Abstract
Objective:
Individuals who are methamphetamine dependent exhibit higher rates of cognitive dysfunction than healthy people who do not use methamphetamine, and this dysfunction may have a negative effect on the success of behavioral treatments for the disorder. Therefore, a medication that improves cognition, such as modafinil (Provigil), may serve as a useful adjunct to behavioral treatments for methamphetamine dependence. Although cognitive-enhancing effects of modafinil have been reported in several populations, little is known about the effects of modafinil in methamphetamine-dependent individuals. We thus sought to evaluate the effects of modafinil on the cognitive performance of methamphetamine-dependent and healthy individuals.
Method:
Seventeen healthy subjects and 24 methamphetamine-dependent subjects participated in this randomized, double-blind, placebo-controlled, crossover study. Effects of modafinil (200 mg, single oral dose) were assessed on participants’ performance on tests of inhibitory control, working memory, and processing speed/attention.
Results:
Across subjects, modafinil improved performance on a test of sustained attention, with no significant improvement on any other cognitive tests. However, within the methamphetamine-dependent group only, participants with a high baseline frequency of methamphetamine use demonstrated a greater effect of modafinil on tests of inhibitory control and processing speed than those participants with low baseline use of methamphetamine.
Conclusions:
Although modafinil produced limited effects across all participants, methamphetamine-dependent participants with a high baseline use of methamphetamine demonstrated significant cognitive improvement on modafinil relative to those with low baseline methamphetamine use. These results add to the findings from a clinical trial that suggested that modafinil may be particularly useful in methamphetamine-dependent subjects who use the drug frequently.
Individuals who are dependent on methamphetamine (MA) exhibit higher rates of cognitive dysfunction than healthy control participants in several domains, including sustained attention, episodic memory, information processing, inhibitory control, and executive functions (Monterosso et al., 2005; Nordahl et al., 2003; Salo et al., 2002; Scott et al., 2007; Simon et al., 2010; Woods et al., 2005). These cognitive deficits have been hypothesized to undermine the addicted individuals’ efforts to stop or reduce MA use and may also negatively affect the outcome of treatment (Vocci and Appel, 2007). For example, during treatment for substance dependence with cognitive behavioral therapy, substance-dependent individuals with low IQs have been shown to improve their coping skills less than those with high IQs, and these differences in coping indirectly affected subsequent treatment outcome (i.e., greater improvement in coping skills led to reduced substance use; Kiluk et al., 2011). In addition to IQ, evidence suggests that inhibitory control function may also be related to the ability of MA-dependent individuals to resist drug-related compulsions when striving to maintain abstinence (Baler and Volkow, 2006; Jentsch and Taylor, 1999).
Given the potential relationships between cognition, treatment, and outcome in MA dependence, treatment approaches that improve cognitive function could be promising for managing the disorder. Recent evidence has indicated that modafinil (Provigil), a U.S. Food and Drug Administration–approved drug for treating narcolepsy and other sleep disorders, produces cognition-enhancing effects in healthy subjects and in some psychiatric patients. The specific mechanism by which modafinil produces these effects has not been identified, but modafinil increases extracellular levels of dopamine, norepinephrine, serotonin, glutamate, and histamine, and it decreases γ-aminobutyric acid levels (Minzenberg and Carter, 2008). In healthy adults, modafinil has been shown to improve working memory (Müller et al., 2004; Turner et al., 2003), sustained attention (Randall et al., 2005b), processing speed/reaction time (Baranski et al., 2004; Randall et al., 2004), and recognition memory (Turner et al., 2003), with particular benefits in individuals who were sleep deprived (Gill et al., 2006; Grady et al., 2010; Hart et al., 2006; Walsh et al., 2004; Wesensten et al., 2002). Positive effects of modafinil on various cognitive functions, particularly sustained attention and working memory, have also been noted in some psychiatric groups, such as individuals with schizophrenia (Rosenthal and Bryant, 2004; Turner et al., 2004b), major depression (DeBattista et al., 2004), and attention-deficit/hyperactivity disorder (ADHD; Greenhill et al., 2006; Turner et al., 2004a). Of note, modafinil has additionally been shown to improve performance on tests of inhibitory control, such as the stop-signal task and Stroop color-naming test, in both healthy individuals and psychiatric patients (DeBattista et al., 2004; Randall et al., 2004; Turner et al., 2003).
Relatively little research has evaluated the effect of modafinil on the cognition of individuals with stimulant use disorders. In a human laboratory study, several acute doses of modafinil (150, 300, and 450 mg) did not significantly modify inhibitory control in cocaine users (n = 11), although the go/no-go task used in this study may not have been optimized to detect improvements in inhibitory control (Vansickel et al., 2008). In a small, between-subjects, inpatient study, immediate verbal memory was improved in MA-dependent participants receiving modafinil (200 mg; n = 7) relative to those receiving placebo (n = 7) (Hester et al., 2010), but differences between the groups were not observed on measures of visual memory, processing speed, or verbal fluency. Last, in a small study of MA-dependent subjects (n = 11; Kalechstein et al., 2010), modafinil (400 mg) did not have a significant effect on working memory and learning/memory performance across subjects. However, when the baseline cognitive functioning of participants was considered, MA-dependent participants with low working memory scores at baseline demonstrated significant improvement with modafinil, whereas those with higher working memory scores did not. This finding suggests that baseline cognitive functioning may moderate the effect of modafinil on cognition. Indeed, similar findings have been noted in healthy college students, in which those with low estimated IQ benefited from modafinil (200 mg) on tests of sustained attention and processing speed, but those with high estimated IQ did not (Randall et al., 2005a).
Modafinil has also been evaluated as a potential pharmacotherapy for stimulant use disorders in clinical trials. Results from these trials have sometimes shown a positive effect of modafinil on stimulant abstinence, but the results have been variable (see Anderson et al., 2009; Heinzerling et al., 2010; McGaugh et al., 2009; Shearer et al., 2009). In particular, moderator variables may play a role in the success of treatment. For example, one study showed that modafinil (400 mg daily) did not improve the treatment outcomes for MA-dependent participants as a group; however, those with a high frequency of baseline MA use showed trends for improved abstinence and study retention during modafinil treatment relative to those with a low baseline frequency of MA use (Heinzerling et al., 2010). The therapeutic effect of modafinil may therefore depend on the frequency of baseline MA use.
Although the effect of modafinil on cognition has been somewhat equivocal in individuals with stimulant use disorders (Hester et al., 2010; Kalechstein et al., 2010; Vansickel et al., 2008), the sample sizes of these studies have generally been small (∼10 participants receiving modafinil). The present study was therefore performed to test the effects of modafinil on the cognitive functioning of MA-dependent (n = 24) and healthy (n = 17) subjects in a randomized, double-blind, within-subjects study. Given the potential importance of inhibitory control in the course of MA dependence and treatment, the cognitive battery included tests of inhibitory control as well as tests of attention, working memory, and psychomotor speed. Because the literature has shown that IQ and frequency of baseline MA use may moderate the effects of modafinil, estimated IQ and frequency of MA use were tested as potential moderators of the effects of modafinil on cognitive performance. The hypotheses for this study were that modafinil would generally improve cognitive performance across subjects, with particular benefits realized in MA-dependent participants with low estimated IQ and in those with a high frequency of MA use.
Method
Participants
The participants were 24 MA-dependent individuals who were not seeking treatment for their stimulant use and 17 healthy control subjects. The study was approved by the University of California, Los Angeles (UCLA), Office for Protection of Research Subjects, and participants signed an informed consent document after receiving a detailed description of the protocol. All participants were fluent in English and were administered the Structured Clinical Interview for the DSM-IV (SCID) for Axis I diagnosis (First et al., 1995) by a master’s-level clinician. Exclusion criteria, based on interview, physician-conducted history, physical examination, and laboratory tests, were neurological disease (e.g., stroke, head trauma with loss of consciousness >30 minutes); frank structural brain abnormalities on magnetic resonance imaging (MRI); systemic disease; cardiovascular disease; pulmonary disease; HIV infection (HIV1/HIV2 antibody screen); abnormal laboratory tests (hematocrit, plasma electrolytes, markers for hepatic and renal function); probable mental retardation (based on the Wechsler Test of Adult Reading); use of psychotropic medications; diagnosis of current abuse or dependence for any substance other than MA, marijuana, or nicotine; and any current non-substance-induced Axis I psychiatric conditions (with the exception of one MA subject with current panic disorder). MA participants provided a urine sample positive for MA metabolite at intake. Two MA participants additionally met criteria for current marijuana dependence (n = 1) or abuse (n = 1). Control participants met the same inclusion/exclusion criteria as MA subjects but did not meet criteria for any current Axis I conditions (substance-related or psychiatric) or test positive for any drugs in urinalysis. All control subjects were naive to MA, amphetamine, and medications typically prescribed for ADHD. A subset of control subjects (n = 5) had a history of experimental use (e.g., a few times) of cocaine, Ecstasy (3,4-methylenedioxymethamphetamine [MDMA]), or diet pills (e.g., ephedrine). The MA group had a greater number of cigarette smokers (n = 22) than the control group (n = 8, p < .01). However, of those who smoked, both the MA and control subjects had modest mean Fagerström Test for Nicotine Dependence scores (M = 2.3, SD = 2.0, and M = 2.9, SD = 2.5, respectively, p > .50). Demographic characteristics of the groups are presented in Table 1.
Table 1.
Characteristics of research participants
| Variable | Control (n = 17) | MA dependent (n = 24) |
| Age, in years, M (SD) | 31.1 (8.1) | 35.9 (10.1) |
| Education, years, M (SD) | 14.5 (1.8) | 12.9 (1.9)* |
| IQ estimate,a standard score, M (SD) | 111.1 (10.3) | 95.3 (12.0)** |
| Gender, male/female, n | 8/9 | 12/12 |
| Ethnicity, n | ||
| White | 10 | 12 |
| Hispanic | 4 | 6 |
| Asian/Pacific Islander | 1 | 2 |
| Other | 2 | 4 |
| Cigarette smoker, n | 8 | 22** |
| Cigarette pack years,b smokers only, M (SD) | 9.5 (11.6) | 14.1 (16.7) |
| Fagerström score,c smokers only, M (SD) | 2.3 (2.0) | 2.9 (2.5) |
| Days of alcohol use in last 30 days, M (SD) | 7.6 (6.8) | 4.0 (6.8) |
| Duration of regular MA use,d in years, M (SD) | 0 | 10.1 (7.9) |
| Days of MA use in last 30 days, n | 0 | 21.4 (8.1) |
| Grams of MA used/week, M (SD) | 0 | 2.6 (2.8) |
Notes: MA = methamphetamine.
IQ estimate = Wechsler Test of Adult Reading;
cigarette pack years = (average cigarettes used per day) × (years smoked) / 20;
Fagerström score = Fagerström Test for Nicotine Dependence score;
regular MA use defined as using three times per week or using heavily for 2 consecutive days per week.
p < .05;
p <.01.
Interview and self-report measures
The following are the interview and self-report measures used in the current study.
Admission and intake form.
Each participant received an intake form to report demographic information, medical information (e.g., exclusion criteria), and a detailed drug use history.
Addiction Severity Index (McLellan et al., 1992).
This is a standardized clinical interviewing instrument widely used to identify problems associated with substance use.
Neurological History Questionnaire.
The 47-item Neurological History Questionnaire (developed by our laboratory) assesses neurological history (e.g., history of stroke), which was used in addition to the physician-conducted neurological history/examination.
Fagerström Test for Nicotine Dependence (Heatherton et al., 1991).
This test is a six-item self-report measure of nicotine dependence that was revised from the earlier Fagerström Tolerance Questionnaire (Fagerström, 1978).
Piper Fatigue Scale—cognitive subscale (Piper et al., 1998).
This measure of fatigue assesses four domains: behavioral/severity, affective meaning, sensory, and cognitive/ mood. Because we were most interested in fatigue associated with cognition, we implemented only the six cognitive/mood items. This subscale was given during the beginning, middle, and end of each cognitive battery, and a mean total score was obtained for each test session.
Neuropsychological measures
Given research suggesting that modafinil has effects on measures of working memory, attention, and processing speed/reaction time (see Minzenberg and Carter, 2008), we implemented several of these tests in our cognitive battery. We also included tests of inhibitory control because of the potential association between this construct and the ability of MA-dependent subjects to maintain abstinence (Baler and Volkow, 2006). Lastly, a test of motor speed (finger tapping) was included to determine if modafinil-associated effects on psychomotor reaction-time measures were discriminable from pure motor speed.
Inhibitory control
Stroop color-word inhibition test (Delis et al., 2001):
This is a test of inhibitory control from the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) in which color words are printed in a different color ink (e.g., “red” printed in green ink), and the examinee must inhibit the reading response to identify the ink color. Total time to complete the task is the outcome variable.
Stroop color-word inhibition/switching test (from D-KEFS; Delis et al., 2001):
This version is identical to the Stroop color-word inhibition test (see above), except that on a subset of items (those presented inside a small box), the examinee must read the word and not identify the ink color.
Continuous Performance Test (CPT; from Consortium for Neuropsychiatric Phenomics [CNP] test battery):
The CPT is a computerized test of sustained attention and inhibitory control in which letters are presented sequentially on a screen and the participant must respond with a button press, as quickly as possible, to every letter except the letter X. The inhibitory control outcome variable is the total number of commission errors (e.g., responding when the X is presented).
Attention Networks Task (CNP test battery, modeled after Fan et al., 2002):
The Attention Networks Task is a computerized measure of inhibitory control. On each trial, an arrow is presented on the screen, surrounded by arrows or lines presented on either side (“flankers”). On some trials, the flanker arrows point in the same direction as the central arrow, and on other trials they point in the opposite direction. The inhibitory outcome variable is the mean reaction time to respond to incongruent trials (i.e., the central arrow and flankers point in opposite directions) minus the mean reaction time to respond to neutral trials (i.e., those flanked by lines rather than arrows), thus factoring out generalized response speed.
Processing speed/attention
Digit Symbol Coding (Wechsler, 1997):
Digit Symbol Coding is a test of symbol substitution in which the examinee must match (through drawing) symbols with numbers as fast as possible. The outcome variable is the total number of items completed in 2 minutes.
Trailmaking—Number Sequencing (Delis et al., 2001):
This is a D-KEFS test of psychomotor speed in which the examinee must connect numbered dots on a sheet of paper using a pencil. Because the D-KEFS has several similar trailmaking subtests, only this subtest and the number/letter switching subtest (see below) were analyzed to avoid redundancy. Total time to complete the task is the outcome variable.
Stroop color-naming test (Delis et al., 2001):
In this D-KEFS subtest, the examinee must identify blocks of ink color on a page as rapidly as possible. Total time to complete the task is the outcome variable.
Stroop word-reading test (Delis et al., 2001):
This is a D-KEFS subtest in which the examinee must read color words out loud as rapidly as possible. Total time to complete the task is the outcome variable.
Continuous Performance Test (from CNP test battery):
This is a test of attention and inhibitory control, described above. The attentional vigilance outcome variable is measured as the variability (i.e., standard deviation) of reaction time on go trials across the task.
Working memory/switching
Letter-Number Sequencing Test (Wechsler, 1997):
A verbal working memory measure in which letters and numbers are presented verbally in random order, the Letter-Number Sequencing Test asks the examinee to recite back the letters in alphabetical order and the numbers in numerical order. The outcome variable is the number of trials performed successfully before two consecutive failures.
Spatial Capacity Delayed Response Test (CNP test battery; based on Glahn et al., 2003; Sternberg, 1969):
This is a computerized test of visual working memory in which yellow circles are presented on a screen for 2 seconds, arranged in a pseudo-random pattern around a fixation point. Following a 4-second delay, a single green circle appears and the examinee must determine if it is in the same location as any one of the previous yellow circles. The memory load is varied across trials by varying the number of yellow circles in each array (1, 3, 5, or 7). The percentage of correct yes/ no responses comprises the primary outcome.
Trailmaking—letter/number switching (Delis et al., 2001):
Using this D-KEFS subtest, we presented circles with numbers and letters on a sheet of paper and the examinee must connect the circles as rapidly as possible with a pencil by alternating between the numbers and letters, in order. Total time to complete the task is the outcome variable.
Motor speed
Finger Tapping Test (based on Halstead, 1947):
The Finger Tapping Test examines the motor speed in which the examinee must tap on a small lever with the forefinger as rapidly as possible. Three trials of 10 seconds each were conducted with each hand; the outcome variable is the mean number of taps for each hand.
Intelligence estimation
Wechsler Test of Adult Reading (Wechsler, 2001):
This is a test of reading/pronunciation of words with atypical grapheme-to-phoneme translations, which is commonly used to estimate intellectual ability. The total number of words pronounced correctly is normed for age to produce a standard score.
Procedure
All MA subjects resided in hospital rooms at the UCLA General Clinical Research Center, in which abstinence from all drugs (aside from nicotine) was confirmed by regular urine drug screens and breath alcohol tests. Control subjects were also tested via urine screen/breath alcohol test for drug use before assessments, but they did not reside at the General Clinical Research Center. Control participants came to the laboratory on separate occasions to complete the two medication/cognitive testing sessions and additional procedures. No participant tested positive for drugs on the days of the cognitive battery. Inclusion/exclusion information was obtained at intake. After intake, the MA participants relaxed on the hospital ward for 3 days and typically slept for significant periods, consistent with the literature on the MA withdrawal syndrome (McGregor et al., 2005). On the fourth day, after MA metabolite was no longer present in urinalyses, the MA participants began research procedures such as structural MRI scanning, IQ estimation, and other assessments as part of a grant-funded study examining neural networks in MA dependence (control subjects also completed these assessments before compound administration). These procedures continued until at least the eighth day of the stay (M = 11.04 days), at which point participants began receiving the within-subjects administration of modafinil or placebo and cognitive testing (thus, all participants were abstinent for at least 8 days before compound administration). The order of test administration in the cognitive battery was fixed as follows: Piper Fatigue Scale, Stroop test, Letter-Number Sequencing, Trailmaking, Digit Symbol Coding, Attention Networks Task, Finger Tapping, Piper Fatigue Scale, Spatial Capacity Delayed Response Task, CPT, and Piper Fatigue Scale. Test order was determined in a pseudo-random fashion, in which demanding cognitive tests (e.g., Attention Networks Task) were interspersed with less-demanding measures (e.g., self-report).
Participants were freely allowed to smoke cigarettes during their stay at the General Clinical Research Center, and smoking breaks were allowed during cognitive testing as needed so that participants were not in a state of nicotine withdrawal. Many participants left the study on the day following the medication procedures, but a subset of participants continued in the study to receive additional structural MRI scanning. On average, the MA participants stayed on the General Clinical Research Center ward for 18.1 days (SD = 8.4).
A double-blind, placebo-controlled, within-subjects design was used in which modafinil (200 mg) or placebo was administered in a single, acute oral dose. Participants first received an acute dose of one compound (200 mg modafinil or placebo) and underwent cognitive testing, followed by at least a 2-day washout period (M = 2.4 days) before receiving the alternate test compound (modafinil or placebo, whichever was not given previously) and repeated cognitive testing. The order of compound administration was randomized. Each compound was administered orally at 8:30 a.m., and cognitive testing occurred at noon on the same day. (MRI scanning was conducted immediately after the administration of modafinil or placebo and resulted in the delay between compound administration and cognitive testing.) After a single oral dose of modafinil, the peak plasma concentration is achieved in approximately 2–4 hours, with an elimination half-life of 12–15 hours (Robertson and Hellriegel, 2003; Wong et al., 1999). The cognitive testing began 3.5 hours after the acute administration of modafinil and lasted for the next 2 hours. During this period of cognitive sessions, plasma concentrations of modafinil were likely high, although not at peak concentrations.
Statistical analyses
Statistical analyses were organized into three main sections: (a) preliminary analyses, in which demographic differences between MA and control subjects were analyzed with t tests or chi-square analyses, as appropriate; (b) primary analyses, in which the effect of compound was examined on each cognitive test while controlling for carryover effects of repeated cognitive testing, using the general linear mixed model (GLMM; this procedure is identical to a repeated measures model [Rencher and Schaalje, 2008]); and (c) moderator analyses, in which the potential moderation of estimated IQ and frequency of recent MA use was evaluated by including interaction terms for each variable into the primary analysis model.
Results
Preliminary analyses
The MA and control groups did not differ significantly in order of modafinil administration, age, gender, ethnicity, or days of alcohol use in the 30 days before the study (ps > .10). However, the control group had more years of education than the MA group (p = .01), higher levels of estimated premorbid IQ (p < .01), and fewer smokers than the MA group (p < .01). Because control participants frequently rescheduled their second assessment/compound session as a result of scheduling conflicts, the control group also had a greater length of time between testing sessions (M = 13.5 days) than the MA participants (M = 2.4 days; p < .01; however, number of days between testing sessions was unrelated to cognitive performance on any of the following GLMM analyses, ps > .20).
Primary analyses
GLMM analyses were conducted using each cognitive test as a dependent variable and group (MA or control), test compound (modafinil or placebo), and their interaction as independent variables. The main effects of test order (i.e., carryover effects), years of education, estimated IQ, and days between assessment sessions were also included to control for these potential confounds. Smoking status was not initially included in the model because this variable was almost totally confounded with group status (i.e., only two MA subjects were nonsmokers).
Across the GLMMs for each cognitive test, no significant interactions between compound and group were observed (ps > .05). With respect to the main effect of compound on each cognitive test, a main effect of compound was found only for CPT reaction time variability, F(1, 38) = 6.56, p = .02, in which participants exhibited less reaction time variability (i.e., better attentional vigilance) after receiving modafinil than after receiving placebo. The main effect of test compound was nonsignificant for all other cognitive tests (ps > .05). With respect to the main effect of group, MA participants underperformed control participants on the Stroop color-word inhibition test, F(1, 35) = 4.10, p = .05; no other significant main effects of group were observed (ps >.05; MA subjects underperformed control subjects on most cognitive tests, but these effects were typically removed when accounting for education and/or IQ). Including the main effect of smoking status in the aforementioned analyses did not alter either the significant main effect of test compound on CPT reaction time variability (p = .02) or the significant main effect of group on the Stroop color-word inhibition test (p = .04). When Piper Fatigue scores were used as the dependent variable in the aforementioned GLMM analyses, no main effects of group, compound, or their interaction were observed (ps > .05). Cognitive performance of the MA and control subjects when receiving modafinil versus placebo is displayed in Table 2.
Table 2.
Cognitive performance during acute administration of modafinil (200 mg) versus placebo in the control and metham-phetamine (MA)-dependent participants
| Control (n = 17) |
MA dependent (n = 24) |
|||
| Cognitive variable | Placebo M (SD) | Modafinil M (SD) | Placebo M (SD) | Modafinil M (SD) |
| Inhibitory control | ||||
| Stroop color-word inhibition, D-KEFSa,† | 39.6 (5.3) | 40.5 (8.0) | 50.9 (12.3) | 52.0 (11.2) |
| Stroop color-word inhibition/switching, D-KEFSa | 42.0 (6.0) | 41.9 (7.7) | 57.3 (14.1) | 59.6 (19.8) |
| CPT percent commission errorsa | 41.0 (20.6) | 42.4 (19.0) | 49.8 (23.3) | 45.6 (17.7) |
| ANT incongruent minus neutral reaction timea | 72.4 (37.7) | 88.0 (28.7) | 105.4 (55.9) | 98.6 (36.7) |
| Processing speed/attention | ||||
| Digit symbol coding, WAIS-III | 91.6 (17.7) | 92.8 (19.6) | 71.0 (20.1) | 74.0 (20.6) |
| Trailmaking—number sequencing, D-KEFSa | 19.1 (7.2) | 18.0 (4.4) | 26.8 (11.4) | 25.2 (11.9) |
| Stroop color naming, D-KEFSa | 24.1 (4.2) | 24.0 (3.3) | 27.9 (4.5) | 27.9 (5.6) |
| Stroop word reading, D-KEFSa | 18.5 (3.4) | 18.4 (3.3) | 21.0 (3.4) | 21.1 (3.4) |
| CPT reaction time variabilitya,* | 91.2 (72.3) | 65.5 (16.0) | 123.9 (64.5) | 93.8 (35.2) |
| Working memory/switching | ||||
| Letter number sequencing, WAIS-III | 13.4 (3.4) | 13.6 (3.3) | 11.1 (2.4) | 11.3 (2.7) |
| Spatial Sternberg total accuracy | 88.5 (6.5) | 88.5 (6.0) | 79.4 (9.3) | 82.9 (13.0) |
| Trailmaking—number/letter switching, D-KEFSa | 52.8 (19.4) | 50.3 (16.0) | 73.7 (35.2) | 74.4 (35.6) |
| Motor speed | ||||
| Finger tapping, dominant hand | 51.5 (5.7) | 53.5 (7.0) | 49.6 (8.4) | 50.2 (8.6) |
| Finger tapping, nondominant hand | 48.1 (6.0) | 48.2 (6.7) | 44.7 (7.2) | 45.5 (7.3) |
Notes: D-KEFS = Delis–Kaplan Executive Function System; CPT = Continuous Performance Test; ANT = Attention Networks Task (flanker task); WAIS-III = Wechsler Adult Intelligence Scale, Third Edition.
Higher scores reflect worse performance;
main effect of group on performance, p = .05;
main effect of modafinil on performance, p = .02.
Moderator analyses
To determine if estimated IQ moderated the effect of modafinil on cognitive performance in either subject group, GLMM models were run for each cognitive test as above but included interaction terms between compound and IQ and among compound, IQ, and group (three-way interaction). Significant moderating effects were not found for IQ on any cognitive test (all interactions, ps > .05).
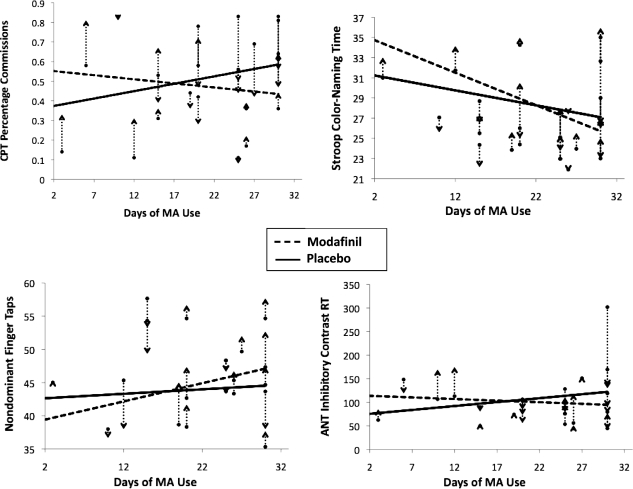
To determine if the frequency of recent MA use moderated the effect of modafinil on cognitive performance, GLMM models were run in an identical fashion to the primary analyses but excluded control participants and the group variable (and all interactions thereof) and included the main effect of MA use frequency (days of MA use in the 30 days before study entry) and the interaction between compound and MA use frequency. Significant interactions between MA use frequency and compound were found for CPT percent commission errors, F(1, 20) = 8.10, p = .01; nondominant hand finger tapping, F(1, 19) = 4.81, p = .04; Stroop color naming, F(1, 20) = 4.18, p = .05; and a non-significant trend with Attention Networks Task incongruent minus neutral reaction time, F(1, 19) = 3.42, p = .08. In each case, relative to the placebo condition, modafinil administration was associated with more pronounced cognitive improvement as the frequency of MA use during the 30 days before the study increased. These effects are illustrated in Figure 1. A significant main effect of MA use frequency was also found on the dominant-hand Finger Tapping Test, F(1, 17) = 7.81, p = .01), in which subjects who used MA more often had better performance. No other main effects of MA use frequency were found. Last, to determine if fatigue accounted for the significant interactions between frequency of MA use and compound, the main effect of Piper Fatigue was entered into each analysis. All results were unchanged.
Figure 1.
Effect of modafinil versus placebo relative to frequency of methamphetamine (MA) use on tests of inhibitory control and processing speed/attention. Vertical dashed lines represent the change in performance between the modafinil and placebo conditions for each participant, and the arrows indicate whether performance went up or down in the modafinil condition. CPT = Continuous Performance Test; ANT = Attention Networks Task (flanker task) incongruent reaction time (RT) minus neutral RT. Days of MA Use = days used MA on the 30 days before study entry. With the exception of Finger Tapping Scores, higher scores on each test represent worse performance. Fit lines were derived from the parameter estimates of the general linear mixed models described in the Results section. Note that a within-subjects design was used so that the same participants received both placebo and modafinil. On each test, individuals who had a greater frequency of baseline MA use showed an enhanced cognitive effect of modafinil relative to those with lower frequency of baseline MA use. All interactionps ≤ .05, with the exception trend-level significance with the ANT (p = .08).
Discussion
The results of this study indicate that the effects of modafinil in MA-dependent subjects are based on the recent drug histories of the participants. Within the MA-dependent group, individuals with a higher frequency of recent MA use showed a greater benefit from modafinil on tests of inhibitory control and processing speed/attention than those with lower rates of MA use. Unexpectedly, there was no generalized modafinil-induced enhancement of cognitive function across subjects, and a main effect of modafinil was found for only one test of attentional vigilance (variability of reaction time on the CPT). On this test, both MA-dependent and control subjects demonstrated improved attention after receiving modafinil compared with placebo.
MA-dependent subjects with a higher frequency of MA use in the month preceding study entry showed an enhanced benefit of modafinil on tests of inhibitory control (CPT commission errors), processing speed/attention (Stroop color naming), and motor speed (nondominant hand finger tapping), with a similar trend-level finding (p = .08) on another measure of inhibitory control (Attention Networks Task). Because these effects occurred in more than one cognitive domain, the cognitive enhancement in frequent MA users appears to be somewhat generalized rather than limited to a specific cognitive function. Inspection of the performance plots (Figure 1) indicates that MA subjects who used MA approximately 20 or more days in the month before entering the study (n = 16) received the most benefit from modafinil and, conversely, performed the worst when administered placebo. Because the frequent MA users performed more poorly when administered placebo than modafinil, this may suggest that frequent MA users generally have lower cognitive functioning than less frequent MA users. However, number of days of MA use was uncorrelated with estimated IQ (r = -.02, p = .92) and years of education (r = .02, p = .93); therefore, it does not appear that MA use frequency was associated with gross cognitive functioning. However, it is nonetheless possible that participants who use MA frequently have selective baseline weaknesses in inhibitory control and processing/motor speed domains relative to the participants who use MA less frequently. An alternative explanation is that modafinil has a more pronounced tendency to improve the untoward effects of abstinence on cognition for frequent MA users compared with less frequent users. This is important considering that the MA participants were abstinent for slightly more than a week at cognitive testing (M = 11 days). Indeed, evidence has suggested that modafinil can curtail the effects of MA withdrawal symptoms in early abstinence, including reduced fatigue, irritability, and craving (McGregor et al., 2008). Our results indicated that the performance enhancement for frequent MA users was not attributable to fatigue effects; therefore, some other aspect of the abstinence syndrome may be implicated (e.g., increased motivation).
It is notable that the results presented here are consistent with those of a clinical trial of modafinil (400 mg daily) for the treatment of MA dependence (Heinzerling et al., 2010). In that trial, modafinil showed trends toward increasing study retention and reducing MA use in participants with high baseline frequency (>18 days of the last 30 days) of MA use relative to those with low baseline frequency of MA use. Thus, the modafinil-induced enhancements in cognitive performance currently demonstrated in participants who frequently use MA may hold promising associations with treatment-relevant outcomes.
The lack of a general effect of modafinil to enhance the cognition of control subjects or MA-dependent subjects when moderating variables were not considered was unexpected. Across research subjects, modafinil improved the cognitive performance on only one test of attentional vigilance (CPT reaction time variability). This test measures the ability to sustain attention over time when stimuli are presented at varying interstimulus intervals. In contrast to the other measures of attention and processing speed administered, the CPT requires attention to be maintained for a longer period (i.e., almost 20 minutes compared with about 2 minutes for the other attentional tasks) and thus requires a greater degree of patience and vigilance. Partly for these reasons, the CPT is one of the tests most commonly found to be improved by psychostimulant medications in both control subjects and those with ADHD (Riccio et al., 2001). Given the particular function assessed by the CPT, it is possible that the significant effect found for modafinil in our study reflects a meaningful improvement. Indeed, our results are consistent with those of several other studies that have shown modafinil to have a beneficial effect on tests of sustained attention in healthy adults and other patient groups (Greenhill et al., 2006; Harsh et al., 2006; Hart et al., 2006; Randall et al., 2005a, 2005b; Rugino and Samsock, 2003; Walsh et al., 2004). However, the enthusiasm regarding this finding is tempered by the number of cognitive tests we evaluated and the high corresponding possibility of Type I error. As such, it must be concluded that the effect of modafinil on the cognitive performance of most participants in our study was relatively small.
The modest effect of modafinil observed across participants in our study may have also been attributable to the dosage used (200 mg). Although this dose has been shown to improve cognitive function in other MA-dependent, psychiatric, and healthy subject samples (Hester et al., 2010; Müller et al., 2004; Turner et al., 2003, 2004a, 2004b; Walsh et al., 2004), higher doses (e.g., 400 mg) have been shown to be safe in MA-dependent participants (Heinzerling et al., 2010) and may produce a more pronounced cognitive effect. In addition, compared with our single-dose design, use of a multiple-dose design would have been preferable for the detection of a dose-response relationship between modafinil and cognitive performance. A single dose may also be insufficient to compensate for the cognitive deficits present in MA-dependent subjects who have used MA for many years. Lastly, not all tests administered in our cognitive battery were given during the period of peak concentration of modafinil in blood (peak plasma concentration of modafinil is reached in 2–4 hours after ingestion; cognitive battery was conducted 3.5–5.5 hours after ingestion). However, the only test that demonstrated significant improvement on modafinil across subjects was the CPT, and this test was the last cognitive test administered in the battery (∼5 hours after compound administration). This finding indicates that although plasma concentrations of modafinil were not likely maximal 5 hours after drug administration, they were large enough to produce behaviorally significant effects in the CPT. Similarly, in the MA-dependent subjects, the significant interactions observed between modafinil and baseline MA use were found on tests that were administered in the beginning, middle, and end of the cognitive battery session (including commission errors on the CPT). Nonetheless, it is possible that larger cognitive effects would have been observed across tests if the cognitive battery was administered exclusively during the peak plasma timeframe.
Although some previous research has indicated that individuals with low IQ or low baseline performance are the most likely to show cognitive benefits with modafinil (Kalechstein et al., 2010; Randall et al., 2005a), we did not find significant interactions between estimated IQ and response to modafinil. Because previous research (Randall et al., 2005a) has found interactions between IQ and modafinil using similar methods of IQ estimation (reading/ pronunciation) and similar doses of modafinil (200 mg acutely), it does not appear that these methodological factors account for the differences in results. However, compared with the examination by Randall and colleagues (2005b), our study had a greater range of IQ scores and included MA-dependent participants, whereas the Randall study was conducted exclusively in university students with relatively high IQ scores. Thus, increased heterogeneity in our sample may account for the different results. Unfortunately, because cognitive functioning other than IQ was not measured before administration of modafinil (or placebo) in our study, it was not possible to determine if low performance on other cognitive functions is predictive of enhancement with modafinil, as shown in a study of working memory (Kalechstein et al., 2010). Independent of baseline functioning, we did not find improvements in working memory in the MA-dependent or control subjects. Kalechstein et al. (2010) administered a higher dose of modafinil (400 mg) for 3 consecutive days, whereas we acutely administered a lower dose of modafinil (200 mg), and therefore dosage and frequency of modafinil administration may have played a role.
Lastly, except for MA-dependent subjects underperforming control subjects on one test of inhibitory control (Stroop color-word inhibition), the MA subjects and control subjects did not significantly differ in cognitive performance after controlling for years of education and estimates of IQ. Because studies controlling for these factors with larger sample sizes have shown that MA-dependent subjects have deficits in several cognitive domains compared with control subjects (e.g., Cherner et al., 2010; Woods et al., 2005), the current null results may be attributable to limited power. However, our results underscore the importance of controlling for these potential confounds when comparing the cognitive performance of MA-dependent subjects with healthy control subjects, particularly given that the literature has not consistently taken into consideration education and estimates of IQ (Scott et al., 2007).
Limitations
The present study assessed the effects of modafinil on cognition after a single, acute administration. A single dose precludes the investigation of dose-response relationships, and chronic drug administration would be more clinically relevant than an acute administration. Also, some of the cognitive tests administered to participants were conducted after the period of peak concentration of modafinil in blood; however, no systematic relationship was observed between cognitive enhancement with modafinil and timing of the cognitive procedures. Lastly, because of scheduling delays associated with control participants returning to the research laboratory, the control participants typically had a longer delay between testing sessions than did the MA subjects who stayed on the hospital ward. Although a balanced design would have been preferable, the delay between testing sessions was not associated with performance on any cognitive test (ps > .20).
Footnotes
This research was supported by National Institutes of Health Grants K23 DA927734 (to Andy C. Dean), P20 DA022539 (to Edythe D. London), R01 DA020726 (to Edythe D. London), R01 DA15179 (to Edythe D. London); and endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies, and the Marjorie M. Greene Trust. Some of the computerized tests used in the study were programmed for use in the Tennenbaum Family Center for the Biology of Creativity and were provided by the University of California, Los Angles (UCLA), Consortium for Neuropsychiatric Phenomics supported by Grants UL1DE019580 (to Robert M.Bilder)andPL1MH083271 (to Robert M. Bilder). The above funding sources had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- Anderson AL, Reid MS, Li S-H, Holmes T, Shemanski L, Slee A, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug and Alcohol Dependence. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Baranski JV, Pigeau R, Dinich P, Jacobs I. Effects of modafinil on cognitive and meta-cognitive performance. Human Psychopharmacology. 2004;19:323–332. doi: 10.1002/hup.596. [DOI] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, Heaton RK the HNRC Group. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug and Alcohol Dependence. 2010;106:154–163. doi: 10.1016/j.drugalcdep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBattista C, Lembke A, Solvason HB, Ghebremichael R, Poirier J. A prospective trial of modafinil as an adjunctive treatment of major depression. Journal of Clinical Psychopharmacology. 2004;24:87–90. doi: 10.1097/01.jcp.0000104910.75206.b9. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (DKEFS): Examiner’ s manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Fagerström K-O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IP) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Gill M, Haerich P, Westcott K, Godenick KL, Tucker JA. Cognitive performance following modafinil versus placebo in sleep-deprived emergency physicians: A double-blind randomized crossover study. Academic Emergency Medicine. 2006;13:158–165. doi: 10.1197/j.aem.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lönnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Grady S, Aeschbach D, Wright KP, Jr, Czeisler CA. Effect of modafinil on impairments in neurobehavioral performance and learning associated with extended wakefulness and circadian misalignment. Neuropsychopharmacology. 2010;35:1910–1920. doi: 10.1038/npp.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill LL, Biederman J, Boellner SW, Rugino TA, Sangal RB, Earl CQ, Swanson JM. A randomized, double-blind, placebo-controlled study of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:503–511. doi: 10.1097/01.chi.0000205709.63571.c9. [DOI] [PubMed] [Google Scholar]
- Halstead WC. Brain and intelligence. A quantitative study of the frontal lobes. Chicago, IL: University of Chicago Press; 1947. [Google Scholar]
- Harsh JR, Hayduk R, Rosenberg R, Wesnes KA, Walsh JK, Arora S, Roth T. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Current Medical Research and Opinion. 2006;22:761–774. doi: 10.1185/030079906X100050. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Gunderson E, Foltin RW. Modafinil attenuates disruptions in cognitive performance during simulated night-shift work. Neuropsychopharmacology. 2006;31:1526–1536. doi: 10.1038/sj.npp.1300991. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson A-N, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Lee N, Pennay A, Nielsen S, Ferris J. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Experimental and Clinical Psychopharmacology. 2010;18:489–497. doi: 10.1037/a0021791. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, II, Newton TF. Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. American Journal on Addictions. 2010;19:340–344. doi: 10.1111/j.1521-0391.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Carroll KM. Relationship of cognitive function and the acquisition of coping skills in computer assisted treatment for substance use disorders. Drug and Alcohol Dependence. 2011;114:169–176. doi: 10.1016/j.drugalcdep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J, Mancino MJ, Feldman Z, Chopra MP, Gentry WB, Cargile C, Oliveto A. Open-label pilot study of modafinil for methamphetamine dependence. Journal of Clinical Psychopharmacology. 2009;29:488–491. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wong-tan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: A comparison of mirtazapine and modafinil with treatment as usual. Journal of Substance Abuse Treatment. 2008;35:334–342. doi: 10.1016/j.jsat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: A review of neuro-chemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Müller U, Steffenhagen N, Regenthal R, Bublak P. Effects of modafinil on working memory processes in humans. Psychopharmacology. 2004;177:161–169. doi: 10.1007/s00213-004-1926-3. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: A review. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncology Nursing Forum. 1998;25:677–684. [PubMed] [Google Scholar]
- Randall DC, Fleck NL, Shneerson JM, File SE. The cognitive-enhancing properties of modafinil are limited in non-sleep-deprived middle-aged volunteers. Pharmacology, Biochemistry, and Behavior. 2004;77:547–555. doi: 10.1016/j.pbb.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Randall DC, Shneerson JM, File SE. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacology, Biochemistry, and Behavior. 2005a;82:133–139. doi: 10.1016/j.pbb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Randall DC, Viswanath A, Bharania P, Elsabagh SM, Hartley DE, Shneerson JM, File SE. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? Journal of Clinical Psychopharmacology. 2005b;25:175–179. doi: 10.1097/01.jcp.0000155816.21467.25. [DOI] [PubMed] [Google Scholar]
- Rencher AC, Schaalje GB. Linear models in statistics. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): Implications for CPT use and interpretation. Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13:326–335. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Robertson P, Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clinical Pharmacokinetics. 2003;42:123–137. doi: 10.2165/00003088-200342020-00002. [DOI] [PubMed] [Google Scholar]
- Rosenthal MH, Bryant SL. Benefits of adjunct modafinil in an open-label, pilot study in patients with schizophrenia. Clinical Neuropharmacology. 2004;27:38–43. doi: 10.1097/00002826-200401000-00011. [DOI] [PubMed] [Google Scholar]
- Rugino TA, Samsock TC. Modafinil in children with attention-deficit hyperactivity disorder. Pediatric Neurology. 2003;29:136–142. doi: 10.1016/s0887-8994(03)00148-6. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, Sullivan EV. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Research. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, Van Beek I, Lewis J, Wodak A. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. Journal of Studies on Alcohol and Drugs. 2010;71:335–344. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. Memory-scanning: Mental processes revealed by reaction-time experiments. American Scientist. 1969;57:421–457. [PubMed] [Google Scholar]
- Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2004a;55:1031–1040. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004b;29:1363–1373. doi: 10.1038/sj.npp.1300457. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Fillmore MT, Hays LR, Rush CR. Effects of potential agonist-replacement therapies for stimulant dependence on inhibitory control in cocaine abusers. The American Journal of Drug and Alcohol Abuse. 2008;34:293–305. doi: 10.1080/00952990802013565. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Randazzo AC, Stone KL, Schweitzer PK. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep. 2004;27:434–439. doi: 10.1093/sleep/27.3.434. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler test of adult reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Wesensten N, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ. Maintaining alertness and performance during sleep deprivation: Modafinil versus caffeine. Psychopharmacology. 2002;159:238–247. doi: 10.1007/s002130100916. [DOI] [PubMed] [Google Scholar]
- Wong YN, Simcoe D, Hartman LN, Laughton WB, King SP, McCormick GC, Grebow PE. A double-blind, placebo-controlled, ascending-dose evaluation of the pharmacokinetics and tolerability of modafinil tablets in healthy male volunteers. Journal of Clinical Pharmacology. 1999;39:30–40. doi: 10.1177/00912709922007534. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Grant I. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]