Abstract
Objective:
We examined the role of anger, hostility, and aggression, in addition to depression and stress, in predicting persistent smoking during pregnancy in a low-income sample.
Method:
The sample consisted of 270 pregnant women (189 smokers, 81 nonsmokers) recruited into a prospective study of prenatal cigarette exposure in the first trimester. Persistent pregnancy smoking was defined as self-reporting daily smoking in at least two trimesters, a positive salivary cotinine level in at least two trimesters, or infant meconium positive for nicotine and/ or its metabolites.
Results:
Persistent smokers reported higher prenatal stress and negative affect symptoms (depression, anger, hostility, aggression) than nonpersistent smokers or nonsmokers. However, in the context of model testing, maternal anger, hostility, and aggression each accounted for unique variance in persistent smoking, whereas symptoms of depression and stress did not.
Conclusions:
To date, interventions for pregnant low-income smokers have been largely ineffective. The current results suggest that anger management interventions may be particularly effective for low-income persistent pregnant smokers and may be more likely to prevent relapse than depression-focused interventions.
Tobacco is one of the most commonly used drugs during pregnancy. In 2006, mean smoking prevalence was 13.2%, with rates as high as 33% among women with a high school education or less (Martin et al., 2006). Smoking during pregnancy is harmful for mother and child, with increased risk of prenatal complications such as risk of abruption, placenta previa, perinatal mortality, impaired fetal growth, higher neonatal stress and irritability, hearing loss, and respiratory problems (Andres and Day, 2000; Cornelius and Day, 2000; Cornelius et al., 2004; Fried, 1998; Mccart-ney et al., 1994; Stroud et al., 2009). Cigarette exposure also has long-lasting effects on developmental outcomes such as externalizing behavior problems (Buschgens et al., 2009; Porath and Fried, 2005; Silberg et al., 2003; Wakschlag et al., 2011) and carries with it a higher risk of smoking in later childhood and adolescence (Cornelius et al., 2004; O'Callaghan et al., 2006).
Despite concerted efforts to increase awareness of the detrimental effects of smoking during pregnancy, a substantial number of women, especially low-income women, continue to smoke during pregnancy (Cnattingius et al., 1992, 2004; Cornelius and Day, 2000; Day et al., 1992; Williamson et al., 1989). Although motivation to quit is typically high during pregnancy (Andres and Day, 2000), only about 25% of smokers are able to remain abstinent throughout pregnancy (Flick et al., 2006). The rates of smoking during pregnancy are especially high among younger, unmarried, low-income women with high school or less than high school education (Cornelius et al., 2004). Thus, maternal cigarette smoking during pregnancy is a significant public health issue that may have profound effects on child development.
Cigarette smoking during pregnancy is associated with higher depression and stress, with rates particularly high among low-income pregnant smokers (Bullock et al., 2001; Ludman et al., 2000; Munafò et al., 2008). Results from prospective studies indicate that lower depression and stress prospectively predict higher quit rates in early but not later pregnancy (Ludman et al., 2000); depression and stress decrease over time among nonsmokers but increase among smokers (Ludman et al., 2000). Depression and stress are also key mediators of the association between socioeconomic status and quitting (Businelle et al., 2010) as well as relapse among pregnant quitters (Reitzel et al., 2010). Thus, depression and stress may be key predictors of lower cessation rates among low-income pregnant smokers. Indeed, new treatment development studies targeted depression-focused treatment for pregnant smokers with some success, particularly for women with higher depressive symptoms (Cinciripini et al., 2010). However, treatment effects have not lasted beyond 6 months.
One explanation for lack of long-lasting effects for depression-focused treatments may be that they have not incorporated other aspects of negative affect that may be particularly salient among low-income pregnant smokers. A number of studies indicate that smoking is positively associated with hostility in the general population. Hostility is characterized by negative attitudes and beliefs toward other people and frequent, intense bouts of anger and aggression (Houston and Vavak, 1991; Miller et al., 1996). Trait hostility consistently predicts higher smoking rates in both men and women (Lipkus et al., 1994; Siegler et al., 1992; Whiteman et al., 1997). In contrast to the large number of studies focusing on the association between maternal depression/ stress and smoking during pregnancy (Bullock et al., 2001; Businelle et al., 2010; Cinciripini et al., 2010; Ludman et al., 2000; Munafò et al., 2008; Reitzel et al., 2010), few investigated anger, hostility, or aggression among pregnant smokers. One exception is the study by Schuetze et al. (2008), which indicated that pregnant smokers report higher hostile mood and maternal hostility. Thus, in addition to symptoms of depression and stress, symptoms of anger/hostility or aggression may be important predictors of continued smoking during pregnancy.
The goal of the current study was to examine if pregnant persistent smokers report higher anger, hostility, and aggression than nonpersistent smokers or nonsmokers and if these negative affect symptoms account for unique variance in continued smoking through pregnancy in addition to depression and stress. We hypothesized that mothers who smoked during pregnancy would have higher scores on angry, hostile, and aggressive mood. We also hypothesized that maternal anger, hostility, and aggression would account for unique variance in persistent smoking during pregnancy and higher intensity of smoking in a model including depression and stress.
Method
Sample selection
Women who presented for care at the prenatal clinic of a large urban hospital were asked to complete a screening form during their first prenatal appointment. Women who were eligible were invited to participate in an ongoing longitudinal study of maternal health and child development. Initial eligibility criteria included the following: less than 20 weeks gestation, maternal age of 18 or older, no illicit drug use (other than cannabis), no heavy alcohol use (more than one drink/day on average or four drinks on one occasion) after pregnancy recognition, and no multiple fetuses. For the purposes of this study, women who agreed to participate were interviewed once at the end of each trimester of pregnancy. At the end of each month, the closest matching nonsmoker (based on age and education) was invited to participate. One nonsmoker was recruited for every two smokers (taking the average of age and education of both).
Participants
The sample consisted of 270 pregnant women (189 smokers and 81 nonsmokers). Women ranged in age from 18 to 39 years (M = 23.96, SD = 4.96). The sample was 51% African American, 19% Hispanic, 29% White, and 1% other or mixed race. Forty-six percent of the women were married or living with their partner, 32% were in a relationship but not living with a partner, and the rest were single. Twenty-nine percent of women had less than a high school education, 29% had earned their high school diploma, 29% had completed some college courses, 9% had completed a vocational degree or technical training degree, and 4% had earned a bachelor's degree. Fifty-nine percent were working at a paid job for 4–45 hours per week. At recruitment, 7.4% had received mental health services in the past 6 months, 15% received Temporary Assistance for Needy Families (TANF), 55% received food stamps, 68% were on Medicaid, and 64% received assistance from the Women, Infants, and Children (WIC) program. Thus, the sample consisted mostly of young, low-income minority pregnant women.
Procedure
All mothers were screened for initial eligibility and matching criteria. Informed written consent was obtained from interested, eligible mothers. Mothers were interviewed at the end of each trimester; maternal oral fluid samples were collected and assayed for cotinine, a primary metabolite of nicotine; and infant meconium samples were collected at delivery. The study was approved by the appropriate institutional review board. Participants were informed that data confidentiality was protected by a U.S. Federal Certificate of Confidentiality issued by the National Institute on Drug Abuse. Participants received a $20.00 check for each prenatal interview.
Maternal substance use.
Maternal smoking status was determined through a combination of self-report, infant meconium results, and maternal oral fluid results. For self-report data, participants were interviewed in a private setting by trained interviewers. At each prenatal interview and at the postnatal interviews, the Timeline Followback (TLFB) interview (Sobell et al., 1986) was used to assess maternal substance use. Participants were given a calendar and asked to identify events of personal interest (i.e., holidays, birthdays, vacations) as anchor points to aid recall. This method has been established as a reliable and valid method of obtaining longitudinal data on substance-use patterns, has good test–retest reliability (with r > .85 across multiple samples), and is highly correlated with other intensive self-report measures, with the majority of correlations in the .80–.97 range (Brown et al., 1998). At each prenatal appointment, the TLFB was used to gather daily tobacco, alcohol, and cannabis use for the previous 3 months. Women who smoked “blunts” were asked how many joints they could have rolled from the amount of marijuana in the blunt. Thus, self-reported data spanned from 3 months before conception through delivery. TLFB yielded a number of different measures of substance use.
Maternal oral fluid was collected at each prenatal interview to provide objective evidence of recent exposure. The oral fluid specimens were analyzed by a commercial laboratory for cotinine, the primary nicotine biomarker. Enzyme-linked immunosorbent assay at 10 ng/mL was used only for the first 32 women recruited into the study (for budgetary reasons). The more sensitive liquid chromatography–tandem mass spectrometry (LC-MSMS) at 5 ng/mL cutoff for cotinine for active smoking was used for the remainder of the sample at the first prenatal interview and for the full sample at the second and third prenatal interviews. LC-MSMS is preferable because of lower detection limits and lower required sample volume (Kellogg et al., 2004).
After birth, meconium specimens were collected from soiled diapers twice daily until the appearance of milk stool, transferred to storage containers, and frozen until transport to the National Institute on Drug Abuse for analysis. Meconium specimens were assayed with a validated LS-MSMS method for nicotine, cotinine, and trans-3’-hydroxycotinine (Gray et al., 2010a, 2010b). Among the 189 pregnant smokers, 96% self-reported smoking during pregnancy; 59% had infants with nicotine and/or metabolites in meconium; and 79% had oral fluid samples that tested positive for cotinine, a primary metabolite of nicotine.
Similar to previous studies (Weaver et al., 2008), persistent pregnancy smoking was defined as self-report of daily smoking in at least two trimesters, a positive salivary cotinine level in at least two trimesters, or infant meconium positive for nicotine or its metabolites. Previous research indicated that a positive infant meconium sample reflects primarily third trimester exposure (Gray et al., 2010a, 2010b). All infants with positive meconium samples had mothers who smoked in at least the first trimester. Previous studies noted high variability in smoking during pregnancy (Pickett et al., 2005), and this definition identified a group of women with relatively stable smoking behavior during pregnancy. In addition, the average number of cigarettes per day over the course of pregnancy was used as the primary measure of smoking intensity.
Depression and stress.
Symptoms of depression during pregnancy were assessed using the Beck Depression Inventory (Beck and Steer, 1984; Sharp and Lipsky, 2002) and the Perceived Stress Scale (Cohen et al., 1983). The Beck Depression Inventory is a widely used self-report measure for assessing the severity of depressive symptoms. It has high reliability in a range of populations and has high internal consistency and well-established construct validity (Beck and Steer, 1984; Sharp and Lipsky, 2002). The internal consistency of the Beck Depression Inventory was quite high for this sample (Cronbach's α = .87). The Perceived Stress Scale is a widely used self-report measure designed to measure perceived stress. Respondents report the occurrence of an item within the last month on a 5-point scale, ranging from never to very often. The Perceived Stress Scale has good reliability and validity with Cronbach's α ranging from .84 to .86 in different samples (Cohen et al., 1983) and internal consistency of .92 for the current sample.
Anger/hostility/aggression.
Symptoms of anger, hostility, and aggression were measured during pregnancy with the Buss–Perry Aggression Questionnaire (Buss and Perry, 1992). This measure yields four subscales: anger, hostility, physical, and verbal aggression. Internal consistency for the four subscales ranged from .72 to .83 for this sample. Confirmatory factor analysis with the four subscales as indicators of a latent variable for anger/hostility/aggression indicated that the four scales loaded on one factor with factor loadings ranging from .72 to .87.
Data analyses.
Analysis of variance (ANOVA) and multivariate analysis of variance (MANOVA) were used to test group differences in demographics and maternal psychological symptoms. Mplus Version 6.0 (Muthén and Muthén, 1998–2004) was used for model testing, allowing for inclusion of related categorical and continuous dependent variables in the same model. The model tested the variance accounted for by depression/stress and anger/hostility/ aggression in a binary outcome (persistent smoking vs. not) with a probit regression while also considering the continuous dependent variable, average number of cigarettes per day during pregnancy. We used weighted least squares mean and variance adjusted (WLSMV) estimation for model testing, as recommended by Muthén and Muthén (1998–2004) for binary outcome data.
Results
Smoking during pregnancy.
Following previous studies (Weaver et al., 2008), combining self-report and cotinine measures indicates that 83% of women (n = 157) in the smoking group were persistent pregnancy smokers (58% of the sample including nonsmokers). On average, persistent smokers reported smoking 11.89 (SD = 7.77), 8.51 (SD = 5.89), 4.89 (SD = 4.76), and 4.44 (SD = 5.33) cigarettes per day for 3 months before conception and during the first, second, and third trimesters, respectively. Of the remaining 32 nonpersistent smokers, 8 denied smoking on the self-report instruments (health screen, TLFB). Of these, 5 were assigned to the smoking group based on positive infant meconium samples and 3 were assigned to the smoking group because their first trimester oral fluid samples were positive, although all other indicators (meconium and self-report) were negative. On average, nonpersistent smokers reported smoking 4.75 (SD = 0.83), 1.68 (SD = 0.29), 0, and 0.02 (SD = 0.02) cigarettes per day for 3 months before conception and during the first, second, and third trimesters, respectively.
Group differences in demographics and negative affect symptoms.
In this predominantly low-income sample, there were no group differences in maternal education, marital status, work status, TANF enrollment, or WIC use among persistent smokers and nonpersistent smokers or nonsmokers. However, persistent smokers were more likely to use food stamps, with 61% of persistent smokers receiving food stamps compared with 40% of nonpersistent smokers and 49% of nonsmokers.
ANOVA with group status (persistent smokers, nonpersistent smokers, and nonsmokers) as the independent variable indicated a significant group difference on symptoms of depression. As indicated in Table 1, persistent smokers reported more symptoms of depression than the other two groups. Similarly, ANOVA with perceived stress as the dependent measures indicated a significant group difference. Persistent smokers reported higher stress than nonsmokers. MANOVA with group status as the independent variable and the four subscales of the Buss–Perry Aggression Questionnaire as the dependent measures yielded a significant multivariate effect of group status, F(8, 528) = 2.69, p < .01, ηp2 = .04. Univariate analyses indicated that persistent smokers reported higher anger, hostility, and aggression than nonsmokers and higher anger and verbal aggression than nonpersistent smokers (Table 1).
Table 1.
Mean differences between smokers and nonsmokers
| Smoker |
||||||||
| Nonsmoker |
Nonpersistent |
Persistent |
||||||
| Variable | M | SD | M | SD | M | SD | F | ηp2 |
| Depressed mood | 14.15a | 7.49 | 14.10a | 6.29 | 16.54b | 7.95 | 3.26 | .02 |
| Perceived stress | 23.31a | 7.50 | 24.41 | 6.98 | 26.38b | 7.01 | 5.08 | .04 |
| Anger | 17.16a | 5.33 | 17.56a | 6.20 | 19.97b | 5.86 | 7.24 | .05 |
| Hostility | 17.35a | 6.38 | 18.65 | 6.23 | 20.15b | 6.33 | 5.25 | .04 |
| Verbal aggression | 14.38a | 3.98 | 14.79a | 4.18 | 16.68b | 4.13 | 9.45 | .07 |
| Physical aggression | 20.10a | 7.33 | 21.06 | 5.77 | 23.14b | 7.10 | 5.28 | .04 |
Notes: Numbers with different superscripts were significantly different from each other at p < .05.
Associations among variables.
Correlations among variables in the model are depicted in Table 2. As indicated in the table, smoking a higher number of cigarettes per day and persistent smoking during pregnancy were significantly associated with higher symptoms of depression, perceived stress, verbal aggression, anger, and hostility. Depression and stress were highly correlated with each other and more moderately correlated with the Buss–Perry Aggression Questionnaire scales.
Table 2.
Correlations among variables
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
| 1. No. of cigarettes/day | |||||||
| 2. Persistent smoking | .63** | ||||||
| 3. Depressed mood | .14* | .15* | |||||
| 4. Perceived stress | .16** | .19** | .63** | ||||
| 5. Physical aggression | .11 | .19** | .31** | .28** | |||
| 6. Verbal aggression | .14* | .26** | .25** | .15* | .54** | ||
| 7. Anger | .21** | .23** | .42** | .41** | .65** | .65** | |
| 8. Hostility | .15* | .19** | .44** | .46** | .63** | .58** | .69** |
p < .05;
p < .01.
Model testing.
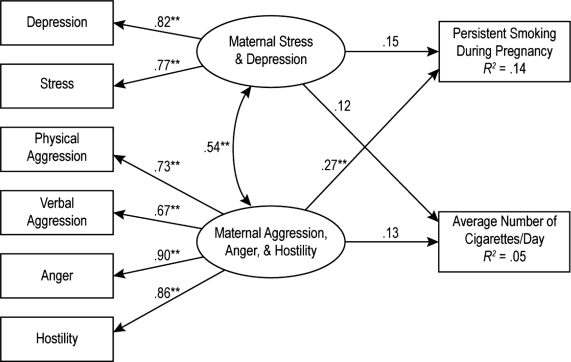
Given the high correlation between depression and stress, we used the total score on the Beck Depression Inventory and the total perceived stress score as measured indicators of a latent variable indicating high depression and stress during pregnancy. To examine whether angry/hostile mood accounted for additional unique variance in pregnancy smoking after including depression and stress, we tested a model with the latent variable for anger/hostility and a latent variable for depression and stress as predictors of persistent smoking (0 = nonsmoker or nonpersistent smoker) and the number of cigarettes per day during pregnancy. Results indicated that there was a significant positive association between the latent variables of anger/hostility and depression/stress, as would be expected from the bivari-ate associations; anger/hostility accounted for unique variance in persistent smoking whereas symptoms of depression and perceived stress did not. This model with standardized path coefficients is displayed in Figure 1.
Figure 1.
Model testing. Persistent smoking during pregnancy was dummy coded as 0 = nonsmoker or nonpersistent smoker, 1 = persistent smoker. The association between the two latent variables for depression and anger/hostility was modeled as the association between the residuals. Maternal anger/hostility accounted for unique variance in persistent smoking. **p < .05.
Discussion
Results supported the hypothesis that cigarette smokers would be associated with higher levels of anger, hostility, and aggression than nonsmokers or nonpersistent smokers, and anger/hostility/aggression accounted for unique variance in persistent smoking. These results are supportive of both theory and research suggesting that smokers may use cigarettes to regulate negative affect and of previous studies indicating that these negative affect symptoms may play a crucial role in continued smoking during pregnancy (Bullock et al., 2001; Ludman et al., 2000; Munafò et al., 2008). However, few studies of pregnant smokers have examined aspects of negative affect other than depression or stress, especially in nontreatment samples. In a study of low-income pregnant adolescent smokers and nonsmokers, Cornelius et al (2004) reported that persistent adolescent smokers scored higher on externalizing behavior problems in general and reported higher aggression and delinquency than nonsmokers. In a study of adults presenting for treatment for substance use disorders, women were reported to have higher levels of hostility than men (Robinson et al., 2001). The results from the current study indicate that issues of aggression, anger, and hostility are apparent in adult low-income pregnant smokers as well and are associated with continued smoking during pregnancy even when considered in the context of higher stress and depression.
Persistent smokers in this study were significantly heavier smokers before conception and did reduce the amount smoked after pregnancy recognition. It is possible that higher aggression, anger, and hostility among persistent smokers is a function of withdrawal effects associated with cutting down on the number of cigarettes. One previous treatment study of pregnant smokers reported that women who abstained from smoking during a clinical trial reported higher anger and impatience during the first 5 days of a cessation attempt (Heil et al., 2006). Although we did not have concurrent assessments of aggressive or angry hostile mood at multiple time points before or during pregnancy, this is one possible explanation for the current results. Another explanation is that women who continue to smoke throughout pregnancy may reflect a broader antisocial phenotype (Silberg et al., 2003), and the association between persistent smoking and higher aggression, anger, and hostility is a reflection of this broader phenotype.
Although data from the current study are not suitable for ascertaining directionality or causation, the association between anger/hostility and continued smoking during pregnancy has significant implications for intervening with low-income persistent smokers. Recent treatment models for low-income pregnant women have focused on negative affect reduction, with the primary focus on depression. The current results indicate that anger management issues may play a more crucial role in continued smoking during pregnancy. This is similar to the role of anger as a significant predictor of relapse for other substances of abuse such as alcohol (Kelly et al., 2010). Anger management interventions developed for substance-using populations may be particularly effective for low-income persistent pregnant smokers (González-Prendes, 2008; Reilly et al., 2000) and may be more likely to prevent relapse than treatments focused on depression alone.
Strengths, limitations, and conclusions.
One strength of this study is that multiple methods identified smokers and nonsmokers. As is evident from the discrepancies between self-reports and biological samples, reliance on one method alone would have misclassified several women in the study. The use of repeated prospective assessments of substance use during pregnancy with self-reports and oral fluid samples is another strength. In addition, examination of other aspects of negative affect in addition to depression fills a gap in the literature.
The study has several limitations. The first is lack of repeated assessments of depression or aggression, anger, and hostility. Thus, it is unclear if there were changes in these mood states across pregnancy that may be predictive of persistent smoking. Future studies with repeated assessment of mood across pregnancy may be better able to answer this question. Second, the sample was restricted to pregnant smokers with low levels of alcohol use, low to moderate marijuana use, and no other illicit substance use during pregnancy. Thus, the results are generalizable only to low-income smokers who meet these criteria. It is possible that heavier smokers who also were more likely to engage in heavier use of other substances have higher levels of psychological symptoms than noted in this more restricted sample. Future studies not investigating prenatal effects on developmental outcomes with less stringent recruitment criteria may yield different results.
However, despite these limitations, the results do have some clear public health implications for pregnant smokers. It is possible that the lack of consistent and long-lasting effects of depression-focused treatment for low-income pregnant smokers may be because of the salience of aggressive, angry, and hostile mood states in addition to depression among these women. Smoking treatment for persistent low-income smokers has been largely ineffective. The current results suggest that negative affect reduction treatments focused on aggressive, angry, hostile mood as well as depression in the context of smoking cessation may be more efficacious.
Acknowledgments
The authors are grateful to the families who participated in the study and to Research Technicians who conducted the prenatal assessments. Special thanks goes to Dr. Lele at Women and Children's Hospital of Buffalo for her collaboration on data collection.
Footnotes
This study was supported by the National Institute on Drug Abuse at the National Institutes of Health (Intramural Research Program and grant number R01 DA 013190).
References
- Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Seminars in Neonatology. 2000;5:231–241. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. Journal of Clinical Psychology. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- Bullock LF, Mears JL, Woodcock C, Record R. Retrospective study of the association of stress and smoking during pregnancy in rural women. Addictive Behaviors. 2001;26:405–413. doi: 10.1016/s0306-4603(00)00118-0. [DOI] [PubMed] [Google Scholar]
- Buschgens CJM, Swinkels SHN, van Aken MAG, Ormel J, Verhulst FC, Buitelaar JK. Externalizing behaviors in preadolescents: familial risk to externalizing behaviors, prenatal and perinatal risks, and their interactions. European Child & Adolescent Psychiatry. 2009;18:65–74. doi: 10.1007/s00787-008-0704-x. [DOI] [PubMed] [Google Scholar]
- Businelle MS, Kendzor DE, Reitzel LR, Costello TJ, Cofta-Woerpel L, Li Y, Wetter DW. Mechanisms linking socio-economic status to smoking cessation: A structural equation modeling approach. Health Psychology. 2010;29:262–273. doi: 10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Blalock JA, Minnix JA, Robinson JD, Brown VL, Lam C, Karam-Hage M. Effects of an intensive depression-focused intervention for smoking cessation in pregnancy. Journal of Consulting and Clinical Psychology. 2010;78:44–54. doi: 10.1037/a0018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, Lindmark G, Meirik O. Who continues to smoke while pregnant? Journal of Epidemiology and Community Health. 1992;46:218–221. doi: 10.1136/jech.46.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cornelius MD, Day NL. The effects of tobacco use during and after pregnancy on exposed children. Alcohol Research & Health. 2000;24:242–249. [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L. Characteristics of persistent smoking among pregnant teenagers followed to young adulthood. Nicotine & Tobacco Research. 2004;6:159–169. doi: 10.1080/14622200310001656975. [DOI] [PubMed] [Google Scholar]
- Day N, Cornelius M, Goldschmidt L, Richardson G, Robles N, Taylor P. The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicology and Teratology. 1992;14:407–414. doi: 10.1016/0892-0362(92)90051-b. [DOI] [PubMed] [Google Scholar]
- Flick LH, Cook CA, Homan SM, McSweeney M, Campbell C, Parnell L. Persistent tobacco use during pregnancy and the likelihood of psychiatric disorders. American Journal of Public Health. 2006;96:1799–1807. doi: 10.2105/AJPH.2004.057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried P. Cigarette smoke exposure and hearing loss. Journal of the American Medical Association. 1998;280:963–964. [PubMed] [Google Scholar]
- González-Prendes AA. Anger-control group counseling for women recovering from alcohol or drug addiction. Research on Social Work Practice. 2008;18:616–625. [Google Scholar]
- Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, Hues-tis MA. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine & Tobacco Research. 2010a;12:658–664. doi: 10.1093/ntr/ntq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TR, Eiden RD, Leonard KE, Connors GJ, Shisler S, Huestis MA. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clinical Chemistry. 2010b;56:1442–1450. doi: 10.1373/clinchem.2010.147876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Mongeon JA, Badger GJ, Bernstein IM. Characterizing nicotine withdrawal in pregnant cigarette smokers. Experimental and Clinical Psychopharmacology. 2006;14:165–170. doi: 10.1037/1064-1297.14.2.165. [DOI] [PubMed] [Google Scholar]
- Houston BK, Vavak CR. Cynical hostility: Developmental factors, psychosocial correlates, and health behaviors. Health Psychology. 1991;10:9–17. doi: 10.1037//0278-6133.10.1.9. [DOI] [PubMed] [Google Scholar]
- Kellogg MD, Behaderovic J, Bhalala O, Rifai N. Rapid and simple tandem mass spectrometry method for determination of serum cotinine concentration. Clinical Chemistry. 2004;50:2157–2159. doi: 10.1373/clinchem.2004.039594. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Stout RL, Tonigan JS, Magill M, Pagano ME. Negative affect, relapse, and Alcoholics Anonymous (AA): Does AA work by reducing anger? Journal of Studies on Alcohol and Drugs. 2010;71:434–444. doi: 10.15288/jsad.2010.71.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkus IM, Barefoot JC, Williams RB, Siegler IC. Personality measures as predictors of smoking initiation and cessation in the UNC Alumni Heart Study. Health Psychology. 1994;13:149–155. doi: 10.1037//0278-6133.13.2.149. [DOI] [PubMed] [Google Scholar]
- Ludman EJ, McBride CM, Nelson JC, Curry SJ, Grothaus LC, Lando HA, Pirie PL. Stress, depressive symptoms, and smoking cessation among pregnant women. Health Psychology. 2000;19:21–27. doi: 10.1037//0278-6133.19.1.21. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. (2006, September 29). Births: Final data for. National Vital Statistics Reports. 2004;55:1–101. [PubMed] [Google Scholar]
- Mccartney JS, Fried PA, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicology and Teratology. 1994;16:269–276. doi: 10.1016/0892-0362(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Miller TQ, Smith TW, Turner CW, Guijarro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psychological Bulletin. 1996;119:322–348. doi: 10.1037/0033-2909.119.2.322. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine & Tobacco Research. 2008;10:1609–1620. doi: 10.1080/14622200802412895. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. M-plus user's guide. Los Angeles, CA: Authors; 1998–2004. [Google Scholar]
- O'Callaghan FV, O'Callaghan M, Najman JM, Williams GM, Bor W, Alati R. Prediction of adolescent smoking from family and social risk factors at 5 years, and maternal smoking in pregnancy and at 5 and 14 years. Addiction. 2006;101:282–290. doi: 10.1111/j.1360-0443.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatric and Perinatal Epidemiology. 2005;19:368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Porath AJ, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicology and Teratology. 2005;27:267–277. doi: 10.1016/j.ntt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Reilly PM, Shopshire MS. Anger management group treatment for cocaine dependence: Preliminary outcomes. American Journal of Drug and Alcohol Abuse. 2000;26:161–177. doi: 10.1081/ada-100100598. [DOI] [PubMed] [Google Scholar]
- Reitzel LR, Vidrine JI, Businelle MS, Kendzor DE, Costello TJ, Li Y, Wetter DW. Preventing postpartum smoking relapse among diverse low-income women: A randomized clinical trial. Nicotine & Tobacco Research. 2010;12:326–335. doi: 10.1093/ntr/ntq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EAR, Brower KJ, Gomberg ESL. Explaining unexpected gender differences in hostility among persons seeking treatment for substance use disorders. Journal of Studies on Alcohol. 2001;62:667–674. doi: 10.15288/jsa.2001.62.667. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Lopez FA, Granger DA, Eiden RD. The association between prenatal exposure to cigarettes and cortisol reactivity and regulation in 7-month-old infants. Developmental Psychobiology. 2008;50:819–834. doi: 10.1002/dev.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. American Family Physician. 2002;66:1001–1008. [PubMed] [Google Scholar]
- Siegler IC, Peterson BL, Barefoot JC, Williams RB. Hostility during late adolescence predicts coronary risk factors at mid-life. American Journal of Epidemiology. 1992;136:146–154. doi: 10.1093/oxfordjournals.aje.a116481. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Parr T, Neale MC, Rutter M, Angold A, Eaves LJ. Maternal smoking during pregnancy and risk to boys’ conduct disturbance: An examination of the causal hypothesis. Biological Psychiatry. 2003;53:130–135. doi: 10.1016/s0006-3223(02)01477-4. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: Utility for alcohol research. Addictive Behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Paster RL, Papandonatos GD, Niaura R, Salisbury AL, Battle C, Lester B. Maternal smoking during pregnancy and newborn neurobehavior: Effects at 10 to 27 days. The Journal of Pediatrics. 2009;154:10–16. doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Henry DB, Blair RJ, Dukic V, Burns J, Pickett KE. Unpacking the association: Individual differences in the relation of prenatal exposure to cigarettes and disruptive behavior phenotypes. Neurotoxicology and Teratology. 2011;33:145–154. doi: 10.1016/j.ntt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K, Campbell R, Mermelstein R, Wakschlag L. Pregnancy smoking in context: The influence of multiple levels of stress. Nicotine & Tobacco Research. 2008;10:1065–1073. doi: 10.1080/14622200802087564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman MC, Fowkes FG, Deary IJ, Lee AJ. Hostility, cigarette smoking and alcohol consumption in the general population. Social Science & Medicine. 1997;44:1089–1096. doi: 10.1016/s0277-9536(96)00236-5. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Serdula MK, Kendrick JS, Binkin NX. Comparing the prevalence of smoking in pregnant and nonpregnant women, 1985 to 1986. Journal of the American Medical Association. 1989;261:70–74. [PubMed] [Google Scholar]