Abstract
Epidemiologic studies on dementia generally have 2 major interacting objectives: descriptive, where rates of dementia and Alzheimer Disease (AD) are calculated for communities and selected populations, and analytic, which attempt to explain the observed phenotypic variations in communities and populations by identifying disease risk factors. The public health benefits derived from descriptive studies are exemplified by the recent published review of the global prevalence of dementia under the auspices of Alzheimer Disease International. This review emphasized the enormous and growing burden associated with dementia particularly for countries in the developing world and outlined strategies to influence policy making, planning, and healthcare allocation. One interesting feature of descriptive studies on dementia is that although the few epidemiologic studies conducted in Africa suggest that rates of dementia and AD are relatively low, rates of AD and dementia have been reported to be relatively high for African Americans. The Indianapolis-Ibadan Dementia Project has reported that the incidence rates for AD and dementia in Yoruba are less than half the incidence rates for AD and dementia in African Americans. Analytic studies are now underway to identify risk factors that may account for these rate differences. The risk factor model being applied, attempts to identify not only putative genetic and environmental factors but also their interactions. So far the major findings have included: apolipoprotein E e4, a major risk factor for AD in most populations, is also a risk factor for AD in African Americans but not for Yoruba; African Americans are at higher risk not only for AD, but also for diseases associated with increased cardiovascular risk such as hypertension, diabetes, and metabolic syndrome; African Americans have higher rates of hypercholesterolemia than Yoruba: there is an interaction between apolipoprotein E e4, cholesterol, and AD risk in both Yoruba and African Americans. We eventually hope to create a risk factor model that will not only account for the dementia rate differences between Yoruba and African Americans, but also help explain dementia rates in other developing and developed countries.
Keywords: Alzheimer disease, Africans, African Americans
This paper consists of 3 sections; a brief review of existing international studies on the epidemiology of dementia: a brief review of the findings from current African American epidemiologic studies; and finally a more in depth review of the results so far from the Indianapolis-Ibadan Dementia project.
The Role of Dr Katzman
No discussion about international epidemiologic studies on dementia, however, can take place without recognizing the major role played by Dr Katzman in the evolution of these studies.
In the late 1980s Dr Katzman began a very successful collaboration with colleagues from the Shanghai Institute of Mental Health to conduct a population-based longitudinal epidemiologic study of dementia in Shanghai China. This study demonstrated that dementia and AD were as common in Shanghai as in Western countries, were associated with increasing age and low education,1 possessed the apolipoprotein E (APOE)e4 allele,2 and constituted a major risk factor for death in the older Chinese.3 It also, perhaps as importantly, demonstrated that collaborations between researchers from developed and developing countries in studies of dementia were feasible.
In the early 1990s Dr Katzman, along with Dr Amaducci, led an ambitious project jointly sponsored by the World Health Organization and the National Institute on Aging to develop multisite comparative epidemiologic studies on dementia.4 Researchers from Italy, Spain, Malta, Chile, and Canada as well as from our Indianapolis Ibadan project were invited to attend. Although the entire collaborative project as originally conceived was not completed, individual studies including the Canadian Study of Health and Aging5 and our own study were successfully funded and conducted. The WHO/National Institute of Aging project did demonstrate that good interrater agreement on the clinical diagnosis of dementia could be reached between researchers from many different countries after an appropriate educational intervention.6
FINDINGS
International Studies of Dementia
Epidemiologic studies on dementia generally have 2 major components, descriptive, where rates of dementia and Alzheimer disease (AD) are calculated for communities and selected populations, and analytic, which attempt to explain the observed phenotypic variations in communities and populations by identifying disease risk factors. The public health benefits derived from descriptive studies are exemplified by the recent published review of the global prevalence of dementia under the auspices of Alzheimer Disease International.7 In this analysis a group of international experts were asked to provide prevalence estimates of dementia in various regions of the world based upon a systematic review of published studies, using a Delphi process. As might be anticipated, large scale well designed epidemiologic studies of dementia were much more common in Europe and North America than in other regions such as Latin America, Russia and Eastern Europe, the Middle East and Africa. Nevertheless the conclusions from this survey indicate the enormity of the public health problem facing the world, and particularly the developing nations, created by dementia. The reviewers concluded that approximately 24.3 million people currently have dementia in the world today with 4.6 million new cases of dementia every year (1 new case every 7 s). The number of these people will double every 20 years to 81.1 million by the year 2040. About 60% of those affected with dementia live in developing countries where it is anticipated that the future increase in cases will be even greater than in the developed world.
Based mainly upon the work of the Alzheimer Disease International and the contributions of that remarkable group of investigators constituting the 10/66 study group8 the survey also addressed issues relating to achieving progress in dementia care in the developing world and what may be the most cost effective approach to provide dementia services. The key public health problem to achieving success in creating programs for dementia care, identified by the reviewers, was poor awareness of the extent of the problem created by dementia by the community and political leaders. The national Alzheimer’s Association are attempting to raise awareness by creating a framework for positive engagement between policymakers, clinicians, researchers, and caregivers. It is likely that for many low-income countries the most cost effective approach to develop services will be through community primary care services and training of family caregivers in simple management techniques.
The estimates of dementia prevalence and incidence for the African region (primarily sub-Saharan Africa) were considerably lower than those for most of the other world geographic regions (prevalence 1.6% for individuals ≤60 y of age, incidence 3.5 per thousand), but it was recognized that these estimates were based upon very few studies (primarily from our Indianapolis Ibadan project which will be discussed in more detail later) that certainly do not constitute a representative sample of all African populations. However, the incidence estimates for dementia from the Indianapolis-Ibadan project are remarkably similar to those derived from studies in Indian rural communities9 suggesting that there may be some environmental characteristics of communities in the developing world that are associated with relatively low rates of dementia.
African American Studies
There are currently at least 4 major on-going large cohort studies of genetic and environmental risk factors for dementia involving African American populations. Two of these are population based and involve multiracial comparisons, the Chicago Study on Health and Aging and the Northern Manhattan Study. One is population based and involves a comparison between African Americans in Indianapolis and Nigerian Yoruba, the Indianapolis-Ibadan dementia project. One is a multicentered clinically based study, the Multi Institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) where the African American population is primarily from South Eastern United States.
The 3 population-based studies have now published incidence data for AD for their African American populations.10–12 A comparison of these incidence rates are shown in Table 1.
TABLE 1.
Age Specific Annual Incidence Rates for AD in 3 African American Communities
| Indianapolis | Chicago | North Manhattan | |
|---|---|---|---|
| 65–74 | 1.38 (0–2.99) | 1.79 (1.05–2.52) | 1.7 (0.7–2.6) |
| 75–84 | 3.29 (1.86–5.01) | 6.06 (4.21–7.91) | 4.4 (2.9–5.8) |
| 85+ | 7.07 (4.54–9.61) | 12.72 (9.48–15.9) | 11.4 (6.3–16.5) |
| Overall rate | 2.52 (1.40–3.64) | 2.95 (2.06–3.84) | Not given |
The incidence rates reported by the North Manhattan and Chicago studies are somewhat higher than those reported from Indianapolis but, except perhaps for the 85 years and over group, do not seem to be significantly different. Both studies involving multiracial comparisons have reported higher incidence rates for AD for African Americans as compared with whites and both groups are actively exploring explanations for this finding.
There is less consistency between these studies of the relationship between possession of the APOEe4 allele and the risk of AD (Table 2).
TABLE 2.
The Association of the APOEe4 Allele and Risk of AD in African American Populations
| Location of Study | Weak or No Association | Robust Association |
|---|---|---|
| North Manhattan | × | — |
| Chicago | × | — |
| Indianapolis | — | × |
| Mirage* | — | × |
| Yoruba | × | — |
Predominately in Southeastern United States.
The Chicago and North Manhattan-based studies report weak (ie, only in persons with the e4/e4 genotype) or no relationship12,13 similar to the reported findings from the Nigerian Yoruba.14 Whereas a robust relationship between the APOEe4 allele (ie, present in persons with 1 or 2 e4 alleles) is reported both from the MIRAGE study15 and African Americans living in Indianapolis.16 The reasons for this discrepancy for the APOEe4 effect on AD between populations of African origin are unclear as yet and will be considered later when the results from the Indianapolis-Ibadan study are discussed. It is unlikely that it is attributable to age effect as suggested by Graff-Radford et al15 because the ages of the North Manhattan, Chicago, and Indianapolis cohorts are similar.
The Indianapolis-Ibadan Dementia Project
Since 1992, research teams from the Indiana University School of Medicine and the University of Ibadan, Nigeria have been collaborating on a longitudinal comparative study of the prevalence and incidence of dementia and AD and their associated risk factors. The study has been supported by the National Institute on Aging and the Alzheimer Association. The study participants, all over the age of 65 years, include African Americans living in Indianapolis and Yoruba living in the Idikan wards of Ibadan Nigeria.
We have reported that the age standardized annual incidence rates were significantly lower among Yoruba than among African Americans for dementia (Yoruba 1.35%, African Americans 3.24%) and for AD (Yoruba 1.15%, African Americans 2.52%).11 It is interesting to note that dementia was associated with an increased risk for mortality for Yoruba of a magnitude similar to African Americans even after adjusting for demographics and chronic disease conditions (Yoruba relative ratio=2.83, African Americans relative ratio=2.05).17 This suggests, somewhat surprisingly given the differences in healthcare access and socioeconomic conditions, that the impact of dementia on mortality risk may be similar for developing and developed countries.
To explore the possible explanation for the rate differences between the Yoruba and the African Americans we have been employing a risk factor model first proposed by Cooper and Kaufman18 which takes into consideration not just putative environmental and genetic risk factors but also how these factors may interact with each other.
Genetic Risk Factors
The major genetic finding of our study so far has been discussed previously. Possession of the e4 allele of APOE is not associated with increased risk for AD in Yoruba14 but is significantly associated with AD risk for African Americans (e3/e4: odds ratio 2.32; 95% confidence interval 1.41–3.82 and e4/e4 odds ratio 7.19; confidence interval 3.00–17.29, compared with the e3e3 genotype).16 Each of these analyses now includes large number of participants with AD (Yoruba 123, African Americans 162) increasing our confidence in the validity of these findings. A differential effect on the e4 allele and mortality has also been reported between the sites. Having APOEe4 alleles significantly increased risk for mortality in African Americans under age 75 years (hazard ratio; 2.00 P=0.0089) but not for African Americans over the age of 75 years or for Yoruba of any age.19 Explanations for the differential effect of e4 and AD risk between African Americans and Yoruba include consideration of possible differences in gene × gene interactions or gene × environmental interactions which may differ between the populations.
Gene × Gene Interactions
It has been proposed that genetic studies of complex diseases including AD in African populations confer certain advantages.20
Genetic diversity is greater in African populations, which makes these populations particularly useful for fine mapping of complex genetic diseases. This diversity was observed by the identification of the fourth APOE haplotype in a Yoruba family with no evidence of AD.21 In addition, many of the environmental risk factors that are associated with AD such as high cholesterol and heart disease are not as frequent in Africans, allowing for better discrimination between genetic and environmental factors.22 Thus it is possible, for example, that gene × gene and gene × environment interactions may be different in Yoruba than in African Americans who may have various degrees of admixture with other populations such as whites and Native Americans. Our genetics group is actively exploring this possibility.
Other Putative Genes
It is unlikely that APOEe4 accounts for all of the genetic risk for AD in African Americans and certainly not in Yoruba.11 We are currently genotyping several single nucleotide polymorphisms located in APOE and other candidate genes to identify other potential genetic risk factors in the 2 populations.
Environmental Risk Factors
There is now considerable evidence that vascular risk factors also increase the risk for AD.23,24 One of the most striking features of our comparative study is that not only is AD less common in Yoruba as compared with African Americans, but so are vascular diseases such as diabetes and hypertension and vascular risk factors such as hypercholesterolemia.25 These differences may in part be dietary intake. The Yoruba, in the Idikan wards, consume a low-calorie, low-fat diet, consisting mainly of grains, roots, and tubers supplemented by small amounts of fish. So far we have not been able to demonstrate a link between cardiovascular disease and AD risk in either population, but we have reported that antihypertensive use in African Americans reduced the odds of incident cognitive impairment by 38%.26 During the 2001 evaluation wave, we added measurements of biomarkers including lipids, inflammatory markers and markers associated with endothelial dysfunction and oxidative stress. We hope that analyses of these markers in our longitudinal study will allow us to explore in more detail the relationship between vascular risk factors and AD.
Genetic/Environmental Interactions
In 2000, we reported finding a significant interaction between serum cholesterol APOE genotype and AD risk for African Americans.27 Increasing cholesterol levels were associated with increased AD risk for the group with no e4 alleles but not for the group with 1 or 2 e4 alleles.
The availability of lipid measurements allowed us to conduct the same analysis with the Yoruba. In this study low-density lipoprotein (LDLs), high-density lipoprotein, and triglyceride levels were included in the analysis as well as cholesterol levels.28
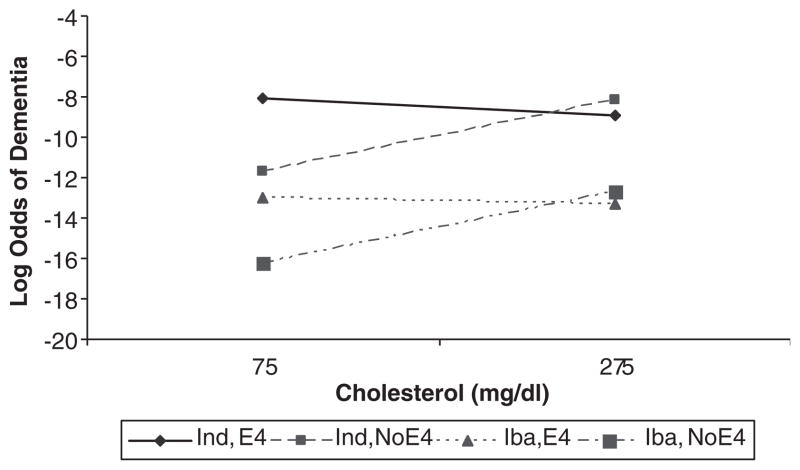
Similar findings were seen in Yoruba as that previously reported for African Americans. Significant interactions were found between increasing cholesterol level, level of LDLs, APOE genotype, and risk for AD. Increasing levels of cholesterol and LDLs were associated with increased risk for AD for individuals without APOEe4 but not for those with APOEe4. There was no association between levels of high-density lipoprotein, APOE genotype, and AD risk (Fig. 1).
FIGURE 1.
APOE genotype and cholesterol interaction effects on AD in Indianapolis and Ibadan.
Figure 1 illustrates the interactions for cholesterol for both sites. It is noteworthy that cholesterol, a potentially modifiable risk factor is associated with increased risk of AD even in a population with relatively low levels of cholesterol. The findings also suggest that any analyses of the association between lipids and AD risk needs to take into consideration APOE status. Our continued analyses of lipid, APOE genotype interactions, and AD risk in African Americans have been confounded by the dramatic increase in statin use in this population over the past 5 years (from about 2% to over 20%). We are now conducting analyses incorporating statin use into our models.
CONCLUSIONS
We believe a risk factor model for AD risk that incorporates not only evaluation of environmental and genetic risk factors, but also how they interact will lead to a better understanding of population phenotypic differences. We also hope that including populations from developing and developed countries will assist in the development of this model.
Acknowledgments
Supported by a grant from the National Institute on Aging R01 AG09956 and the Alzheimer’s Association.
References
- 1.Zhang MY, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 2.Katzman R, Zhang MY, Chen PJ, et al. Effects of apolipoprotein E on dementia and aging in the Shanghai Survey of Dementia. Neurology. 1997;49:779–785. doi: 10.1212/wnl.49.3.779. [DOI] [PubMed] [Google Scholar]
- 3.Katzman R, Hill LR, Yu ES, et al. The malignancy of dementia. Predictors of mortality in clinically diagnosed dementia in a population survey of Shanghai, China. Arch Neurol. 1994;51:1220–1225. doi: 10.1001/archneur.1994.00540240064017. [DOI] [PubMed] [Google Scholar]
- 4.Amaducci L, Baldereschi M, Amato MP, et al. The World Health Organization cross-national research program on age-associated dementias. Aging (Milano) 1991;3:89–96. doi: 10.1007/BF03323983. [DOI] [PubMed] [Google Scholar]
- 5.The Canadian Study of Health and Aging Working Group. The incidence of dementia in Canada. Neurology. 2000;55:66–73. [PubMed] [Google Scholar]
- 6.Baldereschi M, Amato MP, Nencini P, et al. Cross-national interrater agreement on the clinical diagnostic criteria for dementia. WHO-PRA Age-Associated Dementia Working Group, WHO-Program for Research on Aging, Health of Elderly Program. Neurology. 1994;44:239–242. doi: 10.1212/wnl.44.2.239. [DOI] [PubMed] [Google Scholar]
- 7.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi Consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince M. Dementia in developing countries. A consensus statement from the 10/66 Dementia Research Group. Int J Geriatr Psychiatry. 2000;15:14–20. doi: 10.1002/(sici)1099-1166(200001)15:1<14::aid-gps70>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Chandra V, Pandav R, Dodge HH, et al. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 10.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Hendrie HC, Ogunniyi A, Hall KS, et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 12.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 14.Gureje O, Ogunniyi A, Baiyewu O, et al. APOE epsilon4 is not associated with Alzheimer’s disease in elderly Nigerians. Ann Neurol. 2006;59:182–185. doi: 10.1002/ana.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graff-Radford NR, Green RC, Go RC, et al. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch Neurol. 2002;59:594–600. doi: 10.1001/archneur.59.4.594. [DOI] [PubMed] [Google Scholar]
- 16.Murrell JR, Price B, Lane KA, et al. Association of apolipoprotein E genotype and Alzheimer disease in African Americans. Arch Neurol. 2006;63:431–434. doi: 10.1001/archneur.63.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkins AJ, Hui SL, Ogunniyi A, et al. Risk of mortality for dementia in a developing country: the Yoruba in Nigeria. Int J Geriatr Psychiatry. 2002;17:566–573. doi: 10.1002/gps.643. [DOI] [PubMed] [Google Scholar]
- 18.Cooper RS, Kaufman JS. Race and hypertension: science and nescience. Hypertension. 1998;32:813–816. doi: 10.1161/01.hyp.32.5.813. [DOI] [PubMed] [Google Scholar]
- 19.Lane KA, Gao S, Hui SL, et al. Apolipoprotein E and mortality in African-Americans and Yoruba. J Alzheimers Dis. 2003;5:383–390. doi: 10.3233/jad-2003-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tishkoff SA, Williams SM. Genetic analysis of African populations: human evolution and complex disease. Nat Rev Genet. 2002;3:611–621. doi: 10.1038/nrg865. [DOI] [PubMed] [Google Scholar]
- 21.Murrell JR, Price BM, Baiyewu O, et al. The fourth apolipoprotein E haplotype found in the Yoruba of Ibadan. Am J Med Genet: Neuropsychiatr Genet. 2006;14:426–427. doi: 10.1002/ajmg.b.30295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tishkoff SA, Dietzsch E, Speed W, et al. Global patterns of linkage disequilibrium at the CD4 locus and modern human origins. Science. 1996;271:1380–1387. doi: 10.1126/science.271.5254.1380. [DOI] [PubMed] [Google Scholar]
- 23.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Reitz C, Honig LS, et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrie HC. Exploration of environmental and genetic risk factors for Alzheimer’s disease: the value of cross-cultural studies. Curr Directions Psychol Sci. 2001;10:98–101. [Google Scholar]
- 26.Murray MD, Lane KA, Gao S, et al. Preservation of cognitive function with antihypertensive medications: a longitudinal analysis of a community-based sample of African Americans. Arch Intern Med. 2002;162:2090–2096. doi: 10.1001/archinte.162.18.2090. [DOI] [PubMed] [Google Scholar]
- 27.Evans RM, Emsley CL, Gao S, et al. Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: a population-based study of African Americans. Neurology. 2000;54:240–242. doi: 10.1212/wnl.54.1.240. [DOI] [PubMed] [Google Scholar]
- 28.Hall K, Murrell J, Ogunniyi A, et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66:223–227. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]