Abstract
The evaluation of the toxicity of magnetic nanoparticles (MNPs) has attracted much attention in recent years. The current study aimed to investigate the cytotoxic effects of Fe3O4, oleic acid-coated Fe3O4 (OA-Fe3O4), and carbon-coated Fe (C-Fe) nanoparticles on human hepatoma BEL-7402 cells and the mechanisms. WST-1 assay demonstrated that the cytotoxicity of three types of MNPs was in a dose-dependent manner. G1 (Fe3O4 and OA-Fe3O4) phase and G2 (C-Fe) phase cell arrests and apoptosis induced by MNPs were detected by flow cytometry analysis. The increase in apoptosis was accompanied with the Bax over-expression, mitochondrial membrane potential decrease, and the release of cytochrome C from mitochondria into cytosol. Moreover, apoptosis was further confirmed by morphological and biochemical hallmarks, such as swollen mitochondria with lysing cristae and caspase-3 activation. Our results revealed that certain concentrations of the three types of MNPs affect BEL-7402 cells viability via cell arrest and inducing apoptosis, and the MNPs-induced apoptosis is mediated through the mitochondrial-dependent pathway. The influence potency of MNPs observed in all experiments would be: C-Fe > Fe3O4 > OA-Fe3O4.
Keywords: magnetic nanoparticles, BEL-7402, apoptosis, mitochondrial-dependent pathway, cell cycle
Introduction
Over the past few decades, as nanotechnology and materials science has progressed incredibly swiftly, nanomaterials have been widely applied in many fields including medicine, pharmaceuticals, manufacturing technologies, electronics, and telecommunications [1-3]. In particular, the surge of interest in nanomaterials has significantly expanded the breadth of research on magnetic nanoparticles (MNPs) during the recent decade. Due to their multifunctional properties, MNPs are explored for various biomedical applications such as contrast agents for MRI [4,5], targeted drug and gene delivery [6,7], cell sorting [8], hyperthermia [9], or combinations of multiple applications, both diagnostic and therapeutic [10]. Some MNPs, such as bowel contrast agents (Lumiren® and Gastromark®) and liver/spleen imaging (Endorem® and Feridex IV®) [11,12], are already in the market. Moreover, the potential applications of MNPs (e.g., bare Fe3O4 and C-Fe) have expanded into other fields including environmental restoration [13,14] and agriculture [15-18]. Some researches indicate that MNPs would accumulate in aquatic organisms [19], crops [18] for further entry into the food chain. Humans are therefore increasingly exposed to various kinds of MNPs, directly or indirectly.
Along with the expanding applications of MNPs, the potential toxic effects of MNPs have been of wide concern [20-23]. Multiple results show that MNPs significantly reduce cell viability of human macrophage, epithelial cell lines [24], human mesothelioma [25], and inhibit the normal formation of PC12 neuronal cell morphology [26]. At higher concentrations, DMSA-coated MNPs decrease mitochondrial activity of human fibroblasts [27]. Meanwhile, the cytotoxicity of MNPs is found in a dose-dependent manner [26].
Nevertheless, the cytotoxicity data of MNPs is difficult to compare since the toxic effects of MNPs are influenced by many parameters such as size distribution, surface coating, magnetic properties, etc. [27]. Numerous studies can be found that, quite often, report on seemingly contradicting findings since different cell types will interact with the same particle in different ways [28]. Therefore, it is crucial to choose the cell line for the cytotoxicity assessment of specific MNPs. Several pharmacokinetic reports indicate that liver is the most important organ involving the bioaccumulation and clearance procedures of MNPs [29-31]. Furthermore, the cytotoxicity studies of MNPs are limited by the fact that cytology mechanism remained unexplored.
In the present study, human hepatoma BEL-7402 cell line was selected as the model specimen for cytotoxicity assessment, and the aims were to evaluate the cytotoxicity of Fe3O4, OA-Fe3O4, and C-Fe and to elucidate the mechanisms of their cytotoxicities. MNPs internalization was observed by transmission electron microscopy (TEM) and cell viability was determined by tetrazolium salt-based (WST-1) assay. For the study of the mechanism of cytotoxicity, cell cycle and apoptosis were analyzed by flow cytometry. To further elucidate the apoptosis pathway, the mitochondrial membrane potential (MMP), the Bax and cytochrome C protein expression, and caspase-3 activity were investigated.
Results and discussion
MNPs uptake by human hepatoma BEL-7402 cells
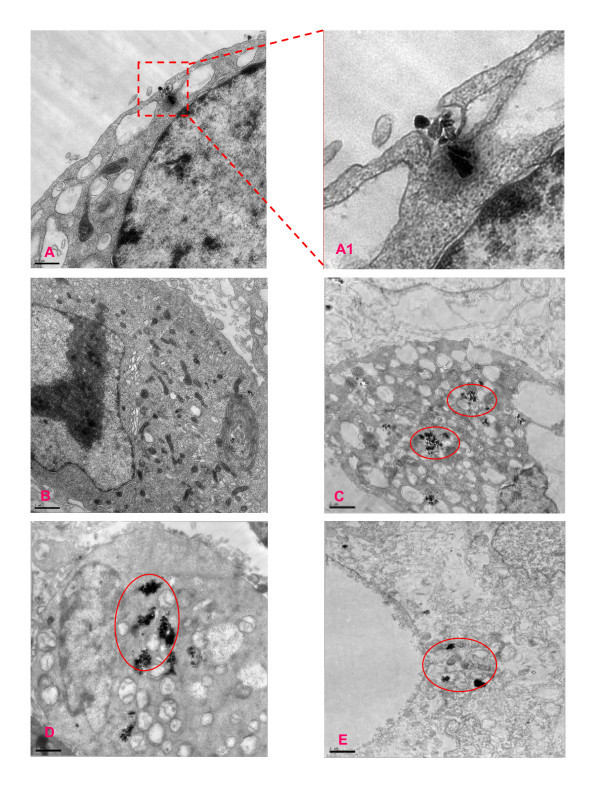
When cells were exposed to MNPs, most nanoparticles were first adhered to the surface, internalized to the cells by endocytosis, and accumulated in digestive vacuoles [32]. Our TEM images results showed that all three kinds of MNPs were incorporated into BEL-7402 cells after 24-h incubation at 0.5 mg/mL of concentration. The MNPs were distributed on the cell membrane and inside of cell. Some MNPs were observed enclosed by the invaginated cell membrane (Figure 1A), suggesting that endocytosis may involve the MNPs internalization process [21]. Lysosomes containing MNPs and swollen mitochondria with lysing cristae were present in MNPs treated cells (Figure 1C-D), coinciding with some results obtained in other MNPs [21,33]. Some cells showed chromatin condensation, typical of apoptotic cell death, and plenty of cytoplasmic vacuoles (Figure 1C-E). Treating with OA-Fe3O4 induced less cell damage than that of Fe3O4, while the C-Fe causes serious cell damage. Untreated cells had none of these features (Figure 1B).
Figure 1.
TEM micrographs of BEL-7402 exposed for 24 h to the different MNPs. (A) TEM micrographs of cell endocytosis; (A1) the enlargement of the rectangular areas on the corresponding images on the left side; (B) untreated cells; (C) cells exposed 0.5 mg/mL Fe3O4 MNPs; (D) cells exposed 0.5 mg/mL OA- Fe3O4 MNPs; (E) cells exposed 0.5 mg/mL C-Fe MNPs. Red circles show MNPs in the BEL-7402 cells.
The dose-dependent cytotoxicity of nanoparticles
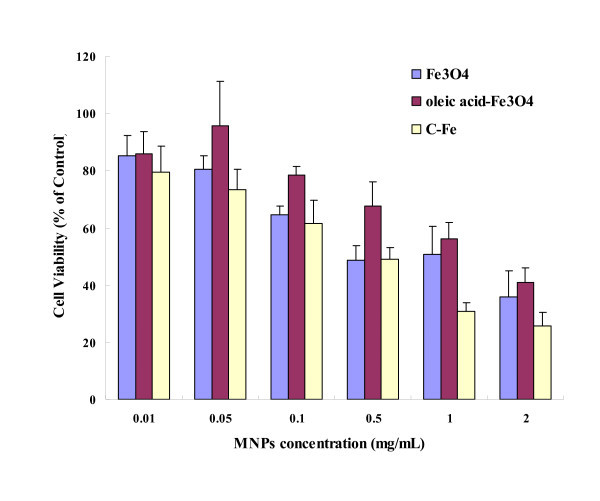
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide and lactate dehydrogenase assays are frequently adopted in assessing nanoparticle toxicity. These assays are used in drug studies, but can lead to aberrant results when using nanoparticles as they can sometimes interfere with the assay components or the readout [34]. Due to its convenience and great sensitivity, recently, the WST-1 assay has become a very popular cytotoxicity assay in the nanotoxicity study [22]. After 24 h exposure at varying doses of Fe3O4, OA-Fe3O4, and C-Fe MNPs, BEL-7402 cell viabilities detected by the WST-1 assay resulted in explicit dose-dependent reduction (Figure 2). The viabilities of BEL-7402 cells exposed to all three types of MNPs were above 60% at the concentration of 0.1 mg/mL and below. When the MNPs concentrations increased more than 1 mg/mL, the cell viabilities dropped to below 60%. The viabilities of cells exposed to Fe3O4 were lower than that to OA-Fe3O4, but higher than to C-Fe at all concentrations, which were correlated with the TEM observations. The cytotoxicity is thus very likely caused by particle overload to cells [35]. It is well known that the surface of BEL-7402 cells is negatively charge. The MNPs absorbed by the cells reduced with the decrease in positively charged surfaces of MNPs due to the electrostatic effects, which could affect the amount of MNPs entering the cells and further affect cytotoxicity. In our results, the surface charge of Fe3O4, OA-Fe3O4, and C-Fe were 14.4, 4.5, and 23.7 mV, respectively, which were consistent with WST-1 data trend.
Figure 2.
The viability of BEL-7402 Cells incubated with MNPs. Cells viability was determined by WST-1 assay after BEL-7402 cells were treated with MNPs (0.01, 0.05, 0.1, 0.5, 1, and 2 mg/mL) for 24 h. The percentage of viable cells was calculated as a ratio of absorbance at 490 nm of treated to control cells.
MNPs influence on the cell cycle
The effects of various concentrations (0.05, 0.1, and 1 mg/mL) of the three kinds of MNPs on cell cycle progression and population distribution in BEL-7402 cells were determined by flow cytometry. MNPs-induced effects were detected by comparing the cell cycle profiles between MNP-treated and -untreated cells. Results demonstrated that all three types of MNPs were able to affect cell cycle distribution of BEL-7402 cells (Table 1). After treated with Fe3O4 and OA-Fe3O4 MNPs at three different concentrations (0.05, 0.1, and 1 mg/mL), the ratio of G0/G1 phase cells increased 3.42%, 18.70%, 28.78% and 4.37%, 3.46%, 15.71% compared with control, respectively. As for C-Fe, 2.85%, 3.21%, and 9.34% G2 phase cell increases were observed. A similar report also showed that single-walled carbon nanotubes also caused a G2 phase arrest in PC12 cells [36]. Therefore, the mechanism of C-Fe MNPs on the cell cycle might be different with that of Fe3O4, and OA-Fe3O4 MNPs.
Table 1.
MNPs affected cell cycle distribution of BEL-7402 cells
| Cell cycle (%) | ||||
|---|---|---|---|---|
| G0-G1 | S | G2 | ||
| Control | 60.13 | 32.65 | 7.22 | |
| Fe3O4 | 0.05 mg/mL | 63.55 | 26.72 | 9.73 |
| 0.1 mg/mL | 78.83* | 14.09* | 7.08 | |
| 1 mg/mL | 88.91 | 3.52** | 7.57 | |
| OA-Fe3O4 | 0.05 mg/mL | 64.50 | 30.37 | 5.13 |
| 0.1 mg/mL | 63.59 | 29.75 | 6.67 | |
| 1 mg/mL | 75.84* | 21 | 3.15 | |
| C-Fe | 0.05 mg/mL | 59.32 | 30.61 | 10.07 |
| 0.1 mg/mL | 65.96 | 23.61 | 10.43 | |
| 1 mg/mL | 56.56 | 26.88 | 16.56* | |
After BEL-7402 cells were treated with MNPs (0.05 and 1 mg/mL) for 24 h, cell cycle assay was carried out by PI staining using flow cytometry. *P < 0.05 vs. control; **P < 0.01 vs. control.
Cells with reversibly damaged DNA will accumulate in G1, S, or G2/M phase [36], while cells that carry irreversibly damaged DNA will undergo apoptosis [37,38]. Hence, it is necessary to further analyze the cell apoptosis to fully interpret the toxic effects of MNPs on BEL-7402 cells.
MNPs-induced apoptosis of BEL-7402 cells
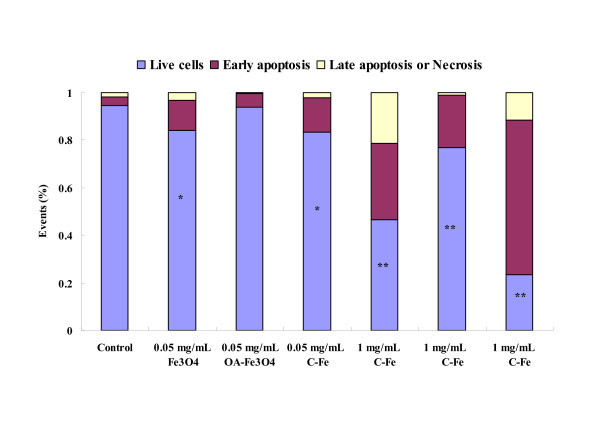
To assess the extent and mode of cell death induced by MNPs, Annexin-V/propidium iodide (PI) staining was performed. Externalization of phosphatidylserine (PS) seems to be a general feature of early stage apoptosis. Annexin V which has a strong Ca2+-dependent affinity for PS [39] was used to measure the apoptotic rate of BEL-7402 cells in response to the treatment of MNPs. The BEL-7402 cells were labeled with annexin V-fluorescein isothiocyanate (FITC)/PI. The Annexin V-/PI- population was regarded as normal cells, while positive staining just for Annexin V was used as a measure of early apoptosis and Annexin V-/PI+ was related to late apoptosis or necrosis [40]. Statistical data were extracted from the dot plots using WinMDI software [37]. As shown in Figure 3, compared with the untreated cells, a significant increase in the ratio of apoptosis cell was observed in Fe3O4 and C-Fe MNPs (0.05 mg/mL) treated cells (P < 0.05, the probability values of P < 0.05 were considered as statistics significance). At high concentration (1 mg/mL), all MNPs cause serious cell apoptosis (P < 0.01). Besides, a dose-dependent apoptosis rate was observed in all three types of MNP-treated cells. Moreover, the apoptosis rate of cells exposed to three types of MNPs would be: C-Fe > Fe3O4 > OA-Fe3O4, in the same concentration.
Figure 3.
MNPs induced apoptosis of BEL-7402 cells by Annexin V/PI assay. Annexin V-FITC/PI assay shows cell apoptosis by flow cytometry. Exposure of BEL-7402 cells to MNPs (0.05 and 1 mg/mL) for 24 h increased cell apoptosis. *P < 0.05 vs. control; **P < 0.01 vs. control.
This apoptosis result is consistent with cytotoxicity trends shown in WST-1 assay. The mechanisms of cytotoxic effects of MNPs on BEL-7402 cells may be implemented through cell cycle arrest and inducing apoptosis.
Assay of mitochondria-dependent apoptosis in BEL-7402 cells after MNPs
Apoptosis is a tightly controlled process in which cell death is executed through the activation of specific signaling pathways [41,42]. Although it is well established that many organelles contribute to apoptosis, extensive research shows that nanoparticles induced cell apoptosis via mitochondria-dependent pathway [43,44]. As an indicative of mitochondria involvement in the apoptosis, the apparently swollen mitochondria with lysing cristae were observed by TEM (Figure 1). Therefore, we speculate that BEL-7402 cell apoptosis was induced by MNPs through mitochondria-dependent pathway.
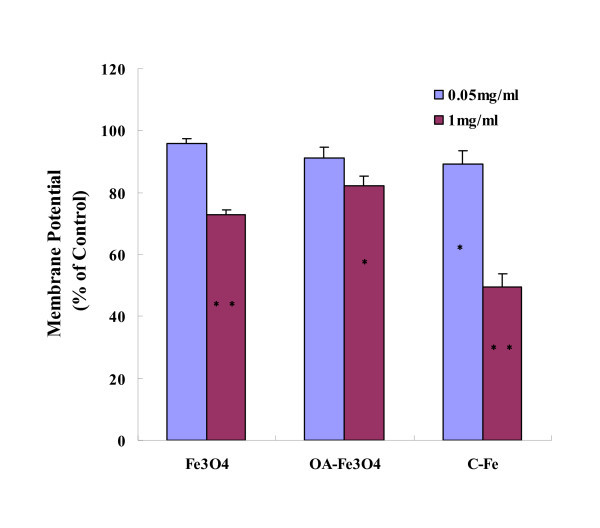
The mitochondrion is an important organelle involved in apoptosis. The loss of MMP is putatively the initial event leading to apoptosis [45]. To further elucidate the molecular mechanism of MNPs-induced apoptosis in BEL-7402, we examined loss of MMP using flow cytometry. As illustrated in Figure 4, after 24-h exposure to MNPs (0.05 mg/mL) for 24 h, a significant decrease in MMP was only observed in C-Fe-treated group (P < 0.05), while at high concentration (1 mg/mL), significant decrease of MMP occurred in all three MNPs-treated groups (P < 0.05).
Figure 4.
MNPs-induced loss of MMP. The MMP was measured by flow cytometry using JC-1 dye. Exposure of BEL-7402 cells to three types of MNPs (0.05 and 1 mg/mL) for 24 h decreased the MMP. *P < 0.05 vs. control; **P < 0.01 vs. control.
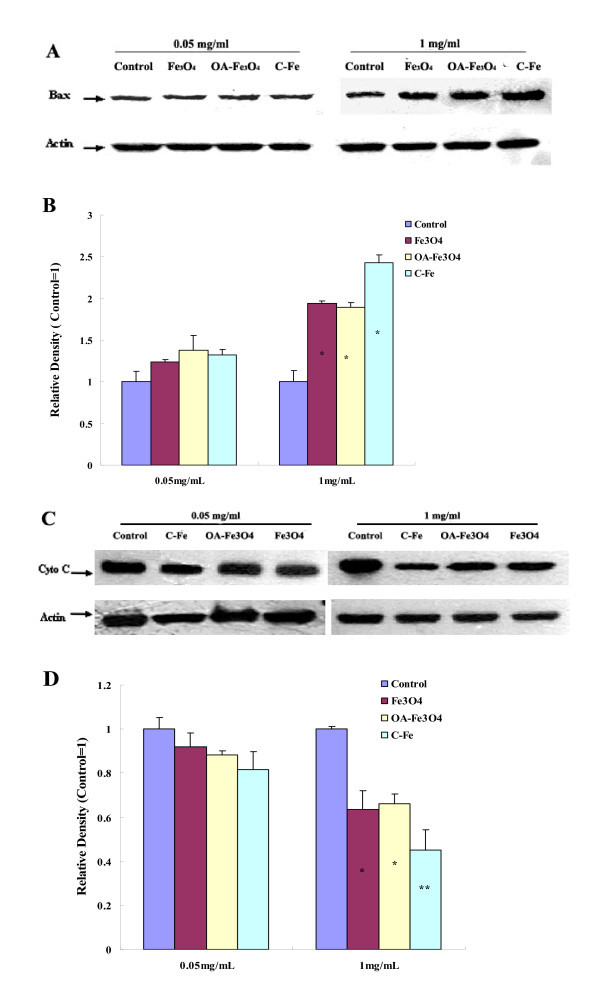
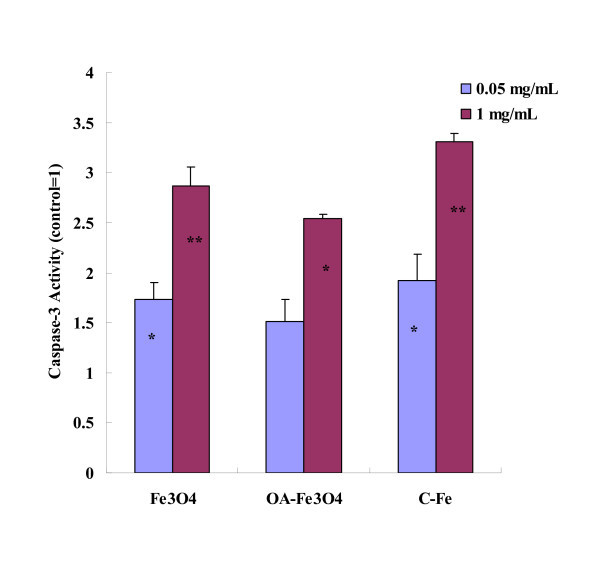
Mitochondrial membrane permeability is regulated through a family of proto-oncogenes. Bax is an important pro-apoptotic protein of the Bcl-2 family members [46]. High level of Bax can translocate to the outer mitochondrial membrane (OMM) and insert into the OMM. Then, Bax forms oligomers that are thought to be important in the formation of the mitochondrial permeability transition pore (PTP) [47,48]. The opening of the mitochondrial PTP can lead to a release of cytochrome C, which is a key event in apoptosis via the mitochondria-mediated pathway [49]. We examined expression of Bax and cytochrome C by Western blot. As shown in Figure 5A-B, after 24 h exposure at low concentration (0.05 mg/mL) of MNPs, the expression of Bax protein slightly increased, while without significant differences (P > 0.05). At high concentration (1 mg/mL), Bax protein expression in Fe3O4, OA-Fe3O4, and C-Fe MNPs-treated groups were about 1.94, 1.89, and 2.43 times compared with the control group, respectively. As for cytochrome C, the protein expression slightly decreased at the low concentration, while without significant differences (P > 0.05). At high concentration, the cytochrome C protein expression of all treated groups decreased dramatically, which is consistent with tendency of MMP. Based on the results mentioned above, we concluded that the three types of MNPs could induce Bax expression, further open PTP, and the PTP opening led to the release of cytochrome C from mitochondria. Once released from the mitochondria, cytochrome C combines with procaspase-9 to form the "apoptosome", which further activates caspase-3 [50-52]. Caspase-3 has been identified as a key mediator of apoptosis of mammalian cells [53]. Its activity is considered to be an appropriate measure of cytotoxic responsiveness [54]. We investigated the activaty of caspases-3 in BEL-7402 after exposure to MNPs for 24 h. As shown in Figure 6, we found that all three types of MNPs can activate caspase-3 in a dose-dependent manner. At low concentration (0.05 mg/mL), the activity of caspase-3 of the experimental groups increased, with significant differences found in Fe3O4- and C-Fe-treated groups (P < 0.05). The activity of caspase-3 was significantly increased in all experimental groups at high concentration (1 mg/mL) (P < 0.05).
Figure 5.
MNPs induced Bax over-expression and Cytochrome C release. Up-regulation of Bax expression in BEL-7402 cells treated with MNPs (A) and plotted as a relative level (B); down-regulation of cytochrome C expression in BEL-7402 cells treated with MNPs (C) and plotted as a relative level (D) *P < 0.05 vs. control; **P < 0.01 vs. control.
Figure 6.
Caspase-3 activity of BEL-7402 cells after incubation with MNPs for 24 h. After BEL-7402 cells were treated with MNPs (0.05 and 1 mg/mL) for 24 h, caspase-3 activity was determined using caspase-3/CPP32 Colorimetric Assay Kit. *P < 0.05 vs. control; **P < 0.01 vs. control.
To sum up, our results indeed suggested that all three types of MNPs can induce apoptosis in BEL-7402 cells through mitochondria-dependent pathway. Moreover, the influence potency of MNPs on the mitochondria-dependent apoptosis would be: C-Fe > Fe3O4 > OA-Fe3O4, and all in a dose-dependent manner.
Conclusion
In this paper, cytotoxic effects and the mechanism of Fe3O4, OA- Fe3O4, and C-Fe MNPs on BEL-7402 cells were studied. A dose-dependent cytotoxicity pattern was found in all three types of MNPs via WST-1 assay. The results of flow cytometric analysis revealed that the cytotoxicity of MNPs is implemented through cell cycle arrest and inducing apoptosis. The results of mitochondrial membrane potential, Western blots for Bax and cytochrome C, and caspase-3 activation further elucidate that MNPs induce apoptosis through mitochondria-dependent pathway. Moreover, the influence potency of MNPs observed in all experiments would be: C-Fe > Fe3O4 > OA-Fe3O4.
Recent studies show that the cytotoxicities of many MNPs could be due to reactive oxygen species (ROS) induction [55,56]. And accompanied with the MNPs degradation, the altered cellular iron pool can then affect cellular functionality by altering the level of transferrin receptor expression and can affect cellular proliferation capacity by altering the expression of cyclins and cyclin-dependent kinases in cell cycle [57,58]. Therefore, the metabolism, ROS determination and transferrin receptor expression will be the next step for further reveal of the cytotoxicities of Fe3O4, OA- Fe3O4, and C-Fe.
Materials and methods
Reagents
RPMI-1640 and fetal bovine serum were purchased from Gibco, Invitrogen Corp., Carlsbad, CA, USA. PI and RNase I were obtained from Sigma, St. Louis, MO, USA. Alexa Fluor® 488 annexin V/Dead Cell Apoptosis Kit was obtained from Invitrogen, USA. The primary antibodies to Bax, cytochrome C, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The goat anti-Mouse IgG-HRP, mouse anti-rabbit IgG-HRP, and Potent ECL kit were purchased from Multisciences, Hanzhou, China. Caspase-3/CPP32 Colorimetric Assay Kit and Mitochondria/Cytosol Fractionation Kit were purchased from BioVision, Mountain View, CA, USA. Total Protein Extraction Kit and BCA Protein Assay Kit were obtained from Applygen Technologies Inc., Beijing, China. The lipophilic cationic dye JC-1 (5, 5, 6, 6-tetrachloro-1, 1, 3, 3-tetraethylbenzimidazol-carbocyanine iodide) was obtained from ChemoMetec, Allerød, Denmark. WST-1 Cell Proliferation and Cytotoxicity Assay Kit was purchased from Beyotime Institute of Biotechnology, Haimen, China. All other reagents are analytical or cultured grade purity.
Cell culture and preparation of MNPs
Human hepatoma BEL-7402 cell line was a gift kindly provided by Medical College of Xiamen University (Xiamen, China). The cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum. Incubation was carried out at 37°C in a humidified 5% CO2 incubator. For all experiments, the cells were in the exponential growth phase. The MNPs used in this study were: (1) Fe3O4 MNPs, purchased from Aladdin (Shanghai, China); (2) OA-Fe3O4 MNPs, purchased from Jinke (Maanshan, China); (3) C-Fe MNPs, purchased from Junye (Shenzhen, China). The purity of three types MNPs are 99.9% and the size distribution of particles are 10-30 nm. Nanoparticle stock suspensions (10 mg/mL) were prepared by UV-sterilization and dispersing a known weight of nanoparticles in RPMI-1640 medium under ultrasonication. The stock suspensions were sonicated for 20 min to distribute the particles, and then dilutions were made in complete media to achieve desired testing concentrations. The test suspensions were sonicated for 20 min before use. Untreated controls were exposed to complete media only, and processed identical to the exposed cells.
TEM analysis
Cells (2 × 106) were seeded into 100-cm2 petri dishes. Cells were allowed to attach for 24 h and were then treated with each MNPs test suspensions for 24 h in a concentration of 0.5 mg/mL. Then, the cells were collected and fixed with 2.5% glutaraldehyde buffered in 0.1 M PBS overnight at 4°C. The samples were washed with PBS, and post-fixed in 1% osmium tetroxide at 4°C for 1 h. After dehydration in series concentrations of ethanol and infiltration in acetone, cells were embedded in Epon 812, and ultra-thin sections cut with glass knives were stained with uranyl acetate and lead citrate, and viewed under JEM 2100 TEM (JEOL, Tokyo, Japan).
WST-1 assay
To determine cell toxicity/viability, BEL-7402 cells (0.5 × 104, 100 μL) were plated onto 96-multiwell plates (Costar, Corning, NY, USA) and incubated for 24 h. Then, cells were exposed to various concentrations (0.01-2 mg/mL) of each MNPs test suspensions for 24 h. Afterwards, the old media was discarded and replaced with 100 μL of new complete media. WST-1 solution (10 μL) was added to each well, followed by incubation for 2.5 h. Absorbance at 490 nm (reference at 630 nm) was measured by a spectrophotometric microplate reader (Bio-tek ELX800, BioTek Instruments, Winooski, VT1, USA). A negative control was provided using the culture medium without the nanoparticles. Each of the particle concentrations and the controls was seeded in eight wells. The percentage cell viability was calculated in term of absorbency in cells treated with MNPs relative to that in cells exposed to culture media alone.
Cell cycle assay
A cell cycle assay was carried out by staining the DNA with PI and analyzing the fluorescence using flow cytometry. Following exposure of the BEL-7402 cells to each MNPs for 24 h, any damaged cells can detach from the plate and become suspended in the medium, necessitating medium storage. Briefly, the cells were harvested, washed with PBS, and fixed in ice-cold 70% of ethanol at -20°C before use. After resuspension, cells were washed, and incubated with 100 μL PI (400 μg/mL) and 100 μL RNase I (1 mg/mL) at 37°C for 15 min. Cells were analyzed with an EPICS XL flow cytometer (Beckman Coulter Inc., Fullerton, CA, USA) and the data were consequently evaluated by Mod-Fit (Verity Software, Topsham, ME, USA).
Detection of apoptosis by annexin V assay
Apoptosis was evaluated using Alexa Fluor® 488 Annexin V/PI Apoptosis Kit. BEL-7402 cells were treated with two concentrations (0.05 and 1 mg/mL) of each MNPs for 24 h. After exposure, the cells (5-10 × 104) were harvested, washed and resuspended with PBS. The Annexin V/PI staining of cells followed the manufacturer's instructions. Then the samples were analyzed with EPICS XL flow cytometer (Beckman Coulter, USA). The results were expressed as the number of apoptotic cells per thousand cells counted.
MMP measurement
The mitochondrial membrane potential was measured by flow cytometry using JC-1 dye. JC-1 changes its fluorescence from green at 535 nm (monomer state) to orange at 590 nm (aggregate state) as it enters in mitochondria of intact cells. When the mitochondrial membrane potential is affected, JC-1 returns to its green monomeric state. All procedures were carried out according to the manufacturer's instructions. The cell treatments were the same with "Detection of apoptosis by annexin V assay" section. In brief, approximately 2 × 106 cells were harvested, washed, resuspended in PBS (1 mL) and stained with 12.5 μL JC-1(200 μg/mL) for 15 min at 37°C in the dark. Both fluorescences emitted by the cells were monitored by flow cytometry and the ratio orange/green fluorescence was calculated. The data was determined by analyzing 10,000 cells using an EPICS XL flow cytometer (Beckman Coulter, Fullerton, CA, USA), and Cell Quest software (Becton Dickinson, San Jose, CA, USA).
Western blot analysis of Bax and cytochrome C
The cell treatments were the same with "Detection of apoptosis by annexin V assay" section. Approximately 1 × 107 cells per sample were harvested. The protein samples of Bax and cytochrome C were extracted using Total Protein Extraction Kit and Mitochondria/Cytosol Fractionation Kit, respectively. Protein contents were quantified using the BCA protein assay kit and stored at -70°C. Protein (20 μg per lane) was resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes (PVDF, Millipore Corporation, Billerica, MA, USA). The transblotted membrane was washed, blocked, and incubated at 4°C overnight with anti-Bax antibody and anti-cytochrome C antibody, respectively. Immunodetection with the secondary HRP-conjugated antibody and chemiluminescence using Potent ECL Kit were performed according to the manufacturer's protocol. Equal protein loading was verified by probing with anti-β actin antibody. Densitometric analysis for the blots was performed with Bandscan image software.
Caspase-3 activity assay
The activity of caspase-3 was determined using caspase-3/CPP32 Colorimetric Assay Kit. The cell treatments were the same with "Detection of apoptosis by annexin V assay" section. All procedures were carried out according to the manufacturer's instructions. Briefly, 2 × 107 BEL-7402 cells were lysed by the solution provided in the assay kit and the protein concentration was measured using BCA protein assay kit. For caspase-3 activity assay, equal amounts of total cell lysates were mixed with a caspase-specific substrate DEVD-pNA in a 96-well plate in triplicate. After incubation at 37°C for 2 h, the caspase-3-mediated cleavage of DEVD-pNA into free pNA was measured using spectrophotometric microplate reader (Bio-tek ELX800, USA) at 405 nm. The results were expressed as absorbance compared with control.
Statistical analysis
All results were expressed as mean values ± S.D. Statistical analysis was performed according to the Student's t test. The probability values of P < 0.05 were considered as significant.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
WK conceived the study, carried out all the experiments and drafted the manuscript. XXJ collected and analysed data, drafted the manuscript and approved the final version. PXM participated in drafting the manuscript. HZQ reviewed the manuscript. ZQQ conceived the study, reviewed the manuscript and approved the final version.
Contributor Information
Wei Kai, Email: weikaiat2001@163.com.
Xu Xiaojun, Email: xuxiaojun13@yahoo.com.cn.
Pu Ximing, Email: puximing@163.com.
Hou Zhenqing, Email: houzhenqing@xmu.edu.cn.
Zhang Qiqing, Email: qiq@xmu.edu.cn.
Acknowledgements
This work was financially supported by the Major Research plan of the National Natural Science Foundation of China (Grant No. 90923042), National Key Technologies R & D Program of China (Grant No. 2007BAD07B05).
References
- M G. Travels to the nanoworld: miniature machinery in nature and technology. New York: Plenum Trade; 1999. [Google Scholar]
- Kim D, El-Shall H, Dennis D, Morey T. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf B Biointerfaces. 2005;40:83–91. doi: 10.1016/j.colsurfb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Perkel JM. Nanoscience is Out of the Bottle. The scientist. 2003;17:20–23. [Google Scholar]
- Boutry S, Laurent S, Elst LV, Muller RN. Specific E-selectin targeting with a superparamagnetic MRI contrast agent. Contrast Media Mol Imaging. 2006;1:15–22. doi: 10.1002/cmmi.87. [DOI] [PubMed] [Google Scholar]
- Mornet S, Vasseur S, Grasset F, Veverka P, Goglio G, Demourgues A, Portier J, Pollert E, Duguet E. Magnetic nanoparticle design for medical applications. Progress in Solid State Chemistry. 2006;34:237–247. doi: 10.1016/j.progsolidstchem.2005.11.010. [DOI] [Google Scholar]
- Dobson J. magnetic nanoparticles for drug delivery. Drug Development Research. 2006;67:55–60. doi: 10.1002/ddr.20067. [DOI] [Google Scholar]
- Mykhaylyk O, Zelphati O, Hammerschmid E, Anton M, Rosenecker J, Plank C. Recent advances in magnetofection and its potential to deliver siRNAs in vitro. Methods Mol Biol. 2009;487:111–146. doi: 10.1007/978-1-60327-547-7_6. [DOI] [PubMed] [Google Scholar]
- Clement JH, Schwalbe M, Buske N, Wagner K, Schnabelrauch M, Gornert P, Kliche KO, Pachmann K, Weitschies W, Hoffken K. Differential interaction of magnetic nanoparticles with tumor cells and peripheral blood cells. J Cancer Res Clin Oncol. 2006;132:287–292. doi: 10.1007/s00432-006-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazeau F, Levy M, Wilhelm C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine (Lond) 2008;3:831–844. doi: 10.2217/17435889.3.6.831. [DOI] [PubMed] [Google Scholar]
- Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, Muldoon LL, Neuwelt EA. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30:15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- Bonnemain B. Superparamagnetic agents in magnetic resonance imaging: physicochemical characteristics and clinical applications. A review. J Drug Target. 1998;6:167–174. doi: 10.3109/10611869808997890. [DOI] [PubMed] [Google Scholar]
- Shen YF, Tang J, Nie ZH, Wang YD, Ren Y, Zuo L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Separation and Purification Technology. 2009;68:312–319. doi: 10.1016/j.seppur.2009.05.020. [DOI] [Google Scholar]
- Zhang D, Wei S, Kaila C, Su X, Wu J, Karki AB, Young DP, Guo Z. Carbon-stabilized iron nanoparticles for environmental remediation. Nanoscale. 2010;2:917–919. doi: 10.1039/c0nr00065e. [DOI] [PubMed] [Google Scholar]
- Scheenen TW, Vergeldt FJ, Heemskerk AM, Van As H. Intact plant magnetic resonance imaging to study dynamics in long-distance sap flow and flow-conducting surface area. Plant Physiol. 2007;144:1157–1165. doi: 10.1104/pp.106.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Melendi P, Fernandez-Pacheco R, Coronado MJ, Corredor E, Testillano PS, Risueno MC, Marquina C, Ibarra MR, Rubiales D, Perez-de-Luque A. Nanoparticles as smart treatment-delivery systems in plants: assessment of different techniques of microscopy for their visualization in plant tissues. Ann Bot. 2008;101:187–195. doi: 10.1093/aob/mcm283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredor E, Testillano PS, Coronado MJ, Gonzalez-Melendi P, Fernandez-Pacheco R, Marquina C, Ibarra MR, de la Fuente JM, Rubiales D, Perez-de-Luque A, Risueno MC. Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol. 2009;9:45. doi: 10.1186/1471-2229-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes Z, Custardoy L, de la Fuente JM, Marquina C, Ibarra MR, Rubiales D, Perez-de-Luque A. Absorption and translocation to the aerial part of magnetic carbon-coated nanoparticles through the root of different crop plants. J Nanobiotechnology. 2010;8:26. doi: 10.1186/1477-3155-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Service RF. Nanotoxicology. Nanotechnology grows up. Science. 2004;304:1732–1734. doi: 10.1126/science.304.5678.1732. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen X, Gu C, Zhang Y, Xu J, Bian Z, Yang D, Gu N. The Effect of Iron Oxide Magnetic Nanoparticles on Smooth Muscle Cells. Nanoscale Res Lett. 2009;4:70–77. doi: 10.1007/s11671-008-9204-7. [DOI] [Google Scholar]
- Ying E, Hwang HM. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Sci Total Environ. 2010;408:4475–4481. doi: 10.1016/j.scitotenv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Wu X, Tan Y, Mao H, Zhang M. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int J Nanomedicine. 2010;5:385–399. doi: 10.2147/ijn.s10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto K, Garza KM, Murr LE. Cytotoxic effects of aggregated nanomaterials. Acta Biomater. 2007;3:351–358. doi: 10.1016/j.actbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006;40:4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- Pisanic TR, Blackwell JD, Shubayev VI, Finones RR, Jin S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials. 2007;28:2572–2581. doi: 10.1016/j.biomaterials.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Auffan M, Decome L, Rose J, Orsiere T, De Meo M, Briois V, Chaneac C, Olivi L, Berge-Lefranc JL, Botta A, Wiesner MR, Bottero JY. In vitro interactions between DMSA-coated maghemite nanoparticles and human fibroblasts: a physicochemical and cyto-genotoxical study. Environ Sci Technol. 2006;40:4367–4373. doi: 10.1021/es060691k. [DOI] [PubMed] [Google Scholar]
- Barua S, Rege K. Cancer-cell-phenotype-dependent differential intracellular trafficking of unconjugated quantum dots. Small. 2009;5:370–376. doi: 10.1002/smll.200800972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P. Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution. J Microencapsul. 1996;13:245–255. doi: 10.3109/02652049609026013. [DOI] [PubMed] [Google Scholar]
- Briley-Saebo K, Bjornerud A, Grant D, Ahlstrom H, Berg T, Kindberg GM. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imaging. Cell Tissue Res. 2004;316:315–323. doi: 10.1007/s00441-004-0884-8. [DOI] [PubMed] [Google Scholar]
- Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5:316–327. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- Tomitaka A, Hirukawa A, Yamada T, Morishita S, Takemura Y. Biocompatibility of various ferrite nanoparticles evaluated by in vitro cytotoxicity assays using HeLa cells. Journal of Magnetism and Magnetic Materials. 2009;321:1482–1484. doi: 10.1016/j.jmmm.2009.02.058. [DOI] [Google Scholar]
- Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115:403–409. doi: 10.1289/ehp.115-a403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soenen SJ, De Cuyper M. Assessing cytotoxicity of (iron oxide-based) nanoparticles: an overview of different methods exemplified with cationic magnetoliposomes. Contrast Media Mol Imaging. 2009;4:207–219. doi: 10.1002/cmmi.282. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Curtis AS. Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials. 2004;25:3029–3040. doi: 10.1016/j.biomaterials.2003.09.095. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun P, Bao Y, Liu J, An L. Cytotoxicity of single-walled carbon nanotubes on PC12 cells. Toxicol in Vitro. 2011;25:242–250. doi: 10.1016/j.tiv.2010.11.010. [DOI] [PubMed] [Google Scholar]
- AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. TEM Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Ishii H, Saito T. DNA damage-dependent cell cycle checkpoints and genomic stability. DNA Cell Biol. 2006;25:406–411. doi: 10.1089/dna.2006.25.406. [DOI] [PubMed] [Google Scholar]
- Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002;54:715–758. doi: 10.1016/S0169-409X(02)00046-7. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Zhao Y, Ren L, Jin LH, Sun LP, Yin P, Zhang YF, Zhang QQ. Novel gelatin-siloxane nanoparticles decorated by Tat peptide as vectors for gene therapy. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/44/445103. [DOI] [PubMed] [Google Scholar]
- Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35:24–27. doi: 10.5483/BMBRep.2002.35.1.024. [DOI] [PubMed] [Google Scholar]
- Ho PK, Hawkins CJ. Mammalian initiator apoptotic caspases. FEBS J. 2005;272:5436–5453. doi: 10.1111/j.1742-4658.2005.04966.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Deng C, Tang S, Zhang M. Mitochondria-dependent apoptosis induced by nanoscale hydroxyapatite in human gastric cancer SGC-7901 cells. Biol Pharm Bull. 2007;30:128–132. doi: 10.1248/bpb.30.128. [DOI] [PubMed] [Google Scholar]
- Woldemariam GA, Mandal SS. Iron(III)-salen damages DNA and induces apoptosis in human cell via mitochondrial pathway. Journal of Inorganic Biochemistry. 2008;102:740–747. doi: 10.1016/j.jinorgbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Petit PX, Susin SA, Zamzami N, Mignotte B, Kroemer G. Mitochondria and programmed cell death: back to the future. FEBS Lett. 1996;396:7–13. doi: 10.1016/0014-5793(96)00988-X. [DOI] [PubMed] [Google Scholar]
- Wang X, Xia Y, Liu L, Liu M, Gu N, Guang H, Zhang F. Comparison of MTT assay, flow cytometry, and RT-PCR in the evaluation of cytotoxicity of five prosthodontic materials. J Biomed Mater Res B Appl Biomater. 2010;95:357–364. doi: 10.1002/jbm.b.31723. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J Biol Chem. 2003;278:48935–48941. doi: 10.1074/jbc.M306289200. [DOI] [PubMed] [Google Scholar]
- Matroule JY, Carthy CM, Granville DJ, Jolois O, Hunt DW, Piette J. Mechanism of colon cancer cell apoptosis mediated by pyropheophorbide-a methylester photosensitization. Oncogene. 2001;20:4070–4084. doi: 10.1038/sj.onc.1204546. [DOI] [PubMed] [Google Scholar]
- Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Vital functions for lethal caspases. Oncogene. 2005;24:5137–5148. doi: 10.1038/sj.onc.1208524. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Urano J, Tamanoi F. Farnesyltransferase inhibitors induce cytochrome c release and caspase 3 activation preferentially in transformed cells. Proc Natl Acad Sci USA. 1998;95:15356–15361. doi: 10.1073/pnas.95.26.15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Sonnemann J, Gange J, Pilz S, Stotzer C, Ohlinger R, Belau A, Lorenz G, Beck JF. Comparative evaluation of the treatment efficacy of suberoylanilide hydroxamic acid (SAHA) and paclitaxel in ovarian cancer cell lines and primary ovarian cancer cells from patients. BMC Cancer. 2006;6:183. doi: 10.1186/1471-2407-6-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soenen SJ, Himmelreich U, Nuytten N, Pisanic TR, Ferrari A, De Cuyper M. Intracellular nanoparticle coating stability determines nanoparticle diagnostics efficacy and cell functionality. Small. 2010;6:2136–2145. doi: 10.1002/smll.201000763. [DOI] [PubMed] [Google Scholar]
- Stroh A, Zimmer C, Gutzeit C, Jakstadt M, Marschinke F, Jung T, Pilgrimm H, Grune T. Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free Radic Biol Med. 2004;36:976–984. doi: 10.1016/j.freeradbiomed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Schäfer R, Kehlbach R, Wiskirchen J, Bantleon R, Pintaske J, Brehm BR, Gerber A, Wolburg H, Claussen CD, Northoff H. Transferrin receptor upregulation: in vitro labeling of rat mesenchymal stem cells with superparamagnetic iron oxide. Radiology. 2007;244:514–523. doi: 10.1148/radiol.2442060599. [DOI] [PubMed] [Google Scholar]
- Huang DM, Hsiao JK, Chen YC, Chien LY, Yao M, Chen YK, Ko BS, Hsu SC, Tai LA, Cheng HY, Wang SW, Yang CS, Chen YC. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials. 2009;30:3645–3651. doi: 10.1016/j.biomaterials.2009.03.032. [DOI] [PubMed] [Google Scholar]