus. Dissection too wide of the endopelvic fascia laterally can result in injury of the nervi erigentes.
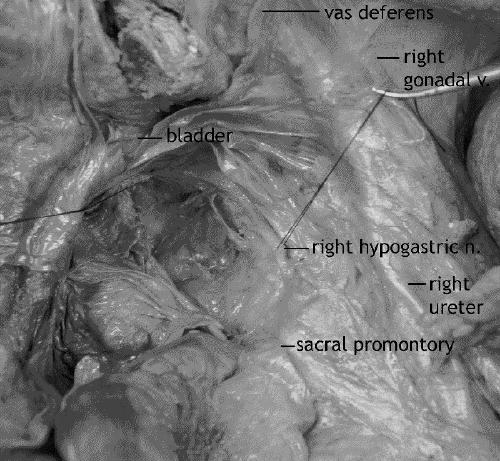
FIG. 5. Cadaver dissection: the right nervi erigentes, behind the endopelvic fascia. The retractor is holding the seminal vesicles.
Preoperative computed tomographic and magnetic resonance imaging provide important information about the closest radial margin from tumour or lymph nodes to the nearest point of the mesorectal fascia. If tumour or lymph node metastases abut onto the mesorectal fascia, wider radial clearance is obtained by dissection wide to the endopelvic fascia with en bloc resection of a cuff of adherent endopelvic fascia.
If possible during this step, the posterior mesorectum is dissected past the tip of the coccyx as the pelvic floor curves upward anteriorly toward the anorectal junction. Complete posterior and posterolateral dissection to the pelvic floor greatly facilitates the following anterolateral dissection and the identification of the lateral stalks.
Steps 4 and 5
Dissection of the right and left midmesorectum laterally and anteriorly (including the cul-de-sac and lateral stalks) constitutes the fourth and fifth steps, respectively. Heald's group3 now approaches the lateral stalks from posterior to anterior, preserving the lateral mesorectal fascia and dividing the lateral stalks with cautery (Fig. 6). Instead of incising the cul-de-sac peritoneum, they dissect a cuff of it off the seminal vesicles or upper vagina en bloc with the mesorectum. Others, including Cohen10 and Enker,11 incise the cul-de-sac peritoneum first, along with dissection of Denonvilliers' fascia and anterior mesorectum, before formally taking the lateral stalks with ligation or vascular linear cutting staplers.
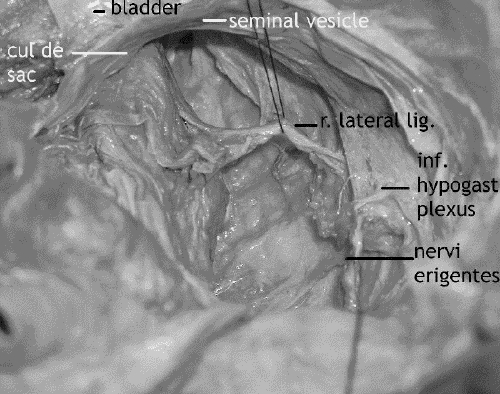
FIG. 6. Cadaver dissection: the right lateral stalk. The seminal vesicles are at the top of the picture.
Most of the time the terminal branches of the middle hemorrhoidal vessels crossing from behind the endopelvic fascia to the mesorectum in the lateral stalks are small enough for cautery to be brief. Occasionally a branch is large enough that ligation is preferred in order to avoid troublesome bleeding and prolonged cautery that could injure the inferior hypogastric nerves.
To date there is no published study of nerve injury in relation to quantity (amperage and duration) of cautery. Division of the lateral stalks at the lateral border of the mesorectal fascia will help to avoid “tenting” and injury to the inferior hypogastric plexus.
Step 6
The last step is dissection of the distal mesorectum and anorectal junction (Fig. 7). With division of the lateral stalks at midmesorectum, continued dissection of the posterior and lateral mesorectum will expose endopelvic parietal fascia covering the midline anococcygeal raphe, levators posterolaterally and Waldeyer's fascia. Again, Waldeyer's is the endopelvic parietal fascia reflecting from levators covering the distal rectal tube and anorectal junction. Branches of the middle hemorrhoidal vessels may be encountered emerging from the distal endopelvic fascia covering the anococcygeal raphe and levators.
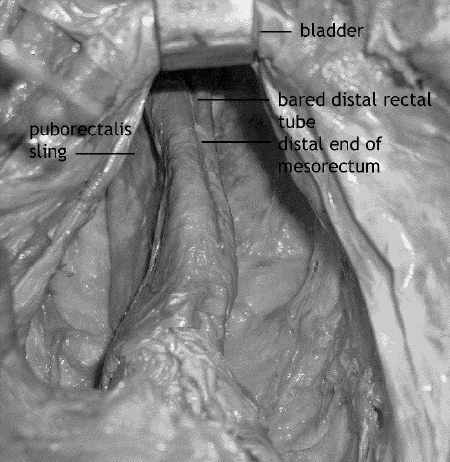
FIG. 7. Cadaver dissection: the complete mesorectal dissection and bared distal rectal tube. The retractor is holding the seminal vesicles.
The anterior rectoprostatic or rectovaginal plane is dissected carefully, preserving the anterior mesorectal fascia and posterior capsule of the prostate or posterior vaginal wall. Small rectoprostatic or rectovaginal blood vessels encountered during this distal anterior dissection are usually sealed with cautery; avoid, however, prolonged cautery on the posterior vaginal wall that could contribute to an anastomotic rectovaginal fistula. Lengthy cautery on the posterior prostate can also injure terminal nerve branches to the prostate and urethra.
At the anorectal junction, Waldeyer's fascia is incised posteriorly and laterally to expose the bared rectal tube past the distal edge of the mesorectum and puborectalis sling. Continued distal anterior rectoprostatic or rectovaginal dissection past the anterior mesorectum will expose the bared rectal tube anteriorly at the anorectal junction. The upper anal sphincter is deficient anteriorly due to the anterior absence of the puborectalis sling.
Variations for tumour locations
For a midrectal cancer the distal resection margin is the distal edge of the mesorectum, with a distal margin of at least 2 cm below the tumour.
For a distal rectal cancer the distal resection margin is the bared rectal tube 1 cm below the tumour.12
For a distal rectal cancer not invading the sphincters, dissection into the intersphincteric plane is sometimes needed to acquire a 1 cm distal margin. This dissection extends in the plane of the bared rectal tube beneath Waldeyer's and within the upper external anal sphincter formed by the puborectalis sling. In females, who have a short anal canal, the anterior circular fibres of the external sphincter may be revealed with further dissection in the anterior intersphincteric plane.
Distal rectal cancer invading the sphincters can be assessed with MRI, endorectal ultrasound and clinical examination under anesthesia. In such cases, Waldeyer's covering the anorectal junction should not be incised to expose the bared distal rectal tube. Instead, an abdominoperineal resection is performed by taking a cuff of puborectalis and its covering endopelvic parietal fascia, resected as the circumferential margin of the anorectal junction en bloc with ischiorectal fossa fat, external sphincters and perianal skin (TME plus APR). On occasion when there is minimal invasion of the puborectalis, a clear distal margin can be taken by resecting the puborectalis while sparing the distal half of the anal canal and the external anal sphincter.
An anterior tumour location is worrying for at least three reasons, first because of the adjacency of the anterior viscera and thinness of the anterior mesorectum. Clinical examination under anesthesia, endorectal ultrasound and MRI are useful to determine whether there is an anterior fat plane that will permit an anterior resection margin thick enough to test negative. If tumour invades the anterior viscera, en bloc resection is required for curative resection: TME plus en bloc partial or complete resection of anterior viscera such as posterior wall of the vagina or possibly pelvic exenteration for en bloc resection of rectal cancer invading the bladder, seminal vesicle and prostate.
A second concern with anterior location of a tumour is an increased risk of “fracturing” of the tumour during dissection. Particular care is needed to avoid tearing and fracturing of the anterior mesorectal fascia and an anteriorly located tumour.
A third worry is that the terminal genitourinary parasympathetic and sympathetic nerve branches residing in the prostate can be injured during dissection of the anterior mesorectum, particularly if the posterior prostatic capsule is breached (especially at the posterolateral capsule).
Consideration should be given to spillage of tumour cells during a distal resection. Many clinical and experimental reports show that viable tumour cells can be found in the lumen of the rectum and anal canal distal to the tumour. To minimize the potential for implantation of these intraluminal cancer cells that could potentially cause recurrent cancer, Moran and colleagues13 have advocated the placement of 2 sequential clamps or staple lines with interval intraluminal washout using a tumouricidal solution. The first clamp or staple line is placed distal to the tumour. The anorectum is irrigated with a tumouricidal or antiseptic solution such as water, betadine or chlorhexidine. After the intraluminal washout, the second clamp or staple line is then placed on the true distal resection margin, thus avoiding implantation of intraluminal cancer cells.
Quality of the TME specimen
Immediate feedback on the quality of the surgery is provided by inspection of the resected specimen for intactness of the fascial mesorectal envelope and distal margin (Fig. 8, Fig. 9). Quirke's group9 suggested 3 grades of mesorectal fascial envelope intactness that have been shown to correlate with the likelihood of local recurrence: poor quality, with deep clefts into the mesorectal fat that expose the bared muscularis of the rectal wall; intermediate quality, with merely superficial clefts into the mesorectal fat that do not expose the muscularis; and good quality, evincing a mesorectal fascial envelope that is intact circumferentially.
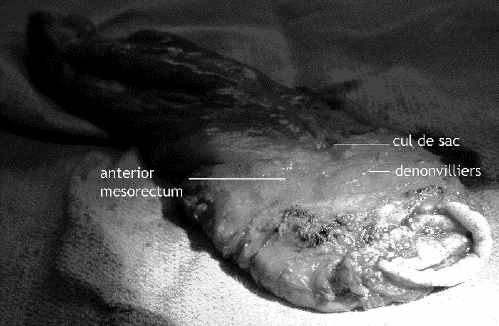
FIG. 8. The resected specimen, showing intact anterior and lateral mesorectal fascia with a cuff of cul-de-sac peritoneum.
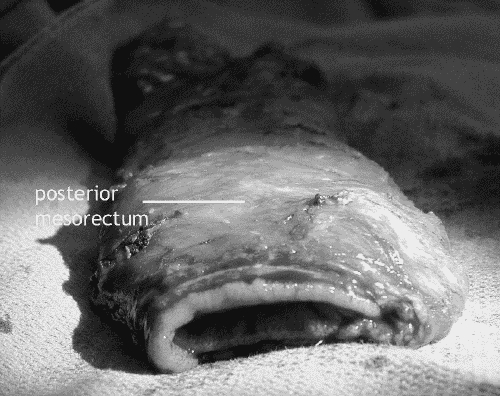
FIG. 9. The resected specimen, showing intact posterior mesorectal fascia covering the posterior mesorectum.
A specimen graded as poor quality is considered an incomplete mesorectal excision and is associated with a local recurrence rate of about 75%, which is similar to local recurrence rates when excised specimens are examined and found to test positive for microscopic cancerous tissue at the radial margins. Intermediate- and good-grade specimens can probably be considered complete mesorectal excisions, associated with a local recurrence rate under 30%, similar to that in patients whose excisions test negative at the radial margins. Furthermore, survival is predicted by intactness of the mesorectal fascial envelope. Patient-survival rates associated with complete mesorectal specimens are around 80%; with incomplete specimens, about 60%.
Minimizing functional problems
After TME, reconstruction requires a coloanal or very distal rectal anastomosis. Because of the loss of rectal reservoir function after TME, use of a colon pouch, coloplasty or end-to-side anastomosis should be considered. Compared to a straight coloanal anastomosis, which tends to produce 3 to 9 stools a day, an end-to-side anastomosis decreases urgency and frequency;14,15 a colon pouch averages 2–4 movements per day. A reservoir technique is therefore recommended for anastomoses within 5 cm of the anal verge. Note that when a colon pouch is longer than 6 cm, evacuations tend to be incomplete.
Stapled anastomosis is appropriate to the level of the anorectal junction. Care must be taken not to incorporate the puborectalis muscle or vagina in the stapler. An anastomosis to the dentate line, however, requires a transanal hand-sewn anastomosis.
Fecal incontinence after TME and coloanal reconstruction can result from loss of rectal reservoir function in the presence of poor anal sphincter function or diarrhea. Preoperative assessment of anal sphincter function by history and a digital assessment of anal tone are necessary predictors of postoperative continence. A preoperative history of fecal incontinence and poor anal sphincter tone are relative contraindications to coloanal reconstruction; in this situation, a colostomy should be considered for a better functional outcome.
Problems and controversies
The disadvantages of TME appear to be threefold. They include a difficult distal pelvic dissection because of the attention required to preserve the pelvic autonomic nerves and to maintain an intact mesorectal fascial envelope.
Because the mesorectal excision is so complete and leaves a “bared” rectal tube distal to the distal edge of the mesorectum, a second difficulty is a difficult low colorectal or coloanal anastomosis. This low anastomosis has a leak rate of about 15%– 20%, which is substantially higher than the 5%–10% leak rate for intraperitoneal colorectal anastomoses.16,17 It is therefore prudent to construct a temporary defunctioning ileostomy or right transverse colostomy to protect the patient from severe sepsis in the event of anastomotic leakage. Once the low anastomosis is proven to have healed, a subsequent surgery to close the defunctioning stoma will be required.
Despite the preservation in TME of the anal sphincter, a third disadvantage is the loss of rectal reservoir function, resulting in increased frequency and urgency and decreased ability to delay defecation, with possible fecal incontinence. Injury to the anal sphincter from radiation therapy will worsen problems of incontinence.
Whether TME is needed for upper rectal cancer is controversial. TME removes all pararectal lymph nodes within the mesorectum, including lymph nodes distal to the cancer. Pathologic examination of mesorectal specimens of upper rectal cancer have not shown metastases to lymph nodes in the mesorectum more than 5 cm distal to the cancer.18,19,20 Therefore a subtotal mesorectal excision with a distal 5-cm margin is likely sufficient to remove all pararectal lymph nodes with potential to contain metastases. In performing a subtotal mesorectal excision it is imperative to resect the distal mesorectal margin at right angles to the long axis of the rectum, so as not to leave behind lymph nodes with this potential; in other words, do not “cone” into the distal mesorectal resection margin.
In summary, TME is a specimen-oriented surgery that requires careful attention to identify and preserve intact the visceral mesorectal fascial envelope and pelvic autonomic nerves. Dissection of the mid- and distal rectum distal to the cul-de-sac is technically more difficult because of the decreasing dimensions of the distal pelvis and access impedance by the anterior pelvic viscera. Rectal cancers within 10 cm of the anus have higher local recurrence rates associated with the increased rate of incomplete TME-resected specimens.9,21 Precise dissection under direct vision is preferred over blunt separation of tissue planes to minimize incomplete TME specimens, bleeding and nerve injury.
Dissection distal to the cul-de-sac takes extra time and care. Although some surgeons dissect anteriorly early in the dissection of the midmesorectum, early posterior dissection to the pelvic floor greatly facilitates identification of the lateral stalks. In some cases where dissection of the mid- and distal mesorectum is made more difficult by increased adherence (e.g., in cases of inflammation or fibrosis), moving the dissection from the right side to the left and vice versa may facilitate the dissection. If the progress of dissection is particularly slow at any point, sometimes leaving that area and dissecting in another area such as the contralateral side of the mesorectum will permit improved distraction of tissue planes upon return to dissection of the original area of non-progress.
The 6 steps proposed for TME dissection clarify and organize how the surgery is conducted, and help to focus attention on the anatomic structures involved at each step. Experience of TME dissection in the cadaver is directly transferable to TME surgery in our patients. The 6 steps can be practised in cadavers as a particularly useful hands-on dissection of pelvic autonomic nerve anatomy.
Competing interests: None declared.
Correspondence to: Dr. P. Terry Phang, St. Paul's Hospital, 1081 Burrard St., Vancouver BC V6Z 1Y6; fax 604 806-8666; tphang@providencehealth.bc.ca
Accepted for publication Oct. 6, 2003
References
- 1.Kapiteijn E, Marijnen CAM, Nagtegall ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [DOI] [PubMed]
- 2.Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedermark B. Effect of a surgical training programme on outcome of rectal cancer in the county of Stockholm. Lancet 2000;356:93-6. [DOI] [PubMed]
- 3.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998;133:894-9. [DOI] [PubMed]
- 4.Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg 2002;89:327-34. [DOI] [PubMed]
- 5.Martling A, Cedermark B, Johansson H, Rutqvist LE, Holm T. The surgeon as a prognostic factor after the introduction of total mesorectal excision in the treatment of rectal cancer. Br J Surg 2002;89:1008-13. [DOI] [PubMed]
- 6.Havenga K, Maas CP, DeRuiter MC, Welvaart K, Trimbos JB. Avoiding long-term disturbance to bladder and sexual function in pelvic surgery particularly with rectal cancer. Semin Surg Oncol 2000;18:235-43. [DOI] [PubMed]
- 7.Sugihara K, Moriya Y, Akasu T, Fujita S. Pelvic autonomic nerve preservation for patients with rectal carcinoma. Oncologic and functional outcome. Cancer 1996;78: 1870-80. [PubMed]
- 8.Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 2002;235:449-57. [DOI] [PMC free article] [PubMed]
- 9.Nagtegaal ID, van de Velde CJH, van der Worp E, Kapiteijn E, Quirke P, van Krieken JHJM, Dutch Colorectal Cancer Group. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol 2002;20:1729-34. [DOI] [PubMed]
- 10.Cohen AM. Operations for colorectal cancer: low anterior resection. In: Zuidema GD, Yeo CJ, editors. Shackelford's surgery of the alimentary tract. Vol. 4. 5th ed. Philadelphia: W.B. Saunders; 2002. p. 245-60.
- 11.Enker WE. Total mesorectal excision with sphincter and autonomic nerve preservation in the treatment of rectal cancer. In: Condon R, editor. Current techniques in general surgery. New York: Mosby; 1996. Volume 5, issue 5: 1-8.
- 12.Karanjia ND, Schach DJ, North WRS, Heald RJ. Close shave in anterior resection. Br J Surg 1990;77:510-2. [DOI] [PubMed]
- 13.Moran BJ, Blenkinsop J, Finnis D. Local recurrence after anterior resection for rectal cancer using a double stapling technique. Br J Surg 1992;79:836-8. [DOI] [PubMed]
- 14.Sailer M, Fuchs KH, Fein M, Thiede. Randomized clinical trial comparing quality of life after straight and pouch coloanal reconstruction. Br J Surg 2002;89:1108-17. [DOI] [PubMed]
- 15.Williams N, Seow-Choen F. Physiological and functional outcome following ultra-low anterior resection with colon pouch–anal anastomosis. Br J Surg 1998;85:1029-35. [DOI] [PubMed]
- 16.Carlsen E, Schlichting E, Guldvog I, Johnson E, Heald RJ. Effect of the introduction of total mesorectal excision for the treatment of rectal cancer. Br J Surg 1998;85:526-9. [DOI] [PubMed]
- 17.Karanjia ND, Corder AP, Bearn P, Heald RJ. Leakage from stapled low anastomosis after total mesorectal for carcinoma of the rectum. Br J Surg 1994;81:1224-6. [DOI] [PubMed]
- 18.Hida J, Yasutomi M, Maruyama T, Fujimoto K, Uchida T, Okuno K. Lymph node metastases detected in the mesorectum distal to carcinoma of the rectum by the clearing method: justification of total mesorectal excision. J Am Coll Surg 1997;184:584-8. [PubMed]
- 19.Reynolds JV, Joyce WP, Dolan J, Sheahan K, Hyland JM. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Br J Surg 1996;83:1112-5. [DOI] [PubMed]
- 20.Scott N, Jackson P, al-Jaberi I, Dixon MF, Quirke P, Finan PJ. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg 1995:82:1031-3. [DOI] [PubMed]
- 21.Phang PT, MacFarlane J, Taylor RH, Cheifetz R, Davis N, Hay J, et al. Effects of positive resection margin and tumor distance from anus on rectal cancer treatment outcomes. Am J Surg 2002;183:504-8. [DOI] [PubMed]


