Abstract
To investigate the short-term (30–240 min) interactions among nitrogenase activity, NH4+ assimilation, and plant glycolysis, we measured the concentrations of selected C and N metabolites in alfalfa (Medicago sativa L.) root nodules after detopping and during continuous exposure of the nodulated roots to Ar:O2 (80:20, v/v). Both treatments caused an increase in the ratios of glucose-6-phosphate to fructose-1,6-bisphosphate, fructose-6-phosphate to fructose-1,6-bisphosphate, phosphoenolpyruvate (PEP) to pyruvate, and PEP to malate. This suggested that glycolytic flux was inhibited at the steps catalyzed by phosphofructokinase, pyruvate kinase, and PEP carboxylase. In the Ar:O2-treated plants the apparent inhibition of glycolytic flux was reversible, whereas in the detopped plants it was not. In both groups of plants the apparent inhibition of glycolytic flux was delayed relative to the decline in nitrogenase activity. The decline in nitrogenase activity was followed by a dramatic increase in the nodular glutamate to glutamine ratio. In the detopped plants this was coincident with the apparent inhibition of glycolytic flux, whereas in the Ar:O2-treated plants it preceded the apparent inhibition of glycolytic flux. We propose that the increase in the nodular glutamate to glutamine ratio, which occurs as a result of the decline in nitrogenase activity, may act as a signal to decrease plant glycolytic flux in legume root nodules.
N fixation, the reduction of N2 to NH4+ by (Brady)rhizobium bacteria, in legume root nodules is catalyzed by the bacterial enzyme nitrogenase. Nitrogenase is O2 labile, but the root nodule provides an environment that protects it from excess O2. Nitrogenase activity in legume root nodules is rapidly inhibited by treatments that affect plant C and N metabolism, such as exposure of the roots to C2H2, replacement of N2 in the rhizosphere with Ar, NO3− fertilization, defoliation, detopping, phloem girdling, and drought (Minchin et al., 1983, 1986; Sheehy et al., 1983; Witty et al., 1984, 1986; Carroll et al., 1987; Durand et al., 1987; Hartwig et al., 1987, 1990, 1994; Hunt et al., 1987; Walsh et al., 1987; Schuller et al., 1988; Vessey et al., 1988; Guérin et al., 1990; King and Layzell, 1991; Denison et al., 1992; Diaz del Castillo et al., 1992, 1994; Heim et al., 1993; Drevon and Hartwig, 1997). In most cases the decline in nitrogenase activity can be overcome by increasing the O2 concentration in the rhizosphere. Thus, the decline in nitrogenase activity has been attributed to a decrease in nodule O2 permeability, which is thought to be regulated by a variable physical barrier to O2 diffusion in the nodule inner cortex (Sheehy et al., 1983; Witty et al., 1984, 1986). The exact mechanism of regulation of this barrier is still poorly understood (Minchin, 1997).
The O2 concentration in the N2-fixing zone of legume root nodules is very low, in the range of 10 to 40 nm (Day and Copeland, 1991; Vance and Heichel, 1991; Hunt and Layzell, 1993). This is significantly lower than the Km(O2) of the terminal oxidase of isolated nodule mitochondria, which is 50 to 100 nm (Rawsthorne and LaRue, 1986; Millar et al., 1995). Thus, C metabolism in the plant fraction of legume root nodules is O2 limited. The main functions of plant C metabolism in legume root nodules are (a) to supply respiratory substrates to the bacteroids and (b) to supply 2-oxo acids to act as C skeletons for the incorporation of fixed N (NH4+) into amino acids (Day and Copeland, 1991; Vance and Heichel, 1991). The main respiratory substrate supplied by the plant host to the bacteroids is malate (Driscoll and Finan, 1993). Malate and the 2oxo acids are synthesized via glycolysis and related reactions. To fully understand the regulation of N2 fixation in legume root nodules, it is important to investigate the interactions between nitrogenase activity and plant glycolysis.
The decline in nitrogenase activity in response to perturbations in plant C and N metabolism occurs within minutes to hours. For example, in alfalfa (Medicago sativa), the decline in nitrogenase activity in response to detopping was complete within 45 min (Denison et al., 1992) and the Ar-induced decline in nitrogenase activity, which occurs when N2 in the rhizosphere is replaced with Ar, was complete within 20 min (Drevon and Hartwig, 1997). Therefore, in the present study we focused on the period 30 to 240 min after the imposition of the inhibitory treatments.
Previous studies have shown that the decline in nitrogenase activity due to phloem girdling, NO3− fertilization, and defoliation is associated with depletion of nodule Suc and starch pools (Walsh et al., 1987; Vessey et al., 1988; Weisbach et al., 1996), but it is still controversial whether the decline in nodule Suc and starch pools is the cause of the decline in nitrogenase activity or whether it reflects the decreased carbohydrate-sink strength of the nodules once nitrogenase activity has already declined. Part of the reason for this ambiguity is the fact that nodule Suc and starch pools are relatively large. In the present study we attempted to overcome this difficulty by focusing on the intermediates of glycolysis. The pool sizes of the glycolytic intermediates were 1 to 3 orders of magnitude smaller than Suc and starch pools and as a result should respond rapidly to treatments that affect nitrogenase activity.
We determined nodule pool sizes for selected glycolytic intermediates and then used these results to make inferences about glycolytic flux. We used two different treatments known to decrease nitrogenase activity: detopping (shoot removal) and Ar:O2 treatment (continuous exposure of the nodulated roots of intact plants to Ar:O2 [80:20, v/v]). We chose these treatments because the primary effect of detopping would be to deprive the nodules of C (i.e. Suc) from the shoots, whereas the primary effect of Ar:O2 treatment would be to deprive the nodules of N (i.e. NH4+) from N2 fixation. Our results provide, to our knowledge, the first insight into the short-term metabolic adaptations of nodule glycolytic flux and primary NH4+ assimilation to alterations in nitrogenase activity.
MATERIALS AND METHODS
Plant Material, Culture, and Growth Conditions
Seeds of alfalfa (Medicago sativa L. cv Resis) were surface-sterilized in 70% (v/v) ethanol for 3 min, rinsed with double-distilled water, allowed to germinate, and then planted. For gas-exchange measurements, plants were grown in gas-tight, sealable pots with a volume of 250 mL. For metabolite analyses, eight to nine plants were grown in 2-L pots and each pot was considered as one replicate. For all experiments pots were filled with silica sand (0.8- to 1.2-mm grain size) and plants were grown in growth chambers (PGR-15, Conviron, Winnipeg, Manitoba, Canada) at 20°C/16°C day/night temperature and 80% RH, with a 16-h photoperiod and a photon flux density of 500 μmol quanta PAR m−2 s−1. Fluorescent light was from 160-W bulbs (Cool White, Sylvania), and incandescent light was from 100-W bulbs (138L, Sylvania). Plants were watered with N-free nutrient solution and inoculated with Rhizobium meliloti strain 102F28. All experiments were performed when plants were 8 to 9 weeks old. This corresponded to the late vegetative or the early flowering stage.
Gas-Exchange Measurements
Nitrogenase (EC 1.7.99.2) activity was measured in situ as H2 evolution using an open flow gas-exchange system similar to that described by Minchin et al. (1983) and modified by Heim et al. (1993). The nodulated root systems of the plants were sealed in their pots and allowed to stabilize overnight for 18 to 20 h in a stream of ambient air at 250 mL min−1. Prior to the imposition of the detopping and Ar treatments, the flow rate was increased to 400 mL min−1 and the root systems were exposed to a stream of N2:O2 (80:20, v/v) for at least 1 h until steady-state rates of H2 evolution were established. For the detopping experiment total nitrogenase activity was determined as the peak H2 evolution in Ar:O2 (80:20, v/v). For the Ar experiment the nitrogenase activity of the Ar:O2-treated plants was measured as H2 evolution in Ar:O2 (80:20, v/v) and for the control plants as H2 evolution in N2:O2 (80:20, v/v).
Detopping and Ar Treatments
The detopping treatment involved the removal of all of the aboveground parts of the plants. For the Ar treatment the nodulated roots of intact plants were exposed to an atmosphere of Ar:O2 (80:20, v/v) as described above. In the detopping experiment the control plants were intact plants harvested at the same time as the detopped plants (i.e. 0, 30, 60, 120, and 240 min after detopping). In the Ar:O2 experiment the control plants were plants whose root systems were exposed to N2:O2. The N2:O2 control plants were harvested at the same time as the corresponding Ar:O2-treated plants. Plants were quickly uprooted and the nodulated root systems were rinsed in distilled water, blotted dry, and immersed in liquid N2. Nodules were picked while still frozen and stored at −80°C.
Preparation of Nodule Extracts for Metabolite Analyses
Extracts were prepared using a method adapted from Paul and Stitt (1993). Nodules were cleaned of all sand particles and root debris under liquid N2. About 400 to 500 mg of frozen, cleaned nodules were ground to a fine powder with a mortar and pestle. A 0.5-mL aliquot of 16% (w/v) TCA in diethyl ether was added to the powder, and the mixture was homogenized and left to stand for 15 min in a bath of liquid N2. A 0.4-mL aliquot of 16% (w/v) TCA in distilled water containing 5 mm EGTA was then added. The samples were homogenized again, and the homogenates were transferred to microfuge tubes, vortexed, and centrifuged at 15,000g for 5 min at 4°C. After the sample was centrifuged the aqueous phase was removed and washed three times with 0.8 mL of diethyl ether. The aqueous phase was neutralized with 5 m KOH:1 m triethanolamine as described by Weiner et al. (1987). Neutralized extracts were immediately frozen, stored in liquid N2, and thawed just prior to the metabolite assays.
Metabolite Assays and Recovery Tests
Metabolites were analyzed using a dual-wavelength spectrophotometer (model ZFP-22, Sigma) and standard enzyme-linked protocols. Glc-6-P and Fru-6-P were assayed as described by Stitt et al. (1989); Fru-1,6-bisP as described by Gerhard (1983); pyruvate and PEP as described by Lamprecht and Heinz (1983); malate as described by Möllering (1983); Glu, Gln, and Suc as described by the technical bulletin “Methods of Biochemical Analysis and Food Analysis” (1989) from Boehringer Mannheim; and starch as described by Fischer et al. (1997). The stability of the metabolites during the extraction procedure was checked by adding a small amount (2- to 3-fold the endogenous concentration) of each metabolite to the finely ground nodule material. The recovery figures for the various metabolites were (as a percentage of the amount added): Glc-6-P, 78%; Fru-6-P, 67%; Fru-1,6-bisP, 78%; PEP, 67%; pyruvate, 82%; malate, 76%; Glu, 87%; and Gln, 112%. The values presented in Figures 2, 3, 5, and 6 are the original data with no adjustment made to take into account differences in recovery between the different metabolites. For most of the metabolites the recovery figures were similar. Therefore, it was considered unnecessary to make any adjustments.
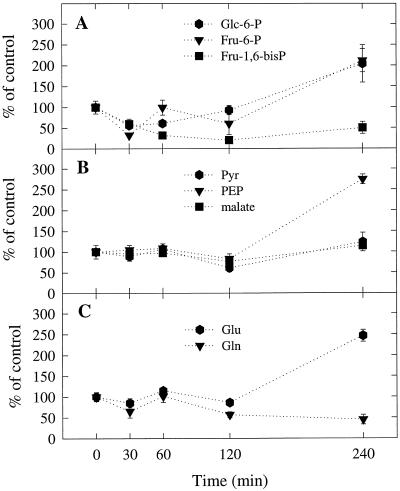
Figure 2.
Effect of detopping on the concentrations of selected metabolites in nodule extracts. Data are the means ± se (n = 3 or 4). Values for the detopped plants are expressed as percentages of the values for the control plants assayed at the corresponding times. Control values did not change significantly over time. The mean values ± se (n = 20) for the control plants averaged across all times were: 1.04 ± 0.07, 0.7 ± 0.06, 0.16 ± 0.01, 0.16 ± 0.01, 0.32 ± 0.02, 13.69 ± 0.5, 16.19 ± 0.9, and 6.11 ± 0.5 μmol g−1 nodule dry weight for Glc-6-P, Fru-6-P, Fru-1,6-bisP, PEP, pyruvate (Pyr), malate, Glu, and Gln, respectively.
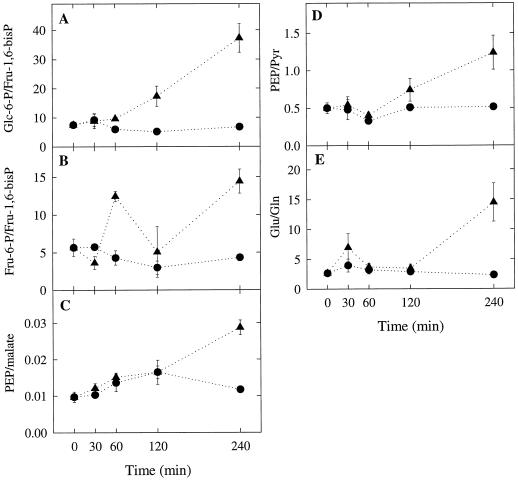
Figure 3.
Effect of detopping on the ratios of Glc-6-P to Fru-1,6-bisP, Fru-6-P to Fru-1,6-bisP, PEP to malate, PEP to pyruvate (Pyr), and Glu to Gln. Data were derived from the results presented in Figure 2. •, Control plants; ▴, detopped plants.
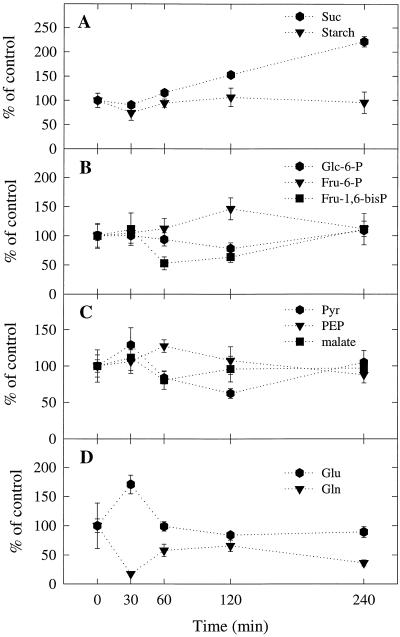
Figure 5.
Effect of Ar:O2 treatment on the concentrations of selected metabolites in nodule extracts. Data are the means ± se (n = 3 or 4). Values for the Ar:O2-treated plants are expressed as percentages of the values for the control plants assayed at the corresponding times. The mean values ± se (n = 17–20) for the control plants averaged across all times were 0.3 ± 0.03 mmol Glc equivalents g−1 nodule dry weight for starch, and 14.36 ± 0.7, 1.19 ± 0.05, 0.48 ± 0.03, 0.13 ± 0.01, 0.27 ± 0.02, 0.42 ± 0.03, 12.86 ± 0.6, 20.20 ± 1.02, and 2.85 ± 0.34 μmol g−1 nodule dry weight for Suc, Glc-6-P, Fru-6-P, Fru-1,6-bisP, PEP, pyruvate (Pyr), malate, Glu, and Gln, respectively.
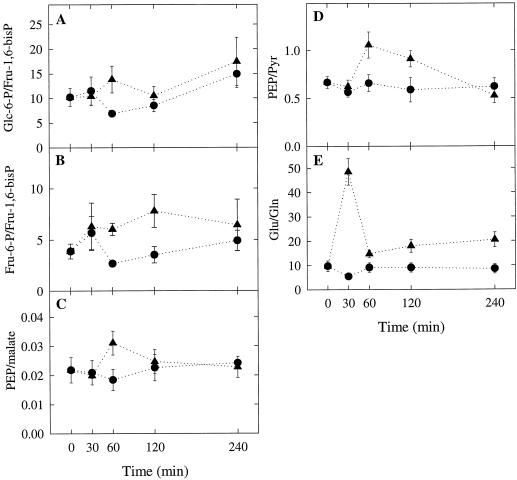
Figure 6.
Effect of Ar:O2 treatment on the ratios of Glc-6-P to Fru-1,6-bisP, Fru-6-P to Fru-1,6-bisP, PEP to malate, PEP to pyruvate (Pyr), and Glu to Gln. Data were derived from the results presented in Figure 5. •, Control plants; ▴, Ar-treated plants.
Statistical Analysis
Data were subjected to analysis of variance using statistical analysis software (SAS Institute, Cary, NC).
RESULTS AND DISCUSSION
Effects of Detopping on Total Nitrogenase Activity
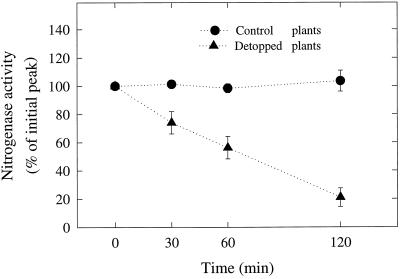
Total nitrogenase activity was assayed as H2 evolution in Ar:O2 (80:20, v/v). The nodulated root systems of both the control and the detopped plants were exposed to N2:O2 for the duration of the experiment, except for brief periods when the gas mixture was switched to Ar:O2 to assay total nitrogenase activity. Detopping caused a steady decline in total nitrogenase activity, which was essentially complete within 2 h of the start of the experiment (Fig. 1). The experiment was terminated at this point because previous studies had shown that after the initial decline, nitrogenase activity in detopped and completely defoliated plants remained low and did not recover until foliage regrowth began several days later (Denison et al., 1992; Weisbach et al., 1996).
Figure 1.
Effect of detopping on nitrogenase activity in alfalfa nodules. Total nitrogenase activity was determined as peak H2 evolution rate in Ar:O2 (80:20, v/v). Data are expressed as percentages of the initial peak activity (total nitrogenase activity) before detopping. Data represent the means ± se of six replicates for the control and nine replicates for the detopped plants. The average total nitrogenase activity value before the detopping treatment was 100.6 ± 17 μmol H2 g−1 nodule dry weight h−1 (n = 15).
Effects of Detopping on Glycolytic Flux
The effects of detopping on glycolytic flux were investigated by determining the nodular concentrations of the substrates and products of the reactions catalyzed by PFK, PK, and PEPC. PFK and PK are the key regulatory enzymes in plant glycolysis (Plaxton, 1996): PFK catalyzes the phosphorylation of Fru-6-P to yield Fru-1,6-bisP, and PK catalyzes the transfer of the phosphate moiety from PEP to ADP to yield pyruvate and ATP. Fru-6-P is in equilibrium with Glu-6-P via phosphohexose isomerase, and PEP can be converted to malate by the concerted action of PEPC and malate dehydrogenase. Alterations in the nodular concentrations of these metabolites are indicative of inhibition and/or activation of glycolytic flux. When there is an increase in the substrate/product concentration ratio for a particular enzyme, this suggests that the enzyme is being inhibited. When the enzyme is a key regulatory enzyme in a metabolic pathway, an increase in the substrate/product concentration ratio for the enzyme suggests that flux through the pathway as a whole is being inhibited. This method of analysis, referred to as crossover point analysis, has been reviewed in detail by Heinrich and Rapoport (1974).
During the first 30 min after detopping, the nodular concentrations of Glc-6-P, Fru-6-P, and Fru-1,6-bisP in the detopped plants declined to about 50% of the corresponding values for the control plants (Fig. 2A). This was probably the result of decreased Suc supply to the nodules from the shoots. During the next 30 min to 2 h, the concentration of Fru-1,6-bisP continued to decline but the concentrations of Glc-6-P and Fru-6-P began to increase. Between 2 and 4 h after detopping there was a dramatic increase in the ratios Glu-6-P to Fru-1,6-bisP and Fru-6-P to Fru-1,6-bisP (Fig. 3, A and B). These results suggest that detopping inhibits glycolytic flux at the level of PFK. The dramatic increase in the hexose-6-P to Fru-1,6-bisP ratio between 2 and 4 h after detopping coincided with an equally dramatic increase in the nodular PEP concentration (Fig. 2B). In in vitro studies it has been shown that PFK from soybean and cowpea nodules is strongly inhibited by PEP (Vella and Copeland, 1993; Lee and Copeland, 1996). Our results suggest that the apparent inhibition of PFK in detopped plants is at least in part due to the accumulation of PEP. The apparent inhibition of PFK was delayed relative to the decline in total nitrogenase activity, indicating that decreased glycolytic flux is a result of the decline in nitrogenase activity and not the cause (Fig. 1).
The increase in the hexose-6-P to Fru-1,6-bisP ratio between 2 and 4 h after detopping (Fig. 3, A and B) coincided with an increase in the PEP to malate ratio (Fig. 3C), the PEP to pyruvate ratio (Fig. 3D), and the Glu to Gln ratio (Fig. 3E). The increase in the PEP to malate ratio suggests that detopping inhibits PEPC activity. Legume nodule PEPC is regulated at the posttranslational level by metabolite effectors and protein phosphorylation (Vance and Stade, 1984; Marczewski, 1989; Schuller et al., 1990; Pathirana et al., 1992; Schuller and Werner, 1993; Vance et al., 1994; Zhang et al., 1995; Wadham et al., 1996; Zhang and Chollet, 1997). Soybean nodule PEPC is activated by hexose-6-P and inhibited by malate, Asp, Glu, citrate, and 2-oxoglutarate (Schuller et al., 1990). The effects of all of these metabolites are greater at pH 7.0 (suboptimal assay pH) than at pH 8.0 (optimal assay pH). The sensitivity of soybean nodule PEPC to inhibition by malate decreases when the enzyme becomes phosphorylated (Schuller and Werner, 1993; Zhang et al., 1995; Wadham et al., 1996; Zhang and Chollet, 1997). The protein kinase responsible for the phosphorylation of soybean nodule PEPC is inhibited by treatments that decrease Suc supply to the nodules, such as detopping, prolonged darkness, and phloem girdling (Zhang et al., 1995; Wadham et al., 1996; Zhang and Chollet, 1997).
Less is known about the posttranslational regulation of alfalfa nodule PEPC, but there are several lines of evidence that suggest that it is similar to soybean nodule PEPC. First, the deduced amino acid sequence of a cDNA encoding the nodule-enhanced form of alfalfa nodule PEPC contains the phosphorylation site motif typical of plant PEPCs regulated by protein phosphorylation (Pathirana et al., 1992). Second, when crude alfalfa nodule extracts were incubated with [γ-32P]ATP, PEPC became labeled with 32P (Vance et al., 1994). Therefore, the apparent inhibition of PEPC activity that we observed in the present study (Fig. 3C) may have been due to decreased PEPC-protein kinase activity as a result of decreased Suc supply to the nodules in detopped plants. This may be superimposed on allosteric inhibition of PEPC by Glu. Glu is a potent inhibitor of soybean nodule PEPC at pH 7.0 (physiological pH) but not at pH 8.0 (optimal pH) (Schuller et al., 1990). In alfalfa nodule PEPC, it is known that Glu has no effect at pH 7.5 (optimal pH), but the effect of Glu at pH 7.0 (physiological pH) has never been tested (Vance and Stade, 1984). If alfalfa nodule PEPC is similar to soybean nodule PEPC, then Glu will be an important allosteric inhibitor of both enzymes.
The increase in the PEP to pyruvate ratio (Fig. 3D) indicates that detopping inhibits glycolytic flux at the level of PK and at the level of PFK (see above). Plant glycolysis is thought to be regulated from the bottom up rather than from the top down, as it is in animal glycolysis (Plaxton, 1996). The primary inhibition of plant glycolysis occurs at the level of PK, leading to accumulation of PEP, which feedback inhibits PFK. Very little is known about the regulation of PK in legume nodules. There is one old report in which it was shown that a partially purified preparation of soybean nodule PK was activated by K+ and inhibited by NH4+ (Peterson and Evans, 1978). The authors tested a broad range of potential metabolite effectors of PK, including Glu, Gln, Asp, 2-oxoglutarate, and malate, but found that none of them had any effect. However, there were some problems with this study. First, the potential metabolite effectors were tested at saturating PEP concentration, so only metabolites that affect Vmax and not those that affect Km(PEP) would have been detected. Second, the PK preparation was heavily contaminated with PEPC, which could have confounded the results, since both PEPC and PK have PEP as a substrate. Clearly, the regulation of PK in legume nodules needs to be reexamined.
The most detailed information about plant PKs comes from work with castor bean. The cytosolic form of PK from germinating castor bean cotyledons is inhibited by Glu, 2-oxoglutarate, citrate, malate, and several other metabolites (Podestá and Plaxton, 1994). This inhibition is pH dependent, being more pronounced at pH 6.9 than at pH 7.5. If legume nodule PK is similar to castor bean cotyledon PK, then we can hypothesize that the accumulation of Glu that we observed in alfalfa nodules between 2 and 4 h after detopping (Fig. 2C) was responsible for the apparent inhibition of PK (Fig. 3D). If we assume a fresh weight:dry weight ratio of 5:1 and a volume-to-fresh-weight conversion of 1.2 g mL−1, then the concentrations of Glu in alfalfa nodules 2 and 4 h after detopping were 2.3 and 6.2 mm, respectively. These values are 1.4- and 3.9-fold greater than the I50 (Glu) value for partially purified PEPC from soybean nodules (Schuller et al., 1990). Clearly, we should also determine the I50 (Glu) value for PEPC and PK from alfalfa nodules. Fougère et al. (1991) reported that the Glu concentration in the plant fraction of alfalfa nodules was 2.6 μmol g−1 fresh weight of nodules, whereas in the bacteroid fraction it was only 0.2 μmol g−1 fresh weight of nodules. Thus, the total nodule Glu pool is a good indicator of the plant fraction Glu pool, and the contribution of the bacteroid Glu pool to the total nodule Glu pool is only minor.
Effects of Detopping on Gln and Glu Pools in the Nodules
There was no significant effect of detopping on nodule Gln and Glu pools in the first 2 h of the experiment (Fig. 2C). However, between 2 and 4 h there was a slight decrease in the nodular Gln concentration and a dramatic increase in the nodular Glu concentration, which resulted in a 5-fold increase in the Glu to Gln ratio (Fig. 3E). As with the other metabolites the changes in the nodular Glu and Gln pools were delayed relative to the decline in nitrogenase activity. NH4+ exported to the host plant by the N2-fixing bacteroids is incorporated into amino acids via the GS/GOGAT cycle. The accumulation of Glu that we observed suggests that the GS/GOGAT cycle is N limited rather than C limited. In other words, the lack of NH4+ limits the cycle, not the lack of 2-oxoglutarate. This was somewhat unexpected, given that detopping decreases the Suc supply to nodules and Suc is the ultimate precursor of 2-oxoglutarate.
Sequence of Events Following Detopping
Our data are consistent with the conclusion that the apparent down-regulation of glycolytic flux following detopping is due to the decline in nitrogenase activity and not vice versa. From our results we can postulate the following sequence of events: Between 30 min and 2 h after detopping, nitrogenase activity declines to a very low level (Fig. 1). Between 2 and 4 h after detopping, NH4+ production by nitrogenase is very low. As a result, the GS/GOGAT cycle becomes N limited, Glu accumulates, and the Glu to Gln ratio increases. The accumulation of Glu inhibits PEPC and/or PK activity. This causes the accumulation of PEP, which inhibits PFK.
Effects of Ar:O2 Treatment on Nitrogenase Activity
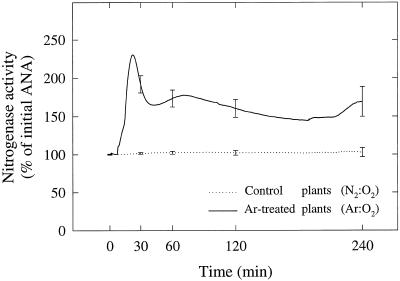
Nitrogenase activity was measured as H2 evolution in either N2:O2 (80:20, v/v, control plants) or Ar:O2 (80:20, v/v, Ar:O2-treated plants; Fig. 4). For the control plants the rate of H2 evolution remained constant throughout the experiment. However, for the Ar:O2-treated plants the results were more complex. During the first 30 min of the experiment there was an initial rapid increase in the rate of H2 evolution in Ar:O2, followed by a sharp decline. Thereafter, H2 evolution remained relatively constant. Similar results have been reported in previous studies with alfalfa (Drevon and Hartwig, 1997). The initial rapid increase was because, in N2:O2, 75% of the electron flux through nitrogenase for N2 fixation and only 25% is used for proton reduction to H2, whereas in Ar:O2, 100% of the electron flux is used for proton reduction (Hunt and Layzell, 1993). The sharp decline in H2 evolution during the first 30 min after the switch from N2:O2 to Ar:O2 was due to the well-known but poorly understood Ar-induced decline in nitrogenase activity attributed to decreased nodule O2 permeability (Minchin et al., 1983; Sheehy et al., 1983; Witty et al., 1984; Hunt et al., 1987).
Figure 4.
Time course of H2 evolution rate during continuous exposure of intact nodulated alfalfa roots to N2:O2 (80:20, v/v, control plants) and Ar:O2 (80:20, v/v, treated plants). Data were plotted as percentages of the respective apparent nitrogenase activity (ANA) value obtained at time 0. The control data were obtained from three replicate plants and the data for the treated plants were obtained from five replicate plants. Bars represent se at chosen times. The average apparent nitrogenase activity value for the control plants at time 0 was 13.4 ± 0.8 μmol H2 g−1 nodule dry weight h−1 (n = 8).
Effects of Ar:O2 Treatment on NH4+ Assimilation and Glycolytic Flux
The switch from N2:O2 to Ar:O2 in the rhizosphere of the Ar:O2-treated plants resulted in a dramatic 70% increase in nodular Glu concentration during the first 30 min and then returned to the control level between 30 and 60 min (Fig. 5D). The increase in nodular Glu concentration was paralleled by a similarly dramatic 80% decrease in nodular Gln concentration (Fig. 5D). There was a partial recovery in nodular Gln concentration between 30 and 60 min and then a further decline between 120 and 240 min. The initial changes in the concentrations of Glu and Gln resulted in a transient 5-fold increase in the Glu to Gln ratio (Fig. 6E). The dramatic increase in the Glu to Gln ratio that occurred within the first 30 min after the switch from N2:O2 to Ar:O2 was most likely due to the cessation of NH4+ production by nitrogenase. The increase in the Glu to Gln ratio appeared to precede the Ar-induced decline in nitrogenase activity (Figs. 4 and 6E), suggesting that it might be the trigger that causes the Ar-induced decline. However, more intense sampling during the first 30 min after the switch from N2:O2 to Ar:O2 would be required to test this hypothesis.
The transient 5-fold increase in the Glu to Gln ratio during the first 30 min after the switch from N2:O2 to Ar:O2 was followed by a transient increase between 30 and 60 min in the ratios Glu-6-P to Fru-1,6-bisP and Fru-6-P to Fru-1,6-bisP (Fig. 6, A, B, and E). There was also a transient increase between 30 and 60 min in the ratios PEP to malate and PEP to pyruvate (Fig. 6, C and D). These results suggest that the transient increase in the Glu to Gln ratio triggers a transient inhibition of glycolytic flux at the steps catalyzed by PFK and PK. This is the same mechanism we have proposed to explain the inhibition of glycolytic flux in detopped plants between 2 and 4 h after detopping (see above). However, in the Ar:O2-treated plants the inhibition was reversible, whereas in the detopped plants it was not.
Effects of Ar:O2 Treatment on Suc and Starch Content of the Nodules
Ar:O2 treatment had no effect on the starch content of the nodules, but by the end of the 4-h treatment Suc content had more than doubled (Fig. 5A). During the first 60 min of the experiment the nodular Suc content of the Ar:O2-treated plants remained the same as for the control plants. However, during the period from 60 min to 4 h, there was a gradual accumulation of Suc in the nodules of the Ar:O2-treated plants, with the final concentration being more than twice that of the control plants. Initially, the accumulation of Suc was probably due to decreased demand for C skeletons for the incorporation of fixed N (i.e. NH4+) into amino acids. Later, however, when the Ar-induced decline in nitrogenase activity had taken place, there was probably also decreased demand for respiratory substrates by the bacteroids. The observation that the accumulation of Suc occurred after the Ar-induced decline in nitrogenase activity (Figs. 4 and 5A) suggests that the main sink for Suc in the nodules is the synthesis of respiratory substrates for the bacteroids.
There has been considerable interest in the possible involvement of nodule carbohydrates, particularly Suc, in the regulation of nitrogenase activity and nodule O2 permeability (Walsh et al., 1987; Vessey et al., 1988; Weisbach et al., 1996; Gordon et al., 1997). Walsh et al. (1987) showed that the decline in nitrogenase activity caused by stem- girdling coincided with a decline in the total soluble-sugar content of nodules. Vessey et al. (1988) found that the decline in nitrogenase activity caused by NO3− treatment coincided with a decrease in the size of the nodule starch pool and in the partitioning of the 14C-labeled photosynthate to nodules. Weisbach et al. (1996) reported that the decline in nitrogenase activity caused by 100% defoliation coincided with a decrease in the size of the nodule Suc pool. From these results one could conclude that decreased Suc supply to the nodules is responsible for the decline in nitrogenase activity. However, it is unclear whether the decline in Suc supply is the primary event or whether it is simply a result of the decline in nitrogenase activity.
Gordon et al. (1997) proposed that the decline in nitrogenase activity caused by various perturbations of plant metabolism could be due to decreased capacity of the nodules to catabolize Suc. This proposal was based on the observation that there was a significant decline in Suc synthase activity in soybean nodules in response to NO3−, salt, and drought treatments. The decline in Suc synthase activity was associated with accumulation of Suc in the nodules of salt- and drought-stressed plants but not in the nodules of NO3−-treated plants. More research, including parallel measurements of nitrogenase activity and Suc synthase activity, is required to determine whether the decline in Suc synthase activity precedes the decline in nitrogenase activity, or whether it is simply a result of decreased Suc sink capacity of the nodules due to decreased nitrogenase activity by some other mechanism. Our results suggest that after Ar:O2 treatment, the decreased capacity of the nodules to catabolize Suc was a result rather than the cause of the decline in nitrogenase activity (Figs. 4 and 5A). This does not exclude the possibility that decreased Suc synthase activity may be involved in the decline in nitrogenase activity in detopped plants.
ACKNOWLEDGEMENTS
We thank Dr. M. Frehner for his generous advice and support and J. P. Almeida for his help with the statistical analyses. We are grateful to W. Wild for invaluable technical assistance and to A. Dürsteler for her help with the starch determinations. We also thank Prof. Dr. N. Amrhein for critically reading the manuscript.
Abbreviations:
- GOGAT
Gln-oxoglutarate aminotransferase
- GS
Gln synthetase
- I50
inhibitor concentration producing 50% inhibition of enzyme activity
- PEPC
PEP carboxylase
- PFK
phosphofructokinase
- PK
pyruvate kinase
Footnotes
This work was supported by a grant from the Swiss Federal Institute of Technology (to U.A.H.) and fellowships from the Human Frontier Science Program and the Organisation for Economic Cooperation and Development (to K.A.S.).
LITERATURE CITED
- Carroll BJ, Hansen AP, McNeil DL, Gresshoff PM. Effect of oxygen supply on nitrogenase activity of nitrate- and dark-stressed soybean (Glycine max L. Merr.) plants. Aust J Plant Physiol. 1987;14:679–687. [Google Scholar]
- Day DA, Copeland L. Carbon metabolism and compartmentation in nitrogen-fixing legume nodules. Plant Physiol Biochem. 1991;29:185–201. [Google Scholar]
- Denison RF, Hunt S, Layzell DB. Nitrogenase activity, nodule respiration, and O2 permeability following detopping of alfalfa and birdsfoot trefoil. Plant Physiol. 1992;98:894–900. doi: 10.1104/pp.98.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz del Castillo L, Hunt S, Layzell DB. O2 regulation and O2 limitation of nitrogenase activity in root nodules of pea and lupin. Physiol Plant. 1992;86:269–278. [Google Scholar]
- Diaz del Castillo L, Hunt S, Layzell DB. The role of oxygen in the regulation of nitrogenase activity in drought-stressed soybean nodules. Plant Physiol. 1994;106:949–955. doi: 10.1104/pp.106.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevon JJ, Hartwig UA. Phosphorus deficiency increases the argon-induced decline of nodule nitrogenase activity in soybean and alfalfa. Planta. 1997;201:463–469. [Google Scholar]
- Driscoll BT, Finan TM. NAD+-dependent malic enzyme of Rhizobium meliloti is required for symbiotic nitrogen fixation. Mol Microbiol. 1993;7:865–873. doi: 10.1111/j.1365-2958.1993.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Durand JL, Sheehy JE, Minchin FR. Nitrogenase activity, photosynthesis and nodule water potential in soybean plants experiencing water deprivation. J Exp Bot. 1987;38:311–321. [Google Scholar]
- Fischer BU, Frehner M, Hebeisen T, Zanetti S, Stadelmann F, Lüscher A, Hartwig UA, Hendrey GR, Blum H, Nösberger J. Source-sink relations in Lolium perenne L. as reflected by carbohydrate concentrations in leaves and pseudo-stems during regrowth in a free air carbon dioxide enrichment (FACE) experiment. Plant Cell Environ. 1997;20:945–952. [Google Scholar]
- Fougère F, Le Rudulier D, Streeter JG. Effects of salt stress on amino acid, organic acid and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.) Plant Physiol. 1991;96:1228–1236. doi: 10.1104/pp.96.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard M (1983) d-Fructose-2,6-bisphosphate, dihydroxyacetone phosphate and d-glyceraldehyde-3-phosphate. In HU Bergmeyer, ed, Methods of Enzymatic Analysis, Ed 3, Vol 6. Weinheim, Basel, Switzerland, pp 342–350
- Gordon AJ, Minchin FR, Skøt L, James CL. Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol. 1997;114:937–946. doi: 10.1104/pp.114.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin V, Trinchant J-C, Rigaud J. Nitrogen fixation (C2H2 reduction) by broad bean (Vicia faba L.) nodules and bacteroids under water-restricted conditions. Plant Physiol. 1990;92:595–601. doi: 10.1104/pp.92.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig U, Boller B, Nösberger J. Oxygen supply limits nitrogenase activity of clover nodules after defoliation. Ann Bot. 1987;59:285–291. [Google Scholar]
- Hartwig U, Boller BC, Baur-Höch B, Nösberger J. The influence of carbohydrate reserves on the response of nodulated white clover to defoliation. Ann Bot. 1990;65:97–105. [Google Scholar]
- Hartwig UA, Heim I, Lüscher A, Nösberger J. The nitrogen-sink is involved in the regulation of nitrogenase activity in white clover after defoliation. Physiol Plant. 1994;92:375–382. [Google Scholar]
- Heim I, Hartwig UA, Nösberger J. Current nitrogen fixation is involved in the regulation of nitrogenase activity in white clover (Trifolium repens L.) Plant Physiol. 1993;103:1009–1014. doi: 10.1104/pp.103.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R, Rapoport TA. A linear steady-state treatment of enzymatic chains. Critique of the crossover theorem and a general procedure to identify interaction sites with an effector. Eur J Biochem. 1974;42:97–105. doi: 10.1111/j.1432-1033.1974.tb03319.x. [DOI] [PubMed] [Google Scholar]
- Hunt S, King BJ, Canvin DT, Layzell DB. Steady and nonsteady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987;84:164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Layzell DB. Gas exchange of legume nodules and the regulation of nitrogenase activity. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:483–511. [Google Scholar]
- King BJ, Layzell DB. Effect of increases in oxygen concentration during the argon-induced decline in nitrogenase activity in root nodules of soybean. Plant Physiol. 1991;96:376–381. doi: 10.1104/pp.96.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht W, Heinz F (1983) d-Glycerate-2-phosphate and phosphoenolpyruvate. In HU Bergmeyer, ed, Methods of Enzymatic Analysis, Ed 3, Vol 6. Weinheim, Basel, Switzerland, pp 555–561
- Lee H-S, Copeland L. Phosphofructokinase from the host fraction of chickpea nodules. Physiol Plant. 1996;96:607–614. [Google Scholar]
- Marczewski W. Kinetic properties of phosphoenolpyruvate carboxylase from lupin nodules and roots. Physiol Plant. 1989;76:539–543. [PubMed] [Google Scholar]
- Millar AH, Day DA, Bergersen FJ. Microaerobic respiration and oxidative phosphorylation by soybean nodule mitochondria: implications for nitrogen fixation. Plant Cell Environ. 1995;18:715–726. [Google Scholar]
- Minchin FR. Regulation of oxygen diffusion in legume nodules. Soil Biol Biochem. 1997;29:881–888. [Google Scholar]
- Minchin FR, Ines Minguez M, Sheehy JE, Witty JF, Skøt L. Relationships between nitrate and oxygen supply in symbiotic nitrogen fixation by white clover. J Exp Bot. 1986;37:1103–1113. [Google Scholar]
- Minchin FR, Witty JF, Sheehy JE, Müller M. A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J Exp Bot. 1983;34:641–649. [Google Scholar]
- Möllering H (1983) l-(−)-Malate: determination with malate dehydrogenase and aspartate aminotransferase. In HU Bergmeyer, ed, Methods of Enzymatic Analysis, Ed 3, Vol 7. Weinheim, Basel, Switzerland, pp 39–47
- Pathirana SM, Vance CP, Miller SS, Gantt JS. Alfalfa root nodule phosphoenolpyruvate carboxylase: characterization of the cDNA and expression in effective and plant-controlled ineffective nodules. Plant Mol Biol. 1992;20:437–450. doi: 10.1007/BF00040603. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Stitt M. Effects of nitrogen and phosphorus deficiencies on levels of carbohydrates, respiratory enzymes and metabolites in seedlings of tobacco and their response to exogenous sucrose. Plant Cell Environ. 1993;16:1047–1057. [Google Scholar]
- Peterson JB, Evans HJ. Properties of pyruvate kinase from soybean nodule cytosol. Plant Physiol. 1978;61:909–914. doi: 10.1104/pp.61.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton WC. The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Podestá FE, Plaxton WC. Regulation of cytosolic carbon metabolism in germinating Ricinus communis cotyledons. II. Properties of phosphoenolpyruvate carboxylase and cytosolic pyruvate kinase associated with the regulation of glycolysis and nitrogen assimilation. Planta. 1994;194:381–387. [Google Scholar]
- Rawsthorne S, LaRue TA. Metabolism under microaerobic conditions of mitochondria from cowpea nodules. Plant Physiol. 1986;81:1097–1102. doi: 10.1104/pp.81.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller KA, Minchin FR, Gresshoff PM. Nitrogenase activity and oxygen diffusion in nodules of soybean cv. Bragg and a supernodulating mutant: effects of nitrate. J Exp Bot. 1988;39:865–877. [Google Scholar]
- Schuller KA, Turpin DH, Plaxton WC. Metabolite regulation of partially purified soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1990;94:1429–1435. doi: 10.1104/pp.94.3.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller KA, Werner D. Phosphorylation of soybean (Glycine max L.) nodule phosphoenolpyruvate carboxylase in vitro decreases sensitivity to inhibition by L-malate. Plant Physiol. 1993;101:1267–1273. doi: 10.1104/pp.101.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy JE, Minchin FR, Witty JF. Biological control of the resistance to oxygen flux in nodules. Ann Bot. 1983;52:565–571. [Google Scholar]
- Stitt M, Lilley RMcC, Gerhardt R, Heldt MW. Determination of metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 1989;174:518–552. [Google Scholar]
- Vance CP, Gregerson RG, Robinson DL, Miller SS, Gantt JS. Primary assimilation of nitrogen in alfalfa nodules: molecular features of the enzyme involved. Plant Sci. 1994;101:51–64. [Google Scholar]
- Vance CP, Heichel GH. Carbon in N2 fixation: limitation or exquisite adaptation. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:373–392. [Google Scholar]
- Vance CP, Stade S. Alfalfa root nodule carbon dioxide fixation. II. Partial purification and characterization of root nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1984;75:261–264. doi: 10.1104/pp.75.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella J, Copeland L. Phosphofructokinase from the host fraction of soybean nodules. J Plant Physiol. 1993;141:398–404. [Google Scholar]
- Vessey JK, Walsh KB, Layzell DB. Oxygen limitation of N2 fixation in stem-girdled and nitrate-treated soybean. Physiol Plant. 1988;73:113–121. [Google Scholar]
- Wadham C, Winter H, Schuller KA. Regulation of soybean nodule phosphoenolpyruvate carboxylase in vivo. Physiol Plant. 1996;97:531–535. [Google Scholar]
- Walsh KB, Vessey JK, Layzell DB. Carbohydrate supply and N2 fixation in soybean. The effect of varied daylength and stem girdling. Plant Physiol. 1987;85:137–144. doi: 10.1104/pp.85.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H, Stitt M, Heldt HW. Subcellular compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim Biophys Acta. 1987;893:13–21. [Google Scholar]
- Weisbach C, Hartwig UA, Heim I, Nösberger J. Whole-nodule carbon metabolites are not involved in the regulation of oxygen permeability and nitrogenase activity in white clover nodules. Plant Physiol. 1996;110:539–545. doi: 10.1104/pp.110.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JF, Minchin FR, Sheehy JE, Ines Minguez M. Acetylene-induced changes in the oxygen diffusion resistance and nitrogenase activity of legume root nodules. Ann Bot. 1984;53:13–20. [Google Scholar]
- Witty JF, Minchin FR, Skøt L, Sheehy JE. Nitrogen fixation and oxygen limitation in legume root nodules. Oxf Surv Plant Mol Cell Biol. 1986;3:275–314. [Google Scholar]
- Zhang X-Q, Chollet R. Phosphoenolpyruvate carboxylase protein kinase from soybean root nodules: partial purification, characterization, and up/down-regulation by photosynthate supply from the shoots. Arch Biochem Biophys. 1997;343:260–268. doi: 10.1006/abbi.1997.0190. [DOI] [PubMed] [Google Scholar]
- Zhang X-Q, Li B, Chollet R. In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1995;108:1561–1568. doi: 10.1104/pp.108.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]