Abstract
Purpose
This phase I study was designed to demonstrate the feasibility, safety, efficacy and predictability of percutaneous cryosurgery, guided under magnetic resonance (MR) imaging, in the treatment of invasive breast carcinoma.
Patients and methods
Under the guidance of near–real-time T1-weighted FSE images of a 0.5-T open-configuration MR system, percutaneous cryosurgery was performed in 25 patients with operable invasive breast carcinoma, 4 weeks prior to their scheduled mastectomy. Predictive assessments by interventional radiologists using 4 breast-imaging techniques (mammography, sonography, scintigraphy and MR) were correlated with postmastectomy results of histopathology and assessed for predictability. Local and systemic morbidity were also evaluated during the month of follow-up preceding mastectomy.
Results
Percutaneous cryosurgery resulted in no serious complications, either local or systemic. All tumoural tissues included in the cryogenic “iceball” were destroyed, with no viable histologic residues. Ablation was total in 13 of the 25 tumours treated. Combining periprocedural MR images with postprocedure scintimammographic findings enabled a 96% rate for predicting the cryosurgical results.
Conclusions
MR-guided cryosurgery of breast carcinoma is feasible, safe and efficient, with predictable results. Major drawbacks are that the cryolesion (a palpable iceball) persists for a month or more after the procedure, undermining the reliability of the physical examination; and that breast imaging (mammography, ultrasound and MR) presents the same difficulty of interpretation as the physical exam even 1 month after the procedure. More studies are required to refine this treatment method.
Abstract
Objet
Cette étude de phase I devait démontrer la faisabilité, la sécurité, l'efficacité et la prévisibilité de la cryochirurgie percutanée guidée par imagerie à résonance magnétique (RM) dans le traitement du cancer du sein envahissant.
Patientes et méthodes
En se guidant sur des séquences d'écho de spin rapide en pondération T1 et en temps quasi réel d'un système RM à configuration ouverte de 0,5-T, on a procédé à une cryochirurgie percutanée chez 25 patientes qui avaient un cancer du sein envahissant opérable, quatre semaines avant la mastectomie prévue. On a établi un lien entre les évaluations prédictives de radiologistes d'intervention qui ont utilisé quatre techniques d'imagerie du sein (mammographie, échographie, scintigraphie et RM) et les résultats d'histologie après la mastectomie et on en a évalué la prévisibilité. On a aussi évalué la morbidité locale et systémique au cours du mois de suivi qui a précédé la mastectomie.
Résultats
La cryochirurgie percutanée n'a causé aucune complication grave, locale ou systémique. Tous les tissus de la tumeur inclus dans la «masse de glace» cryogénique ont été détruits et n'ont laissé aucun résidu histologique viable. L'ablation a été totale dans 13 des 25 tumeurs traitées. La combinaison d'images RM périprocédurales et des résultats de scintimammographies après l'intervention ont permis de prédire les résultats de la cryochirugie à 96 %.
Conclusions
La cryochirurgie guidée par RM d'un cancer du sein est faisable, sûre et efficace, et donne des résultats prévisibles. Les principaux inconvénients sont le fait que la cryolésion (masse de glace palpable) persiste pendant un mois ou plus après l'intervention, ce qui mine la fiabilité de l'examen physique, et le fait que l'imagerie du sein (mammographie, échographie et RM) présente la même difficulté d'interprétation que l'examen physique même un mois après l'intervention. D'autres études s'imposent pour raffiner cette méthode de traitement.
Cryosurgery is a procedure in which in vivo tissue ablation is achieved through freezing with biocompatible cryogenic probes. This therapeutic approach has been used for almost 150 years in many anatomical sites,1 particularly to treat nonresectable liver metastasis.
In situ cryosurgery has been shown in animals to be useful in treating breast cancer.2 Not only is it minimally invasive, but local tumour necrosis in cryosurgery has potential to induce a cytoimmune reaction against surviving or recurrent neoplastic cells.3,4,5 Thus far, however, successful clinical trials in women have been limited to a study by Staren and colleagues.2
When breast tumours are treated cryosurgically, monitoring is of practical concern. During these cryosurgical procedures, “iceball” growth is routinely monitored by ultrasound (US). The main drawback of this imaging modality is total US reflection at the ice interface; no structure can be seen beyond the edge of the iceball because of this acoustic shadow,6 so that the iceball's exact dimensions remain unkown.
MRI offers several advantages that make it suitable for guidance and monitoring in cryosurgical procedures. It has high spatial and time resolution and (unlike US) is sensitive to temperature, providing good contrast between frozen and unfrozen tissues. This allows accurate delineation of the whole circumference of the growing iceball.7 Furthermore, along with the availability of open-configuration MRI systems (Fig. 1), the development of new pulse sequences and magnetic resonance (MR)- compatible treatment devices have led to a breakthrough in the use of MRI in the guidance of minimally invasive therapies, particularly in cancer cryotherapy.8,9

FIG. 1. Open-configuration MRI system (Signa-SP, General Electric Medical Systems, Milwaukee, Wis.).
Before percutaneous cryosurgery of breast tumours under near–real-time MRI guidance can be safely offered to patients as a therapeutic option, it is essential to demonstrate not only the safety and effectiveness of the thermal treatment, but also the predictability of MR monitoring, to ensure that the monitored cryolesion accurately reflects the extent of tumour necrosis.
Patients and methods
Patient population
To ensure that local complications would not convert a possible partial mastectomy into a total mastectomy, 25 women were selected over a period of 2 years from a pool of almost 300 new cases of trocart-proven invasive breast cancer. Most of them (21) needed a total mastectomy for various reasons. For example, one patient had a new cancer in a breast already treated by partial mastectomy and radiotherapy; others had multicentric tumours or an invasive tumour with extensive in situ carcinoma. Our goal in these patients was not to eradicate all neoplastic tissues but to destroy the “dominant” invasive tumour. The MR-guided percutaneous breast cryosurgery was approved by our institute's review board. An informed-consent form approved by our ethics committee was signed by all patients.
Methods
Before beginning this in vivo procedure study, we did check that the radiologic iceball corresponded to the pathologic iceball by an ex vivo study of 15 breast-cancer specimens obtained by partial or total mastectomy. All iceballs were found to be of the same size to within a millimetre.
Four types of breast imaging were used on all patients: mammography, ultrasound, scintigraphy and MR. (For scintimammography we used the Mibi-Tc99m Miraluma, Dupont-Pharma Radiopharmaceuticals, North Billerica, Mass.) These exams were carried out before the cryoprocedure and repeated 1 month later, before definitive surgery. Additional criteria for inclusion in the study were negative results from chest x-ray, abdominal US and bone scan. To avoid cryo-injury, the target lesion had to be at least 1 cm away from the skin and chest wall.
Percutaneous cryosurgery procedures were guided in the near– real-time imaging mode of a 0.5-T open-configuration Signa-SP MRI system (General Electric Medical Systems, Milwaukee, Wis.). A standard flexible linear surface square coil (LOOPI) placed directly over the treated breast (Fig. 2) served as a radiofrequency transmitter and receiver. The MR protocol used slices 5 mm thick, an FOV of 24 х 18 cm2 and a matrix of 256 х 128. A T 1-weighted fast spin-echo sequence was used with a repetition time: effective echo time ratio (TR/TEeff) of 350/ 17 ms and an echo train length of 4, leading to a refresh rate of 9 s/image. One procedure was performed upon each lesion, to a total of 25.

FIG. 2. Breast magnetic resonance (MR) antenna.
Freezing was done with a cryotherapy delivery system (CryoHit, Galil Medical Ltd., Yokneam, Israël) with high-pressure cooling argon converted to a low-pressure liquid by means of the Joule–Thompson effect (Fig. 3).
FIG. 3. Cryotherapy delivery system (CryoHit, Galil Medical Ltd., Yokneam, Israel).
During the cryosurgery protocol itself, a lethal thermal stress was imposed on the tumour cells until the resulting radiologic iceball completely encompassed the “dominant” tumour. A systematic attempt was made to “overfreeze” each tumour to ensure an oncologic security margin of 1 cm.
Typically, cryogenic probes delivered 2 or 3 freezing cycles of up to 15 minutes each at a target temperature of –180°C. Freezing cycles alternated with 15-minute periods of passive thawing with the cryogenic probes turned off.
Cryosurgery was done with the patients under local anesthesia and intravenous narcosis. The first 10 patients were observed in the hospital for 24 hours afterward; subsequent procedures were performed on an outpatient basis.
Each procedure began with insertion of a cryoprobe into the targeted lesion. For the first 6 patients, the only probes available were 6 mm in diameter, which failed to adequately penetrate the tumour. Therefore, beginning with the seventh patient (for whom 3-mm probes became available), the procedure first involved transfixing the breast completely through the long axis of the target tumour under US guidance with an 18-gauge puncture needle. Then a 0.35-mm Amplatz guidewire (Boston Scientific Corp., Watertown, Mass.) was inserted to pierce the breast tumour entirely. This permitted placement of a 9F introducer (Terumo Medical Corp., Elkton, Md.) at either end of the guidewire. The distal ends of the introducers were brought into contact at the centre of the tumour to allow for the placement of 1 or 2 MR-compatible cryogenic probes 3 mm in diameter (Galil Medical Ltd.) in a head-on configuration at the heart of the lesion (Fig. 4).
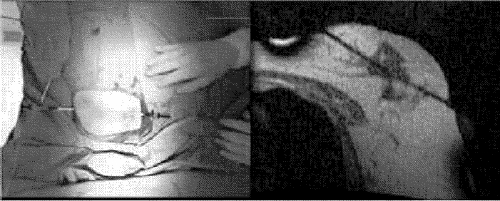
FIG. 4. Left, introducers inserted into the breast just before cryoprobe insertion. Right, a near–real-time image to guide cryoprobe placement.
Each procedure typically took 3 hours, followed by at least 2 hours of recovery. The interventionist's periprocedural assessment of the result was recorded immediately for future comparison.
During the 1-month recovery period, patients were followed weekly with blood tests, a physical examination and an appropriate questionnaire (given by interview). The 4 types of breast imaging used before the cryoprocedure were repeated before mastectomy to evaluate any changes induced by cryosurgery.
Surgery and pathology
Twenty-one patients underwent total mastectomy and 4, partial mastectomy. All had axillary nodes excised. No particular complications were noted in the postoperative period.
Immediately after surgery, each surgical specimen was analyzed histopathologically to assess the final ablative effect of the cryoprocedure. After a macroscopic exam, a complete cephalocaudal section centred on the tumour was performed. The 2 pieces were analyzed and the dimensions of the cryolesion recorded, along with its position relative to the skin and nipple. Tissue slices were then cut parallel to the long axis of the specimen. Histological cross-sections of the slices containing the trajectory of the probes (Fig. 5) were specifically studied to detect possible tumour-seeding during the insertion of the devices.
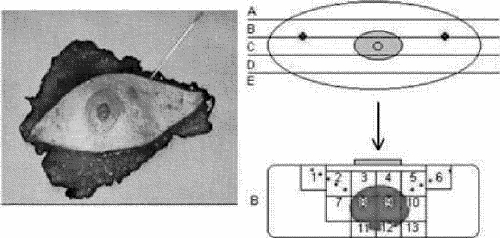
FIG. 5. Schematic for preparation of surgical specimens.
Results
MR-guided percutaneous cryosurgery was well tolerated by all patients. Some reported a local sensitivity in the immediate recovery period. All patients were discharged within 24 hours. The weekly blood tests revealed no significant changes. During the 1 month of follow-up, the most frequent complaint was a sensation of heaviness in the breast treated. Physical signs noted locally were a minimal skin burn at the entry points of the probes and signs of a traumatic mastitis that persisted until the mastectomy. No abscess formation was observed, even though no antibiotic prophylaxis was given.
Table 1 summarizes the results obtained. Importantly, no viable tumour cells were found in any of the pathologic iceballs. Pathologic tumour ablation was technically total in 13 of the 25 tumours treated. Of the 12 failures, 10 were declared for various reasons by the interventionist during the procedure. In the remaining 2 failures (cases 20 and 23) the interventionist overestimated his result, whereas scintimammography was positive for residual tumour. Only in case 13 did the interventionist underestimate his result (scintigraphy predicted the total ablation actually obtained). Only in case 22 was scintigraphy unpredictive, showing a negative result when residual tumour in fact remained.
Table 1
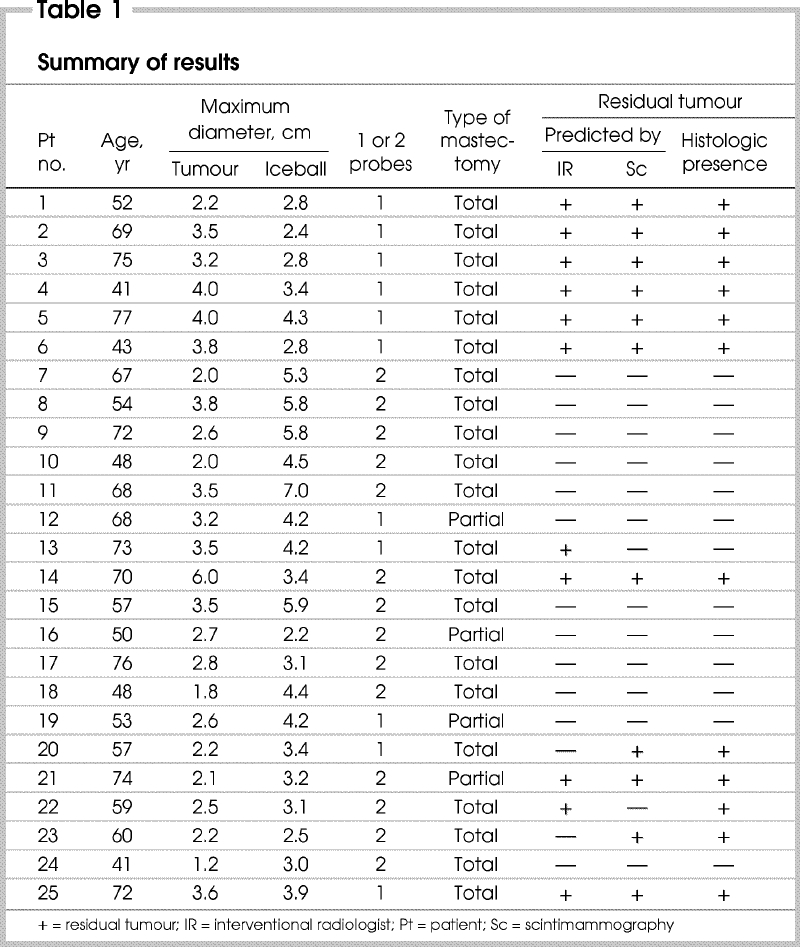
Combining the interventionist's periprocedureal assessment and presurgical scintimammography findings enabled the correct prediction of 96% of the histopathological results.
Conclusions
Percutaneous MR-guided cryosurgery of breast cancer has minimal morbidity. MR imaging seems very adequate to delineate tumour versus iceball (Fig. 6). Scintimammography seemed to be the simplest means of predicting results: images showing only a residual halo (Fig. 7) seem to predict total ablation.10,11 The cryoprocedure's major drawback is that the clinically palpable iceball persists for a month or more, undermining the reliability of the physical examination.
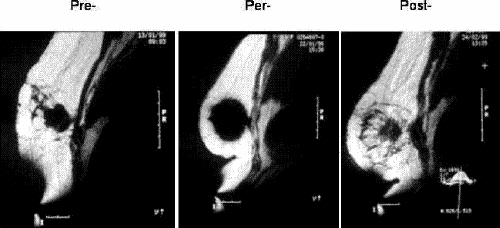
FIG. 6. Typical cryosurgical perioperative MR images. Left, the viable tumour before the cryoprocedure. Middle, the growing “iceball” (not completed). Right, the residual iceball and dead tumour 1 month later (with a pre-existing traumatic lesion to the pectoral muscle behind).
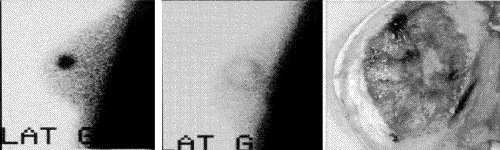
FIG. 7. Scintimammographic exam performed (left) 1 week prior to and (middle) 4 weeks after the cryosurgery, with (right) the corresponding pathologic sample.
The cryoprocedure of 2 of our patients whose tumours were totally ablated had been synchronized with their trocart biopsy; they therefore had their definitive histologic diagnosis with local destruction of the tumour during about a 3-hour period under local anesthesia, as outpatients.
In conclusion, MRI-guided percutaneous cryosurgery was shown to be both feasible and safe. These results support this innovative technique as a promising, minimally invasive approach to the treatment of malignant breast lesions. Further clinical trials with long-term follow-up are warranted.
Competing interests: None declared.
Correspondence to: Dr. Christian Moisan, iMRI Unit, CHUQ–Hôpital St-François d'Assise, Bureau/suite A0-212d, 10, rue de L'Espinay, Québec QC G1L 3L5; fax 418 525-4411; christian.moisan@rad.ulaval.ca
Accepted for publication Mar. 5, 2004
References
- 1.Gage AA. Cryosurgery in the treatment of cancer. Surg Gynecol Obstet 1992;174:73-92. [PubMed]
- 2.Staren ED, Sabel MS, Gianakakis LM, Wiener GA, Hart VM, Gorski M, et al. Cryosurgery of breast cancer. Arch Surg 1997;132:28-33. [DOI] [PubMed]
- 3.Neel HB 3rd. Immunotherapeutic effect of cryosurgical tumor necrosis. Vet Clin North Am Small Anim Pract 1980;10: 763-9. [DOI] [PubMed]
- 4.Neel HB 3rd, Ritts RE Jr. Immunotherapeutic effect of tumor necrosis after cryosurgery, electrocoagulation, and ligation. J Surg Oncol 1979;11:45-52. [DOI] [PubMed]
- 5.Tanaka S. Immunological aspects of cryosurgery in general surgery. Cryobiology 1982;19:247-62. [DOI] [PubMed]
- 6.Rabin Y, Coleman R, Mordovich D, Ber R, Shitzer A. A new cryosurgical device for controlled freezing. Cryobiology 1996;33: 93-105. [DOI] [PubMed]
- 7.Baust J, Cage A, Ma H, Zhang CM. Minimally invasive cryosurgery — technological advances. Cryobiology 1997;34:373-84. [DOI] [PubMed]
- 8.Matsumoto R, Selig AM, Colucci VM, Jolesz FA. MR monitoring during cryotherapy in the liver: predictability of histologic outcome. J Magn Reson Imaging 1993;3:770-6. [DOI] [PubMed]
- 9.Silverman SG, Tuncali K, Adams DF, vanSonnenberg E, Zou KH, Kacher DF, et al. MR imaging-guided percutaneous cryotherapy of liver tumors: initial experience. Radiology 2000;217:657-64. [DOI] [PubMed]
- 10.Dumont M, Morin J, Marois M, Duchesne N, Dufour M, Dionne G, et al. Scintimammographic findings from short term follow up of breast cancer cryosurgery. J Nucl Med 1999;40:249.
- 11.Pérusse K, Dumont M, Morin J, Fouquet B, Marois M, Dionne G, et al. Diagnostic value of scintimammography in following up of MR-guided percutaneous cryosurgery of breast cancer. J Nucl Med 2001;42:292.11216529


