Abstract
Objective:
The incidence rate of hepatocellular carcinoma has been rising in the United States during the last 2 decades. Heavy alcohol use has been widely recognized as one of the major etiological factors of hepatocellular carcinoma. This study sought to assess the extent to which heavy alcohol use contributed to premature death from hepatocellular carcinoma on a population scale in the United States.
Method:
We analyzed the Multiple Cause of Death public-use data sets. Using codes from the International Classification of Diseases, 1 0th Revision, hepatocellular carcinoma death was defined based on the underlying cause of death, and heavy alcohol use was indicated by the presence of any alcohol-induced medical conditions among the contributing causes of death. During 1999–2006 in the United States, 51,400 hepatocellular carcinoma deaths were identified from 17,727,245 natural deaths of persons age 25 or older. We conducted Poisson regression, life table, and multiple linear regression analyses to compare prevalence ratios, cumulative probabilities, and mean ages of death, respectively, from hepatocellular carcinoma by heavy alcohol use status across sex and race/ethnicity.
Results:
Heavy alcohol use decedents had higher prevalence ratios of dying from hepatocellular carcinoma than from non–chronic liver diseases compared with those decedents without heavy alcohol use. Heavy alcohol use was associated with decreased mean ages and increased cumulative probabilities of death among hepatocellular carcinoma decedents across racial/ethnic groups in both sexes. This association was stronger among women than men and stronger among non-Hispanic Whites than non-Hispanic Blacks.
Conclusions:
This study provides mortality-based empirical evidence to further establish heavy alcohol consumption as one of the key risk factors contributing to premature deaths from hepatocellular carcinoma in the United States, and its effect appears more prominent among women and non-Hispanic Whites.
Hepatocellular carcinoma (HCC) is among the deadliest cancers worldwide, affecting about half a million people per year and ranking as the third leading cause of cancer mortality (Parkin et al., 2001, 2005). In the United States, the age-adjusted incidence rates rose from 3.1/100,000 during 1992–1993 to 5.1/100,000 during 2003–2005, with an overall annual percentage change of 4.3% (Altekruse et al., 2009). Because the prognosis for HCC remains uniformly poor regardless of its underlying etiology, the mortality rate for liver cancer (excluding intrahepatic bile duct) increased correspondingly during the same period (Altekruse et al., 2009; El-Serag et al., 2008; Raoul, 2008).
Numerous studies have examined the causal link between heavy alcohol use (HAU) and HCC risk. Because chronic alcohol consumption has long been associated with progressive liver disease that can lead to the development of liver cirrhosis, it has been widely recognized as one of the major etiological factors of HCC, in addition to chronic infections with hepatitis B virus and hepatitis C virus and intake of aflatoxin (El-Serag and Mason, 1999; Yu et al., 2008). Epidemiological studies also have suggested that HAU, diabetes, and viral hepatitis may exert synergistic effects on the risk for HCC and that these factors are likely contributors to the rising incidence of HCC in the United States (Hassan et al., 2002; Singal and Anand, 2007; Voigt, 2005).
Recently, increasing numbers of studies have reported substantial racial/ethnic variations in the rising incidence and survival outcomes of HCC by showing greater increases in incidence among middle-aged Black, Hispanic, and White men (Altekruse et al., 2009; Davila and El-Serag, 2006; Harrison et al., 2004; Wong and Corley, 2008). Nonetheless, to our knowledge, to date no study has systematically evaluated the effect of HAU on premature death from HCC on a population scale. Given that close to 18 million adults in the United States have an alcohol use disorder (Grant et al., 2004)—a prevalence almost fivefold that for hepatitis C virus (Armstrong et al., 2006)—more research is needed to provide information on the national burden of alcohol-related HCC, to clarify whether the differences in the effect of HAU on HCC premature death vary with demographic factors, and to help identify populations at risk for premature death from HCC to facilitate prevention.
To fill the gap in the existing literature, we performed a comprehensive analysis of the Multiple Cause of Death (MCD) public-use data compiled by the National Center for Health Statistics (NCHS). These data are based on information from resident death certificates filed in the 50 states and the District of Columbia, which cover more than 99% of deaths occurring in the United States (Heron et al., 2009). The MCD files are a valuable data source for estimating the disease burden and assessing population health disparities for several reasons. They have large case numbers from the U.S. total population; they include basic demographic information; they contain detailed diagnostic codes of the conditions that underlie or contribute to the final death; and they span the whole age spectrum. These unique features made it possible to overcome some limitations in the existing studies because of small case numbers and/or short follow-up time. The large case numbers also allowed us to stratify our analysis by sex and race/ethnicity with enough power to investigate the relationship between HAU and premature death from HCC, as well as how this relationship may differ across these subpopulations.
Method
Data sources
The primary data sources used in this study were the MCD data sets for 1999–2006 obtained from the NCHS Web site (at www.cdc.gov/nchs/data_access/Vitalstatsonline.htm). In the MCD files, causes of death were coded according to the International Classification of Diseases, 10th Revision (ICD-10), coding scheme (World Health Organization, 1992). For each death record, MCD data include codes for one underlying cause and up to 20 contributing causes of death. Other available MCD data elements include age at death, sex, education, race, ethnicity, marital status, and place of death. In the present study, deaths at ages younger than 25 years were excluded given that the carcinogenesis of HCC involving multiple stages and chronic alcohol consumption is very rare for young people. In addition, we excluded deaths from unnatural causes (ICD-10 codes V01 through Y89) to minimize the bias in death age for reasons such as intentional and unintentional injuries and poisonings that are unrelated to the natural course of progression of chronic diseases. This led to a total of 17,727,245 natural deaths between 1999 and 2006 in the final analysis.
Midyear population data from 1999 to 2006 used for the calculation of mortality rates and life table parameters were obtained from two sets of the U.S. population estimates developed by the U.S. Census Bureau for NCHS: the bridged-race intercensal resident population estimates for 1999 (NCHS, 2004) and the bridged-race vintage postcensal estimates of the resident population for 2000 through 2006 (NCHS, 2008).
Hepatocellular carcinoma death and heavy alcohol use ascertainment
The ICD-10 codes used in the present analysis are presented in Table 1. Based on the underlying cause, deaths were divided into three groups: HCC, other chronic liver diseases (CLD), and non-CLD (i.e., all other causes). Using the underlying cause for HCC identification is an effort to ensure the inclusion of primary liver cancer cases only. According to NCHS, the underlying cause of death is the disease or injury that initiated the train of morbid events leading directly to death or the circumstances of the accident or violence that produced the injury (NCHS, 2010). Furthermore, HCC was identified by two ICD-10 codes on the underlying cause of death—C22.0 (liver cell carcinoma) or C22.9 (liver cancer, unspecified)—if a contributing cause of hepatitis C virus infection, hepatitis B virus infection, or liver cirrhosis was present. These two ICD-10 codes are designated for primary liver cancer (World Health Organization, 1992) and are rarely present in the contributing causes. (A separate code, C78.7, is reserved for secondary malignant neoplasm of liver—i.e., other cancers metastatic to the liver.) Following Kim et al. (2005), we included C22.9 (liver cancer, unspecified) conditional on the presence of liver diseases to minimize the inclusion of secondary liver cancers. Using this definition for HCC death, Kim et al. (2005) found good agreement of the estimate of HCC deaths from MCD with that based on hospitalization data from the National Inpatient Sample. Accordingly, a total of 51,400 deaths were identified as HCC deaths in the current analysis after excluding 233 HCC cases with death age less than 25 years.
Table 1.
International Classification of Diseases, 10th Revision, codes for liver cancer, other chronic liver diseases, alcohol-induced medical conditions, heavy tobacco use, diabetes, hypertension, HIV infection
| Liver cancer (HCC)a | |
| C22.0 | Liver cell carcinoma, hepatocellular carcinoma |
| C22.9b | Liver cancer, unspecified |
| Other chronic liver diseases (CLD)a | |
| K70 | Alcoholic liver disease |
| K71 | Toxic liver disease |
| K72 | Hepatic failure, not elsewhere classified |
| K73 | Chronic hepatitis, not elsewhere classified |
| K74 | Fibrosis and cirrhosis of liver |
| K75.1–K75.9 | Other inflammatory liver diseases, excluding abscess of liver (K75.0) |
| K76 | Other diseases of liver |
| K83 | Other diseases of biliary tract |
| B16–B19 | Viral hepatitis |
| B94.2 | Sequelae of viral hepatitis |
| D73.1 | Hypersplenism |
| D73.2 | Chronic congestive splenomegaly |
| E80 | Disorders of porphyrin and bilirubin metabolism |
| E83 | Disorders of mineral metabolism |
| I81 | Portal vein thrombosis |
| I82.0 | Budd-Chiari syndrome |
| I85 | Esophageal varices |
| R16 | Hepatomegaly and splenomegaly, not elsewhere classified |
| R17 | Unspecified jaundice |
| R18 | Ascites |
| Alcohol-induced medical conditions | |
| E24.4 | Alcohol-induced pseudo-Cushing's syndrome |
| F10 | Mental and behavioral disorders due to use of alcohol |
| G31.2 | Degeneration of nervous system due to alcohol |
| G62.1 | Alcoholic polyneuropathy |
| G72.1 | Alcoholic myopathy |
| I42.6 | Alcoholic cardiomyopathy |
| K29.2 | Alcoholic gastritis |
| K70 | Alcoholic liver disease |
| K86.0 | Alcohol-induced chronic pancreatitis |
| R78.0 | Finding of alcohol in blood |
| Heavy tobacco use | |
| F17 | Mental and behavioral disorders due to use of tobacco |
| Diabetes | |
| E10–E14 | Diabetes mellitus |
| Hypertension | |
| I10-I13 | Hypertensive diseases, excluding secondary hypertension |
| Human immunodeficiency virus infection (HIV) | |
| B20–B24 | Human immunodeficiency virus (HIV) disease |
Notes: HCC = hepatocellular carcinoma.
Codes included in the HCC and other CLD categories are defined as chronic liver diseases according to Manos et al. (2008).
C22.9 was included as HCC if a contributing cause of hepatitis C or B virus infection or liver cirrhosis was present (Kim et al., 2005). Source: International Classification of Diseases and Related Health Problems, Tenth Revision. Geneva, Switzerland (World Health Organization, 1992).
We further ascertained the primary nature of the HCC among the 51,400 HCC deaths by examining the presence of any other cancers on their lists of contributing causes of death. Among these decedents, only about 5.6% (or 2,889 deaths) had other cancers recorded in the contributing causes, of which about 75% (or 2,154 deaths) were clearly coded as secondary cancers, possibly metastasized from HCC or other co-occurring cancers (data not shown).
Following the definition suggested by Manos et al. (2008) for CLD, there were 214,253 deaths identified as other CLD deaths (i.e., all deaths from CLD except deaths from HCC; see ICD-10 codes in Table 1).
Because there is no information on alcohol drinking behaviors in the MCD data sets, alcohol-induced medical conditions listed on the death certificates were used as a proxy measure for HAU. These conditions include mental/ behavioral disorders because of use of alcohol and alcoholic cirrhosis (Table 1). This proxy measure has a high predictive value in identifying heavy drinkers because these medical conditions are 100% attributable to alcohol use, but it also is a conservative measure because not all heavy drinkers develop those alcohol-induced medical conditions (Chen et al., 2007).
Statistical analysis
We performed descriptive analysis to summarize the total numbers and rates of mortality and the prevalence of HAU by cause-of-death categories (i.e., HCC, other CLD, and non-CLD causes) for each of the subpopulation groups (defined by sex and race/ethnicity). Mean ages at HCC death and their standard errors also were calculated by HAU status. To facilitate comparisons across subpopulation groups, age standardized mortality rates of HCC were calculated using the 2000 U.S. population age distribution as the standard (Ingram et al., 2003).
A set of Poisson regressions was conducted to calculate adjusted prevalence ratios to evaluate the effect of HAU on the risks for HCC death, other CLD death, and death from all other causes. These models adjusted for demographic characteristics and other potential confounders for premature death, including marital status (married, single, divorced, and widowed), education (≤8 years, 9–12 years, and ≥13 years), year of death (1999, 2000, 2001, 2002, 2003, 2004, 2005, and 2006), place of death (inpatient, outpatient, nursing home, decedent's home, and other), heavy tobacco use (yes/no), diabetes (yes/no), hypertension (yes/ no), and HIV (yes/no). Contributing causes of death were used to identify the presence of heavy tobacco use, diabetes, hypertension, and HIV (see ICD-10 codes in Table 1). The SAS procedure GENMOD (SAS Version 9.2, SAS Institute Inc., Cary, NC; Spiegelman and Hertzmark, 2005) was used to estimate the Poisson regression models, which generated the adjusted prevalence ratios and their 95% confidence intervals.
Two approaches—multiple-cause life table analysis and multiple linear regression analysis—were used to assess the effect of HAU on premature mortality (as represented by age at death) among HCC decedents. First, multiple-cause life tables were constructed to reveal the differences in age patterns between HCC decedents with and without HAU. As an extension of a single-cause life table, a multiple-cause life table generates cause-specific survival probabilities or cumulative probabilities of death by age for subpopulation groups to assess the effect of specific causes (Selvin, 1996). In addition to life table analysis, a multiple linear regression model was used to calculate adjusted mean age at death based on predictive margins, which are directly standardized to the distribution of each covariate in the model (Graubard and Korn, 1999). This analysis included the same set of covariates (marital status, educational attainment, year of death, place of death, heavy tobacco use, diabetes, hypertension, and HIV) and was performed with the statistical software package SUDAAN, Version 10 (Research Triangle Institute, 2008).
To generate sex- and race/ethnicity-specific estimates, all analyses were conducted by sex and race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and other races). All statistical tests were two-sided, and a type I error level of less than .05 was considered significant.
Results
Numbers and age-adjusted rates of hepatocellular carcinoma deaths
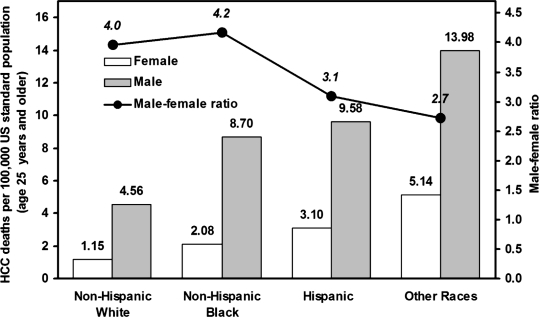
From 1999 to 2006, there were 51,400 HCC deaths at age 25 years or older, including 38,650 among men and 12,750 among women (Table 2). Although more than 60% of these decedents were non-Hispanic White, the annual age-adjusted HCC mortality rate was lowest in this group for both sexes (4.56/100,000 men and 1.15/100,000 women), followed by non-Hispanic Blacks, Hispanics, and other races (Figure 1). Across all race/ethnicity groups, the age-adjusted HCC mortality rate was much higher for men than women (with the male to female ratio ranging from 2.7 to 4.2).
Table 2.
Number of cases and prevalence of heavy alcohol use (HAU) among decedents who died from hepatocellular carcinoma (HCC), other chronic liver diseases (CLD), a and non-CLD at age 25 and older: United States, 1999–2006
| HCC |
Other CLD |
Non-CLD |
|||||||
| w/HAU |
w/HAU |
w/HAU |
|||||||
| Sex and race/ethnicity | Total n | n | %of total | Total n | n | %of total | Total n | n | %of total |
| Male | |||||||||
| Non-Hispanic White | 24,696 | 1,682 | 6.81 | 159,592 | 57,032 | 35.74 | 6,677,497 | 81,759 | 1.22 |
| Non-Hispanic Black | 5,590 | 350 | 6.26 | 23,558 | 7,727 | 32.80 | 937,924 | 18,437 | 1.97 |
| Hispanic | 4,597 | 552 | 12.01 | 28,042 | 12,765 | 45.52 | 367,514 | 9,522 | 2.59 |
| Other races | 3,767 | 127 | 3.37 | 7,837 | 3,201 | 40.84 | 194,887 | 4,064 | 2.09 |
| All | 38,650 | 2,711 | 7.01 | 219,029 | 80,725 | 36.86 | 8,177,822 | 113,782 | 1.39 |
| Female | |||||||||
| Non-Hispanic White | 7,929 | 202 | 2.55 | 96,471 | 21,469 | 22.25 | 7,597,579 | 21,964 | 0.29 |
| Non-Hispanic Black | 1,687 | 44 | 2.61 | 14,584 | 3,456 | 23.70 | 1,017,989 | 4,498 | 0.44 |
| Hispanic | 1,588 | 53 | 3.34 | 13,095 | 2,477 | 18.92 | 351,068 | 1,234 | 0.35 |
| Other races | 1,546 | 29 | 1.88 | 5,660 | 1,539 | 27.19 | 182,548 | 1,094 | 0.60 |
| All | 12,750 | 328 | 2.57 | 129,810 | 28,941 | 22.29 | 9,149,184 | 28,790 | 0.31 |
| Total | 51,400 | 3,039 | 5.91 | 348,839 | 109,666 | 31.44 | 17,327,006 | 142,572 | 0.82 |
Source: Multiple Cause of Death data files, 1999–2006 (National Center for Health Statistics).
Figure 1.
Average annual age-standardized mortality rate of hepatocellular carcinoma (HCC) for “Non-Hispanic White,” “Non-Hispanic Black,” “Hispanic,” and “Other Races” by sex for adults ages 25 years and older in the United States, 1999–2006. Source: Multiple Cause of Death data files, 1999–2006 (National Center for Health Statistics).
Prevalence of heavy alcohol use among deaths from hepatocellular carcinoma, other chronic liver diseases, and non-chronic liver diseases
As shown in Table 2, across all race/ethnicity groups for both sexes, HCC decedents had higher HAU prevalence than decedents with non-CLD causes but much lower HAU prevalence than decedents with other CLD causes. Within HCC deaths, HAU prevalence was higher in men than in women for all race/ethnicity groups, and higher for both sexes in Hispanics (12.01% in men and 3.34% in women) than any of the other race/ethnicity groups. Among deaths from other CLD or non-CLD causes, for men, HAU prevalence was higher in Hispanics and non-Hispanic others (i.e., the other races category) than in non-Hispanic Whites and non-Hispanic Blacks; for women, it was higher in non-Hispanic Blacks and non-Hispanic others than in non-Hispanic Whites and Hispanics.
Effect of heavy alcohol use on risks for hepatocellular carcinoma and other chronic liver diseases death
Table 3 presents the adjusted prevalence ratios estimated from Poisson models for HAU status. The prevalence ratios for HCC relative to non-CLD causes were all greater than 1 (ranging from 2.04 to 8.09) and statistically significant (p < .001) in both men and women across all race/ethnicity groups, indicating that HAU was associated with elevated risks for dying from HCC versus non-CLD causes. However, HAU showed a much stronger association with other CLD death than with HCC death. This is evidenced by the much larger prevalence ratios (ranging from 10.91 to 35.61, all ps < .001) for other CLD causes relative to non-CLD causes. Also, the prevalence ratios for HCC relative to other CLD were well below 1 (ranging from 0.08 to 0.22, all ps < .001), indicating largely reduced prevalence ratios of dying from HCC versus other CLD if the decedents had HAU.
Table 3.
Adjusted prevalence ratios (PR) of hepatocellular carcinoma (HCC) deaths, other chronic liver diseases (CLD) deaths, and non-CLD deaths for heavy alcohol use (HAU) relative to non-HAU among decedents who died at age 25 and older: United States, 1999–2006
| HCC vs. other CLD deaths |
HCC vs. non-CLD deaths |
Other CLD vs. non-CLD deaths |
||||
| Sex and race/ethnicity | PRa | [95% CI] | PRa | [95% CI] | PRa | [95% CI] |
| Male | ||||||
| Non-Hispanic White | 0.18*** | [0.17,0.19] | 4.81*** | [4.56, 5.07] | 20.50*** | [20.26, 20.74] |
| Non-Hispanic Black | 0.19*** | [0.17,0.21] | 3.11*** | [2.78, 3.49] | 15.04*** | [14.60, 15.49] |
| Hispanic | 0.22*** | [0.20, 0.24] | 4.42*** | [4.03, 4.86] | 10.91*** | [10.64,11.20] |
| Other races | 0.11*** | [0.09,0.13] | 2.04*** | [1.69,2.46] | 16.20*** | [15.36, 17.08] |
| All | 0.17*** | [0.16,0.18] | 4.15*** | [3.98,4.33] | 18.65*** | [18.46, 18.83] |
| Female | ||||||
| Non-Hispanic White | 0.10*** | [0.09,0.12] | 6.38*** | [5.52, 7.38] | 35.61*** | [35.02, 36.20] |
| Non-Hispanic Black | 0.10*** | [0.08,0.14] | 5.22*** | [3.84, 7.08] | 29.75*** | [28.53,31.01] |
| Hispanic | 0.19*** | [0.14,0.25] | 8.09*** | [6.10, 10.73] | 17.27*** | [16.48, 18.10] |
| Other races | 0.08*** | [0.06,0.12] | 2.68*** | [1.81,3.96] | 19.24*** | [17.98, 20.59] |
| All | 0.11*** | [0.10,0.12] | 5.71*** | [5.09, 6.39] | 31.58*** | [31.13,32.04] |
Notes: aAdjusted for covariates including education, marital status, place of death, year of death, heavy tobacco use, diabetes, hypertension, and HIV using Poisson regression. Source: Multiple Cause of Death data files, 1999–2006 (National Center for Health Statistics).
p <.001.
Effect of heavy alcohol use on age patterns of hepatocellular carcinoma deaths
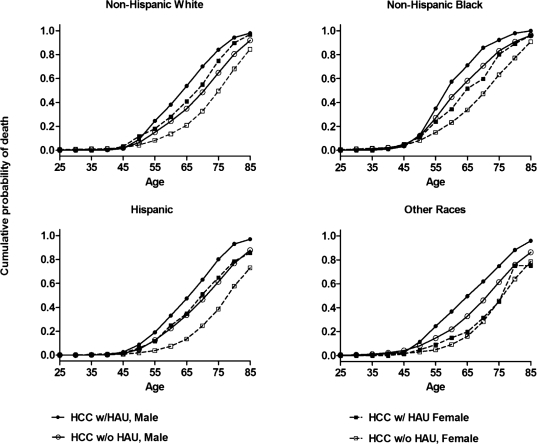
Figure 2 provides the cumulative probabilities of death from the life table analysis to display the age pattern of HCC death by sex, race/ethnicity, and HAU status. Before age 45, the cumulative probabilities of death remained very low and similar for all of these subgroups. However, the cumulative distributions revealed distinct patterns of HCC mortality associated with HAU status and sex. Generally, after age 45, men showed faster increases in cumulative probabilities of death than women. Within each sex, without exception, decedents with HAU showed faster increases in cumulative probabilities of death than those without HAU across race/ ethnicity groups. This pattern clearly indicates that HAU resulted in dying at younger ages from HCC. Nonetheless, some variations in cumulative probabilities of death were observed across the race/ethnicity groups. Among men, non-Hispanic Whites showed the largest difference (.19) in cumulative probabilities of death before age 65 between decedents with HAU (.54) and those without HAU (.35), whereas non-Hispanic Blacks showed the smallest difference (.13) in cumulative probabilities of death before age 65 between decedents with HAU (.71) and those without HAU (.58). In contrast, among women, the difference in cumulative probabilities of death before age 65 between HAU and non-HAU decedents was .20 or more in both Hispanics (.35 vs. .13) and non-Hispanic Whites (.41 vs. .21) but only .04 in non-Hispanic others (.20 vs. .16).
Figure 2.
Cumulative probability of death from hepatocellular carcinoma (HCC) based on complete multiple-cause life tables, by sex, for Non-Hispanic White, Non-Hispanic Black, Hispanic, and other races, and heavy alcohol use (HAU) status for adults ages 25 years and older in the United States, 1999–2006. Source: Multiple Cause of Death data files, 1999–2006 (National Center for Health Statistics).
Effect of heavy alcohol use on mean age at hepatocellular carcinoma deaths
In addition to the age structure for HCC deaths throughout the life span, the effect of HAU on premature mortality was evidenced by the mean age at death. Table 4 presents the unadjusted and adjusted mean ages at HCC death by sex and race/ethnicity. Overall, compared with their counterparts without HAU, male decedents with HAU died 4.20 years younger (M = 64.12, SE = 0.07, vs. M = 59.92, SE = 0.20) and female decedents died 6.72 years younger (M = 70.24, SE = 0.11, vs. M = 63.52, SE = 0.67). A similar pattern is seen across all subpopulation groups with some variations. Because non-Hispanic Whites without HAU had the oldest mean age at HCC death among both men (M = 66.07, SE = 0.08) and women (M = 71.77, SE = 0.14), the reduction in life for their HAU counterparts (4.99 and 7.60 years for men and women, respectively) also was generally larger than that for minority groups except for Hispanic women.
Table 4.
Unadjusted and adjusted mean ages at death among hepatocellular carcinoma (HCC) decedents who died at age 25 and older: United States, 1999–2006
| Unadjusted mean age at HCC death |
Adjusted meana age at HCC death |
|||||||||||||
| w/o HAU |
w/HAU |
Contrast |
w/o HAU |
w/HAU |
Contrastb |
|||||||||
| Sex and race/ethnicity | M | SE | M | SE | Diff. | SE | p | Ma | SE | Ma | SE | Diff. | SE | p |
| Male | ||||||||||||||
| Non-Hispanic White | 66.07 | 0.08 | 61.08 | 0.25 | 4.99 | 0.31 | <.0001 | 65.75 | 0.08 | 62.24 | 0.24 | 3.52 | 0.25 | <.0001 |
| Non-Hispanic Black | 59.00 | 0.16 | 56.64 | 0.49 | 2.36 | 0.63 | <.0001 | 60.68 | 0.15 | 58.92 | 0.47 | 1.76 | 0.49 | .0003 |
| Hispanic | 62.20 | 0.19 | 58.89 | 0.45 | 3.31 | 0.53 | <.0001 | 61.64 | 0.17 | 58.46 | 0.43 | 3.18 | 0.47 | <.0001 |
| Other races | 61.34 | 0.22 | 58.11 | 0.99 | 3.23 | 1.20 | .002 | 60.83 | 0.21 | 58.87 | 0.88 | 1.96 | 0.91 | .03 |
| All | 64.12 | 0.07 | 59.92 | 0.20 | 4.20 | 0.25 | <.0001 | 64.05 | 0.07 | 60.98 | 0.20 | 3.07 | 0.20 | <.0001 |
| Female | ||||||||||||||
| Non-Hispanic White | 71.77 | 0.14 | 64.17 | 0.85 | 7.60 | 0.88 | <.0001 | 71.54 | 0.14 | 66.08 | 0.73 | 5.46 | 0.73 | <.0001 |
| Non-Hispanic Black | 65.20 | 0.34 | 60.86 | 1.80 | 4.34 | 2.08 | .04 | 66.69 | 0.30 | 63.54 | 1.45 | 3.15 | 1.48 | .03 |
| Hispanic | 69.94 | 0.31 | 62.09 | 1.66 | 7.85 | 1.69 | <.0001 | 69.01 | 0.29 | 63.94 | 1.46 | 5.07 | 1.48 | .0006 |
| Other races | 68.22 | 0.31 | 65.62 | 2.30 | 2.60 | 2.29 | .26 | 68.26 | 0.28 | 68.46 | 1.99 | -0.19 | 2.01 | .92 |
| All | 70.24 | 0.11 | 63.52 | 0.67 | 6.72 | 0.71 | <.0001 | 70.18 | 0.11 | 65.77 | 0.58 | 4.42 | 0.59 | <.0001 |
Notes: Diff. = difference; HAU = Heavy alcohol use, as indicated by alcohol-induced medical conditions.
Adjusted for covariates including education, marital status, place of death, year of death, heavy tobacco use, diabetes, hypertension, and HIV based on predictive margins estimated from multiple linear regression.
Multiple R2 was .23 and .29 for all predictors in the regression model for male and female decedents, respectively. Source: Multiple Cause of Death data files, 1999–2006 (National Center for Health Statistics).
Table 4 also reports the adjusted mean age at HCC death estimated by predictive margins from linear regression models. These results confirm the patterns observed with the unadjusted mean ages. Although the differences in mean age between HAU and non-HAU groups were generally reduced after adjusting (i.e., standardizing) for demographic characteristics and other risk factors, they remain statistically significant across all sex and race/ethnicity groups except non-Hispanic women of other races.
Further analysis was conducted to examine the joint effects of HAU and hepatitis B or C (B/C) virus infection on the mean age at HCC death by sex after controlling for race/ ethnicity and other demographic and risk factors. Interestingly, the results indicate that the interaction effect of the two risk factors on age at death was not significant for men (b = 0.3673, p = .3523) but was significant for women (b = -2.7462, p = .0328) (data not shown). Therefore, the joint effects of the two risk factors tend to be additive in men and multiplicative in women. Table 5 shows the adjusted mean age at HCC death by HAU and hepatitis B/C status. Among men, compared with HCC decedents without HAU and hepatitis B/C, the average reduction in life was 2.78, 5.63, and 8.04 years for HCC decedents with HAU only, hepatitis B/C only, and both HAU and hepatitis B/C, respectively. In contrast, among women, compared with HCC decedents without HAU and hepatitis B/C, the average reduction in life was 3.55, 2.97, and 9.25 years for HCC decedents with HAU only, hepatitis B/C only, and both HAU and hepatitis B/C, respectively.
Table 5.
Adjusted mean ages at death by heavy alcohol use and hepatitis B/C status among hepatocellular carcinoma (HCC) decedents who died at age 25 and older: United States, 1999–2006
| Adjusted meana age at HCC death |
Contrastb |
||||||
| Sex | HAU | Hepatitis B/C | Adjusted M | SE | Difference | SE | p |
| Male | − | − | 65.17 | 0.07 | Ref. | ||
| + | − | 62.39 | 0.22 | −2.78 | 0.23 | <.0001 | |
| − | + | 59.54 | 0.13 | −5.63 | 0.14 | <.0001 | |
| + | + | 57.13 | 0.30 | −8.04 | 0.31 | <.0001 | |
| Female | − | − | 70.64 | 0.12 | Ref. | ||
| + | − | 67.09 | 0.66 | −3.55 | 0.67 | <.0001 | |
| − | + | 67.67 | 0.25 | −2.97 | 0.27 | ||
| + | + | 61.39 | 1.08 | −9.25 | 1.09 | <.0001 | |
Notes: HAU = Heavy alcohol use, as indicated by alcohol-induced medical conditions.
Adjusted for covariates including education, marital status, place of death, year of death, heavy tobacco use, diabetes, hypertension, and HIV based on predictive margins estimated from multiple linear regression.
Multiple R2 was .27 and .30 for all predictors in the regression model for male and female decedents, respectively. Source: Multiple Cause of Death data files, 1999–2006 (National Center for Health Statistics).
Discussion
In the present study, our primary interest was to examine the association of HAU, as indicated by the presence of alcohol-induced medical conditions, with premature death from HCC. To our knowledge, this is the first such study conducted on a population scale in the United States.
Our results confirmed the increased risk of HCC conferred by HAU, showing that decedents with HAU had higher prevalence ratios of dying from HCC than dying from non-CLD than did those without HAU. This is consistent with findings from previous studies that alcohol consumption is a risk factor for primary liver cancer (Bagnardi et al., 2001; El-Serag, 2004; Mueller et al., 2009). The results also indicated a much stronger association between HAU and risks for dying from other CLD than from HCC. This finding exhibits a pattern of competing mortality risks: Compared with those without HAU, those with HAU were more likely to die of other CLD before they had a chance to develop HCC. This is not surprising, given that excessive alcohol consumption may have direct and indirect effects on HCC carcinogenesis that involve a sequential process, with cirrhosis as an intermediate key step (Boffetta and Hashibe, 2006; Schafer and Sorrell, 1999). Although this has been suggested in the literature, our study is among the first to present systematic data on the three-group contrasts of competing mortality risks for HCC, other CLD, and all other causes with respect to HAU (Table 3).
Also of significance, our findings provided empirical evidence that HAU is associated with a reduction in the mean age at HCC death and an increased cumulative probability of HCC death across all race/ethnicity groups among both sexes. Chronic alcohol consumption may induce cytochrome P450 2E1 (CYP2E1) to lead to increased acetaldehyde production and reactive oxygen species generation, which alter cell integrity through the formation of adducts with DNA and cellular proteins (Brooks and Theruvathu, 2005; Freeman et al., 2005). Reactive oxygen species also can react with lipid molecules in the cell membrane, leading to the formation of biologically reactive aldehyde molecules that share structural and reactive properties similar to those of acetaldehyde (Das and Vasudevan, 2007; Seitz and Becker, 2007) and stimulate collagen synthesis in hepatic stellate cells. This is a key step in progressive hepatic scarring toward cirrhosis (Tuma and Casey, 2003). Chronic, heavy alcohol consumption also increases intrahepatic lipopolysac-charide levels and the activation of the liver's Kupffer cells. Once activated, Kupffer cells synthesize and release a range of proinflammatory cytokines.
Hepatic insults because of increases in proinflammatory cytokines and oxidative stress lead to rapid activation of hepatic stellate cells. The activation of hepatic stellate cells is characterized by transdifferentiation of cells to a myofi-broblastic state during which increased proliferation and collagen synthesis/deposition occurs. The continued deposition of collagen results in the emergence of hepatic scarring and the progression toward cirrhosis (Friedman, 2008). In addition, chronic alcohol consumption causes an imbalance in endothelin and nitric oxide signaling, resulting in increased vasoconstriction and portal pressure, and the changes in the microcirculation in and around transformed cells may play a role in the initiation and/or progression of angiogenesis in dysplastic nodules (hepatic foci). It appears that neoangio-genesis is a prerequisite for hepatic foci to expand to HCC (Sugimachi et al., 2003). These mechanisms link heavy chronic alcohol use with the increased rate of liver fibrosis, cirrhosis, and tumorigenesis and their progression. They therefore provide biologically plausible explanations for the age difference at HCC death reported in the present study.
By analyzing the data for men and women separately, our findings revealed that HAU affected women more strongly than men regarding premature HCC death, although overall, men had higher HCC mortality rates and died younger than women. Specifically, after controlling for demographic characteristics and other risk factors, those with HAU had greater reductions in adjusted mean age at HCC death among women than among men for non-Hispanic Whites (5.46 vs. 3.52 years), non-Hispanic Blacks (3.15 vs. 1.76 years), and Hispanics (5.07 vs. 3.18 years). Similarly, the difference in cumulative probability of HCC death between decedents with HAU and those without HAU was larger in women than in men after age 45 for these three racial/ethnic groups. In addition, interaction analysis of HAU and hepatitis B/C revealed that their combined effect tends to be additive in men but multiplicative in women. These patterns further support the previous observation that alcohol has more detrimental effects on the progression of liver disease in women than in men, given similar exposure levels (Becker et al., 1996).
A number of mechanisms have been cited to explain why women appear to be more vulnerable than men to many adverse consequences of alcohol use (Müller, 2006). First, men and women absorb and metabolize alcohol differently. In general, women have less body water than men of similar body weight and would achieve higher concentrations of alcohol in the blood than men after drinking equivalent amounts of alcohol (Taylor et al., 1996). Compared with men, women have a lower level of total gastric alcohol de-hydrogenase, an enzyme involved in alcohol metabolism, and thus a weaker first-pass barrier to prevent excess alcohol from penetrating the body. Although alcoholism reduces the first-pass metabolism in both men and women, the effect is more severe in women, resulting in a total loss of the first-pass protection (DiPadova et al., 1987; Frezza et al., 1990). Moreover, given that alcohol is metabolized almost entirely in the liver, women appear to eliminate alcohol from the blood faster because of their higher liver volume per unit of lean body mass (Kwo et al., 1998; Li et al., 2000). Although the mechanisms underlying the relationship between alcohol elimination rates and liver damage still are not clear, it has been speculated that faster alcohol elimination rates in women may result in higher concentrations of acetaldehyde in liver, a toxic product of alcohol metabolism (Thomasson, 2000). Findings from animal research also indicate that elevated female risk for liver damage from alcohol could be attributable to the physiological effects of the female reproductive hormone estrogen, which increases the sensitivity of hepatic Kupffer cells to endotoxin (Ikejima et al., 1998). Although studies continue to explore other explanations for sex differences in the susceptibility to alcohol's effects (e.g., differences in inflammatory cytokines and CYP2E1 expression), findings from our study underscore the importance of this line of research.
Taking advantage of the large case numbers in the mortality data, we were able to further break down the analysis by race/ethnicity, and we observed substantial racial/ethnic disparities both in the HCC mortality rate and in the mean age at death. Several explanations have been proposed for racial/ethnic differences in alcohol susceptibility, including metabolic, pharmacokinetic, and genetic mechanisms (Li et al., 2000; Lieber, 2000). Our results show that, compared with non-Hispanic White decedents, non-Hispanic Black decedents had a smaller reduction in mean age at death by HAU, especially among men. This is consistent with previous observations that Blacks had lower alcohol elimination rates than Whites (Thomasson, 1995) because of their lower liver weight per unit body weight (Li et al., 2000). Furthermore, the variability of the HAU effect on premature death from HCC may be partly attributable to the different patterns of comorbid conditions, such as co-infection with hepatitis C virus, hepatitis B virus, and HIV across different racial/ ethnic groups. For instance, our additional analysis revealed a higher prevalence of comorbidity with hepatitis B virus, hepatitis C virus, and HIV among non-Hispanic Black HCC decedents than among their non-Hispanic White counterparts (data not shown). These co-infections accelerate disease progression of HCC (García-Samaniego et al., 2001; Michielsen et al., 2005) and may have played a more important role in the younger age at death in this subpopulation group regardless of HAU status.
In most prospective studies, the duration of follow-up is insufficient to provide complete outcome data on the progression of HCC because it is not feasible to conduct large-scale prospective studies that follow subjects for a lifetime. The current study draws strength from mortality data, with all death records in the United States covering the full age spectrum of a life course. The large number of death certificates provided a study sample that is unparalleled in prospective studies. The detailed ICD-10 codes of the conditions that underlie or contribute to the final death allowed us to assess common combinations of events or conditions such as HAU and hepatitis C virus as risks for HCC. Additionally, to minimize the confounding, we excluded unnatural deaths from the analysis and adjusted our estimates for demographic factors that may be associated with the HCC risk or mortality.
At the same time, because of the nature of the mortality data, the present study has several limitations. First, we used alcohol-induced medical conditions as a proxy for HAU, and we lacked information on specific levels of alcohol consumption. Therefore, we were not able to assess the dose-response relationship between alcohol use (especially light and moderate alcohol use) and HCC mortality. Similarly, this limitation also applies to tobacco use and other risk factors because of the unavailability of information on the actual exposure levels in the MCD data.
Second, using alcohol-induced medical conditions as a proxy for heavy drinking may underidentify heavy drinkers because not all heavy drinkers develop these medical conditions, and alcohol-induced medical conditions are generally underreported on death certificates (Hanzlick, 1988; Pollock et al., 1987). Therefore, this measurement bias could lead to a conservative estimate of the impact of HAU on premature HCC death. Nonetheless, the underreporting problem should not significantly affect the validity of our findings because our focus is on comparisons among identified cases and not on prevalence estimates.
Third, because there was no information on treatment history in the death records, we were not able to control for the potential confounding effect of treatment differentials, although this bias should be small on a population scale because the prognosis of HCC has been uniformly poor, except when the patient receives liver transplantation (which is limited by resources).
Fourth, we could not control for a potential effect of state because beginning in 2005, state identification has no longer been included in the MCD. However, our sensitivity analyses based on data from 1999 to 2004 did not find substantial changes in the effect size estimates of HAU when including state as an additional covariate or in the standard error estimates of the HAU effect when including state as a cluster variable (i.e., considering deaths as being nested in states) in the models.
Fifth, the exclusion of unnatural deaths, although necessary in isolating external factors (e.g., injury and poisoning) that are unrelated to the natural course of disease progression, could result in an underestimation of the prevalence of HAU among non-CLD deaths presented in Table 2. The exclusion of deaths before age 25 may also introduce a potential bias. Given that the number of HCC decedents with death age less than 25 years is rare (only 233 in our data), this bias, if any, should be ignorable.
Finally, it is not possible for us to check the accuracy of the HCC diagnosis recorded on the death certificates or to access supplemental data, such as decedents’ medical histories, which could shed light on the information revealed by death certificate data (Redelings et al., 2007). Again, this potential measurement error should be small because previous research has found a close correspondence between the mortality data and the Surveillance, Epidemiology, and End Results incidence data (Altekruse et al., 2009) and hospital-ization data (Kim et al., 2005). The cross-validation of the MCD data and the National Inpatient Sample data by Kim et al. (2005) indicated a high consistency between death certificates and medical practitioners’ assessments recorded on the hospital records for HCC diagnoses among HCC decedents. Our own examination of other comorbid cancers among the HCC decedents also suggests that there is a very low chance that secondary liver cancer deaths were included in our analysis.
In conclusion, we have provided population mortality-based evidence that HAU contributes to the premature death from HCC in the United States, and we have shown that alcohol consumption affects men and women differently in terms of HCC mortality. Our findings suggest that abstinence from HAU not only will decrease the risk for HCC but also reduce the loss of life because of HCC.
Footnotes
This article is based on a study conducted for the Alcohol Epidemiologic Data System project funded by the National Institute on Alcohol Abuse and Alcoholism through Contract No. HHSN267200800023C to CSR Incorporated. The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of the sponsoring agency or the federal government.
References
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of Clinical Oncology. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of Internal Medicine. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. Alcohol consumption and the risk of cancer: A meta-analysis. Alcohol Research & Health. 2001;25:263–270. [PMC free article] [PubMed] [Google Scholar]
- Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Müller CF, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: A prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Hashibe M. Alcohol and cancer. The Lancet Oncology. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: Implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–193. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Chen CM, Yoon Y-H, Yi H-Y, Lucas DL. Alcohol and hepatitis C mortality among males and females in the United States: A life table analysis. Alcoholism: Clinical and Experimental Research. 2007;31:285–292. doi: 10.1111/j.1530-0277.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sciences. 2007;81:177–187. doi: 10.1016/j.lfs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Davila JA, El-Serag HB. Racial differences in survival of hepatocellular carcinoma in the United States: A population-based study. Clinical Gastroenterology and Hepatology. 2006;4:104–110. quiz 4–5. [PubMed] [Google Scholar]
- DiPadova C, Worner TM, Julkunen RJ, Lieber CS. Effects of fasting and chronic alcohol consumption on the first-pass metabolism of ethanol. Gastroenterology. 1987;92:1169–1173. doi: 10.1016/s0016-5085(87)91073-0. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. The New England Journal of Medicine. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- Freeman TL, Tuma DJ, Thiele GM, Klassen LW, Worrall S, Niemelä O, Preedy VR. Recent advances in alcohol-induced adduct formation. Alcoholism: Clinical and Experimental Research. 2005;29:1310–1316. doi: 10.1097/01.alc.0000171484.52201.52. [DOI] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women—The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. New England Journal of Medicine. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiological Reviews. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Samaniego J, Rodríguez M, Berenguer J, Rodríguez-Rosado R, Carbó J, Asensi V, Soriano V. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. American Journal of Gastroenterology. 2001;96:179–183. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and Alcohol Dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- Hanzlick R. Death certificates, natural death, and alcohol. The problem of underreporting. American Journal of Forensic Medicine and Pathology. 1988;9:149–150. doi: 10.1097/00000433-198806000-00011. [DOI] [PubMed] [Google Scholar]
- Harrison LE, Reichman T, Koneru B, Fisher A, Wilson D, Dela Torre A, Korogodsky M. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Archives of Surgery. 2004;139:992–996. doi: 10.1001/archsurg.139.9.992. [DOI] [PubMed] [Google Scholar]
- Hassan MM, Hwang L-Y, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Patt YZ. Risk factors for hepatocellular carcinoma: Synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. National Vital Statistics Reports. 2009. Deaths: Final data for 2006. 57(14), Hyattsville, MD: National Center for Health Statistics. Available at http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. [PubMed] [Google Scholar]
- Ikejima K, Enomoto N, Iimuro Y, Ikejima A, Fang D, Xu J, Thurman RG. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. American Journal of Physiology. 1998;274:G669–G676. doi: 10.1152/ajpgi.1998.274.4.G669. [DOI] [PubMed] [Google Scholar]
- Ingram DD, Parker JD, Schenker N, Weed JA, Hamilton B, Arias E, Madans JH. Vital Health Stat. 2003. United States Census 2000 population with bridged race categories (DHHS Publication No. PHS 2003-1335) 2 (135). Hyattsville, MD: National Center for Health Statistics. Available at http://www.cdc.gov/nchs/data/series/sr_02/sr02_135.pdf. [PubMed] [Google Scholar]
- Kim WR, Gores GJ, Benson JT, Therneau TM, Melton LJ., III Mortality and hospital utilization for hepatocellular carcinoma in the United States. Gastroenterology. 2005;129:486–493. doi: 10.1016/j.gastro.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Kwo PY, Ramchandani VA, O'Connor S, Amann D, Carr LG, Sandrasegaran K, Li T-K. Gender differences in alcohol metabolism: Relationship to liver volume and effect of adjusting for body mass. Gastroenterology. 1998;115:1552–1557. doi: 10.1016/s0016-5085(98)70035-6. [DOI] [PubMed] [Google Scholar]
- Li T-K, Beard JD, Orr WE, Kwo PY, Ramchandani VA, Thomasson HR. Variation in ethanol pharmacokinetics and perceived gender and ethnic differences in alcohol elimination. Alcoholism: Clinical and Experimental Research. 2000;24:415–416. [PubMed] [Google Scholar]
- Lieber CS. Ethnic and gender differences in ethanol metabolism. Alcoholism: Clinical and Experimental Research. 2000;24:417–418. [PubMed] [Google Scholar]
- Manos MM, Leyden WA, Murphy RC, Terrault NA, Bell BP. Limitations of conventionally derived chronic liver disease mortality rates: Results of a comprehensive assessment. Hepatology. 2008;47:1150–1157. doi: 10.1002/hep.22181. [DOI] [PubMed] [Google Scholar]
- Michielsen PP, Francque SM, van Dongen JL. Viral hepatitis and hepatocellular carcinoma. 2005. World Journal of Surgical Oncology, 3(1), 27. Available at http://www.wjso.com/content/3/1/27. [DOI] [PMC free article] [PubMed]
- Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: A frequently underestimated combination. World Journal of Gastroenterology. 2009;15:3462–3471. doi: 10.3748/wjg.15.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C. Liver, alcohol and gender. Wiener Medizinische Wochen-schrift. 2006;156:523–526. doi: 10.1007/s10354-006-0348-8. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Bridged-race intercensal estimates for July 1, 1990–July 1, 1999, United States resident population by county, single-year of age, sex, race, and Hispanic origin. 2004. Prepared by the U.S. Census Bureau with support from the National Cancer Institute. Available at http://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm#july1999. [Google Scholar]
- National Center for Health Statistics. Postcensal estimates of the resident population of the United States for July 1, 2000–July 1, 2007, by year, county, age, bridged race, Hispanic origin, and sex (Vintage 2007) 2008. Prepared under a collaborative arrangement with the U.S. Census Bureau; released August 7, 2008. Available at http://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm#vintage2007. [Google Scholar]
- National Center for Health Statistics. Instructions for classifying the underlying cause-of-death, ICD-10: Part 2a. 2010. Available at http://www.cdc.gov/nchs/data/dvs/2A_2010acc.pdf.
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. International Journal of Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pollock DA, Boyle CA, DeStefano F, Moyer LA, Kirk ML. Underreporting of alcohol-related mortality on death certificates of young US Army veterans. Journal of the American Medical Association. 1987;258:345–348. [PubMed] [Google Scholar]
- Raoul JL. Natural history of hepatocellular carcinoma and current treatment options. Seminars in Nuclear Medicine. 2008;38:S13–S18. doi: 10.1053/j.semnuclmed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Redelings MD, Wise M, Sorvillo F. Using multiple cause-of-death data to investigate associations and causality between conditions listed on the death certificate. American Journal of Epidemiology. 2007;166:104–108. doi: 10.1093/aje/kwm037. [DOI] [PubMed] [Google Scholar]
- Research Triangle Institute. SUDAAN: Software for the Statistical Analysis of Correlated Data, Version 10. 2008 Research Triangle Park, NC. [Google Scholar]
- Schafer DF, Sorrell MF. Hepatocellular carcinoma. The Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Research & Health. 2007;30:38–41. 44–47. [PMC free article] [PubMed] [Google Scholar]
- Selvin S. Statistical analysis of epidemiologic data. 2nd ed. New York, NY: Oxford University Press; 1996. [Google Scholar]
- Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. Journal of Clinical Gastroenterology. 2007;41:761–772. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. American Journal of Epidemiology. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- Sugimachi K, Tanaka S, Taguchi K, Aishima S, Shimada M, Tsuneyoshi M. Angiopoietin switching regulates angiogenesis and progression of human hepatocellular carcinoma. Journal of Clinical Pathology. 2003;56:854–860. doi: 10.1136/jcp.56.11.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Dolhert N, Friedman L, Mumenthaler M, Yesavage JA. Alcohol elimination and simulator performance of male and female aviators: A preliminary report. Aviation, Space, and Environmental Medicine. 1996;67:407–413. [PubMed] [Google Scholar]
- Thomasson H. Alcohol elimination: Faster in women? Alcoholism: Clinical and Experimental Research. 2000;24:419–420. [PubMed] [Google Scholar]
- Thomasson HR. Gender differences in alcohol metabolism. Physiological responses to ethanol. In: Galanter M, editor. Recent developments in alcoholism. Volume 12, Alcoholism and Women. New York, NY: Plenum Press; 1995. pp. 163–179. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Casey CA. Dangerous byproducts of alcohol breakdown—focus on adducts. Alcohol Research & Health. 2003;27:285–290. [PMC free article] [PubMed] [Google Scholar]
- Voigt MD. Alcohol in hepatocellular cancer. Clinics in Liver Disease. 2005;9:151–169. doi: 10.1016/j.cld.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. American Journal of Medicine. 2008;121:525–531. doi: 10.1016/j.amjmed.2008.03.005. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International classification of diseases and related health problems, tenth revision. Geneva, Switzerland: Author; 1992. [Google Scholar]
- Yu MC, Yuan J-M, Lu SC. Alcohol, cofactors and the genetics of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 2008;23(Supplement s1):S92–S97. doi: 10.1111/j.1440-1746.2007.05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]