Abstract
The literature on the association between apolipoprotein E (ApoE) and mortality across ethnic and age groups has been inconsistent. No studies have looked at this association in developing countries. We used data from the Indianapolis-Ibadan Dementia study to examine this association between APOE and mortality in 354 African-Americans from Indianapolis and 968 Yoruba from Ibadan, Nigeria. Participants were followed up to 9.5 years for Indianapolis and 8.7 years for Ibadan. Subjects from both sites were divided into 2 groups based upon age at baseline. A Cox proportional hazards regression model adjusting for age at baseline, education, hypertension, smoking history and gender in addition to time-dependent covariates of cancer, diabetes, heart disease, stroke, and dementia was fit for each cohort and age group. Having ApoE ε4 alleles significantly increased mortality risk in Indianapolis subjects under age 75 ( hazard ratio: 2.00; 95% CI: 1.19–3.35; p = 0.0089). No association was found in Indianapolis subjects 75 and older (hazard ratio: 0.71; 95% CI: 0.45–1.10; p = 0.1238), Ibadan subjects under 75 (hazard ratio: 1.04; 95% CI: 0.78 to 1.40; p = 0.7782), or Ibadan subjects over 75 (hazard ratio: 1.21; 95% CI: 0.83 to 1.75; p = 0.3274).
Keywords: Apolipoprotein E, survival analysis, African-Americans
1. Introduction
The effect of apolipoprotein E (ApoE) on mortality has been reported to be age-related. Schacter et al. and Louhija et al. have found smaller frequencies of the ApoE ε4 allele in French and Finnish centenarians compared to younger cohorts, suggesting an increased mortality with ApoE ε4 [11,16]. Corder et al. found in subjects 75–85 years old with and without cognitive impairment and cognitively impaired subjects 85 years or older that ApoE did not significantly predict survival, while among 85 year or older subjects with good cognition, mortality increased with a ε3/ε4 genotype and decreased with an ε2/ε3 genotype relative to the ε3/ε3 genotype [1]. Tilvis et al. found that the presence of the ApoE ε4 allele was associated with 5-year mortality in 75 and 80 year old Finish subjects but not with 85 year old subjects [18].
The association between ApoE and mortality has been inconsistent across ethnic groups in the literature. Lee et al. found different associations between mortality risk and ApoE between Caucasians, Hispanics and African Americans aged 65 and older living in northern Manhattan. He reported that the ε2/ε3 genotype was associated with the lowest mortality risk in the Caucasians and Hispanics, while the ε3/ε4 genotype was associated with the lowest mortality risk in African-Americans, even after stratifying by age group [10]. With Caucasians, no association was found between mortality risk and ApoE ε4 in citizens from Vantaa age 85 and older or Finnish subjects age 69–78 [8,9]. However, Rea et al. found that longevity is negatively associated with the ApoE ε4 allele in Belfast [14].
Few studies have looked at the association between mortality risk and ApoE in either African-Americans or people in developing countries. Using information from the Indianapolis-Ibadan Dementia Project, we investigate the association of ApoE with mortality in African Americans and Yoruba 65 years and older after adjusting for other known risk factors of age, education, gender, smoking history, hypertension, cancer, diabetes, heart disease, stroke, and dementia.
2. Materials and methods
2.1. Subjects
The study populations come from the Indianapolis-Ibadan Dementia study, a longitudinal community-based cohort study on the prevalence and incidence of dementia on elderly African-Americans living in Indianapolis and Yoruba living in Ibadan, Nigeria. The study began in 1992 and details of the study design have already been published [5,6]. In brief, both sites had a baseline wave and 2 follow-up waves. During each wave that lasted approximately 1.5–2 years, a two-stage (screening and clinical assessment) sampling was employed. The subjects were first screened and then a subset, based upon their score from the screening interview, was selected for clinical assessment and diagnosis. Baseline began in 1992, follow-up 1 began in 1994 and follow-up 2 began in 1997. As of early 2002, the screening stage of follow-up 3 for Indianapolis has been completed and will be considered as follow-up 3. For Ibadan, this stage is ongoing but has not been completed. However, in late 1999–2000, all subjects were re-contacted about future participation where only contact information and whether or not the subject was still alive were collected. This will be considered as Follow-up 3 for Ibadan. At baseline, there were 2212 subjects in Indianapolis and 2487 in Ibadan screened.
This project was approved by the Indiana University-Purdue University Indianapolis institutional review board and by the University of Ibadan institutional review board.
Throughout the study, information on patient characteristics and medical comorbidities has been collected. Cancer, diabetes, heart disease, and stroke status were collected at baseline, follow-up 2 and follow-up 3 from both the subject and informant (if available). If either mentioned the disease presence at either the screening or clinical assessment stage, then the disease was considered present and irreversible. Another comorbidity of interest was dementia. Subjects who were clinically assessed were diagnosed by consensus conference of the study physicians at baseline, follow-up 1 and follow-up 2. Both DSM-III-R and ICD-10 criteria were met to diagnose dementia.
Information on mortality has been collected throughout the entire study period. In Indianapolis, deaths were recorded using obituaries and the Indiana State Vital Statistics. In Ibadan, the interviewers continuously monitored the study population through daily visits and recorded all deaths.
A portion of the subjects from both sites gave blood samples in which their ApoE status could be identified. In Indianapolis only clinically assessed subjects gave blood samples, while Ibadan genotyped subjects who were clinically assessed at follow-up 1 or either screened or clinically assessed at follow-up 2. Therefore, not all Ibadan genotyped subjects had a clinical diagnosis.
2.2. Genotyping
DNA was eluted from dried blood spots collected on filter paper or isolated from whole blood samples. ApoE genotypes were determined by Hhal digestion of the amplified product [7].
2.3. Statistical analysis
Descriptive statistics were calculated for all variables. For continuous variables, these included means and standard deviations. For categorical variables, descriptive statistics included proportions for the binary data and the number in each category for the categorical variables. Subjects were divided into 2 groups based upon their age at baseline and all analyses were performed within each age group within each site. The two groups were those under age 75 and those who were 75 years and older, as 74 was the median age for the Indianapolis genotyped subjects.
Subjects were followed from the time of their screening at the baseline visit until their endpoint. The endpoint is reached when the subject dies or when the subject completes his final visit. Subjects who didn’t die are considered censored. Survival curves were constructed using the Kaplan-Meier product limit method and median survival time was calculated for each cohort. Log rank tests were used to compare the survival times of those with and without ApoE ε4 alleles within each age group. The proportions of deaths at each site were compared based upon having ApoE ε4 alleles using chi-squared tests. Cox proportional hazards regression models were used to model the survival time since baseline for each cohort as a function of ApoE status after adjusting for age at entry, education, smoking history and gender. Cox proportional hazards regression models with time dependent variables were also used to model the survival time since baseline for each cohort as a function of ApoE status. The effect of ApoE was considered as a dose response for the number of ApoE ε4 alleles. The models were adjusted for age at entry, education, hypertension, smoking history and gender as well as the time dependent covariates of cancer, diabetes, heart disease, stroke, and dementia collected at each visit. Other than at Baseline, each time dependent covariate is treated as beginning at the midpoint between the visit where it is observed and the prior visit. As these conditions are considered irreversible, once the subject has the disease, they continue to have it throughout all future visits until their endpoint is reached. Subjects without a clinical assessment but with a screening visit at that wave are considered not demented for that visit unless previously diagnosed with dementia. In Ibadan, very few subjects had cancer so this time dependent covariate was not used in the models.
Within each cohort and age group, subjects with ApoE genotyping were compared to subjects who were eligible to donate blood but did not using t-tests for continuous variables and chi-squared tests for categorical variables. The variables tested were age, gender, education, hypertension, smoking history, and rate of death.
All analyses used SAS for Windows version 8.2 [15]. Graphs were created using Splus version 6.0 [17].
3. Results
There were 396 African Americans in Indianapolis and 985 Yoruba living in Ibadan, Nigeria that had been ApoE genotyped. Of these, we excluded 41 subjects in Indianapolis and 17 in Ibadan who had dementia at Baseline, and 2 subjects in Indianapolis who were missing covariates used in the models. Thus, 353 Indianapolis and 968 Ibadan subjects were used in these analyses. In Indianapolis, there were 179 in the under age 75 group and 174 in the 75 and older group. In Ibadan, there were 697 in the younger group and 271 in the older age group. Overall, the Indianapolis subjects were followed for an average of 6.1 ± 2.4 years with a range from 0.5 to 9.5 years. The Ibadan subjects were followed on average for 6.7 ± 1.2 years with a range from 1.8 to 8.7 years. The average age in Indianapolis of all subjects was 75.5 ± 7.0 with a median value of 74.0 years. The average age in Ibadan of all subjects was 72.1 ± 7.2 with a median of 70.0 years. Overall, all but one Indianapolis and 440 Ibadan subjects had been clinically diagnosed at any visit. In Ibadan, 329 (47.2%) of the under 75 and 199 (73.4%) of the over 75 groups had been clinically diagnosed.
Subject characteristics for both sites by age group are shown in Table 1. The ApoE allele distribution was nearly identical between the two sites and age groups. The majority of subjects had no ApoE ε4 alleles. Over 60% of the African-Americans reported having hypertension at baseline compared to less than 25% in Ibadan. Similarly, over 60% of the African-Americans had a history of smoking while only 23.9% of the Yoruba did.
Table 1.
Baseline Subject Characteristics
| Indianapolis
|
Ibadan
|
|||
|---|---|---|---|---|
| Under 75 (n = 179) | 75 and Older (n = 174) | Under 75 (n = 697) | 75 and Older (n = 271) | |
| Age | 69.9 ± 2.7 | 81.3 ± 5.1 | 68.4 ± 2.7 | 81.7 ± 6.3 |
| Education* | 9.7 ± 2.9 | 8.8 ± 3.5 | 109 (15.6%) | 54 (19.9%) |
| Male (%) | 72 (40.2%) | 55 (31.6%) | 224 (32.1%) | 118 (43.5%) |
| Smoking History | 121 (67.6%) | 98 (56.3%) | 145 (20.8%) | 86 (31.7%) |
| Hypertension | 124 (69.3%) | 110 (63.2%) | 174 (25.0%) | 57 (21.0%) |
| ApoE – have 2 ε4 alleles | 8 (4.5%) | 8 (4.6%) | 30 (4.3%) | 15 (5.5%) |
| ApoE – have 1 ε4 allele | 65 (36.3%) | 58 (33.3%) | 239 (34.3%) | 96 (35.4%) |
| ApoE – have 0 ε4 alleles | 106 (59.2%) | 108 (62.1%) | 428 (61.4%) | 160 (59.0%) |
| ApoE – have any ε4 alleles | 73 (40.8%) | 66 (37.9%) | 269 (38.6%) | 111 (41.0%) |
Mean years of education (SD) for Indianapolis; Number of subjects (%) with any schooling for Ibadan.
Table 2 shows the total number of subjects seen, the number who died before each follow-up wave, and the number of subjects with various medical conditions at each wave for Indianapolis and Ibadan. The characteristics include the time dependent covariates and death. In total, Indianapolis had 113 (32.0%) subjects die and Ibadan had 205 (21.2%) subjects die. Throughout the study, heart disease was present in 136 (38.5%) Indianapolis subjects and 313 (32.3%) Ibadan subjects.
Table 2.
Number of subjects with various medical conditions at each wave for Indianapolis and Ibadan
| Indianapolis
|
Ibadan
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up 1a | Follow-up 2 | Follow-up 3b | Baseline | Follow-up 1a | Follow-up 2 | Follow-up 3c | |
| Age < 75 | ||||||||
| Total seen | 179 | 169 | 137 | 80 | 697 | 694 | 645 | 548 |
| Died | NA | 5 | 15 | 24 | NA | 0 | 40 | 87 |
| Cancer | 21 | 20 | 17 | 12 | 6 | 6 | 6 | |
| Diabetes | 51 | 50 | 54 | 27 | 24 | 23 | 28 | |
| Heart disease | 55 | 53 | 60 | 34 | 221 | 220 | 225 | |
| Stroke | 30 | 28 | 28 | 18 | 12 | 11 | 19 | |
| Dementia | NA | 10 | 17 | NA | NA | 4 | 23 | |
| Age 75+ | ||||||||
| Total seen | 174 | 166 | 111 | 45 | 271 | 267 | 233 | 187 |
| Died | NA | 4 | 36 | 29 | NA | 3 | 25 | 50 |
| Cancer | 26 | 25 | 19 | 12 | 4 | 4 | 4 | |
| Diabetes | 36 | 32 | 27 | 13 | 7 | 7 | 13 | |
| Heart disease | 50 | 47 | 41 | 18 | 56 | 55 | 56 | |
| Stroke | 18 | 18 | 20 | 7 | 7 | 7 | 5 | |
| Dementia | NA | 20 | 27 | NA | NA | 12 | 20 | |
Only Dementia and Death were ascertained during this follow-up wave.
Follow-up 3 for Indianapolis was the screening phase.
Follow-up 3 for Ibadan was a contact phase and only death was ascertained during this interval.
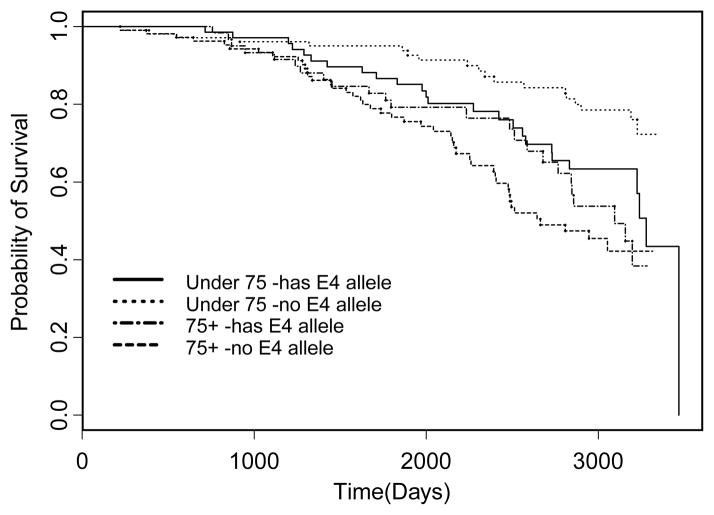
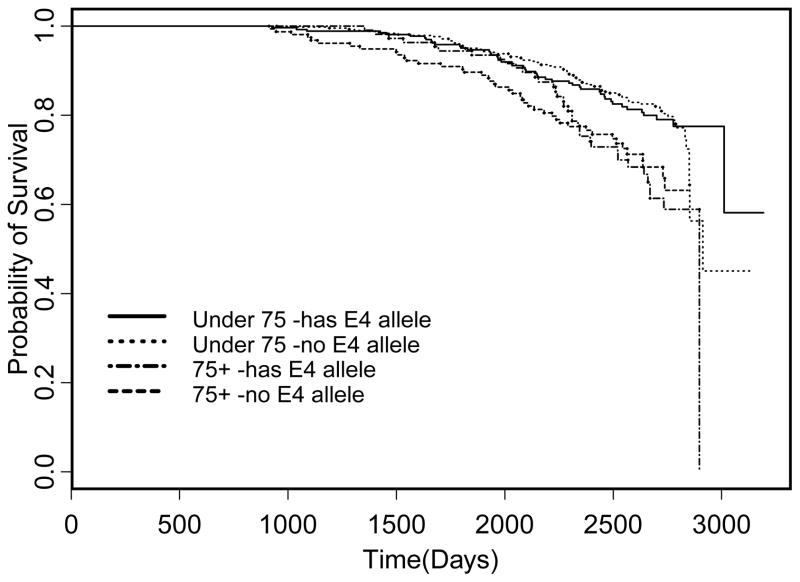
Figures 1 and 2 show the survival curves for Indianapolis and Ibadan, respectively. The log rank test found a significant difference in the under 75 years old group, but not in the 75 and over group (p = 0.0191, p = 0.4239, respectively). In the under 75 group, having an ε4 allele was detrimental to survival. In the older group, having an ε4 allele appears protective, although not significantly. The log rank test did not find any significant differences in either age group between having an ε4 allele or not (p = 0.8546 for under 75, p = 0.8597 for 75 and older).
Fig. 1.
Kaplan Meier Plot for Indianapolis based on whether the subject has any ApoE ε4 alleles stratified by age group.
Fig. 2.
Kaplan Meier Plot for Ibadan based on whether the subject has any ApoE ε4 alleles stratified by age group.
The ApoE ε4 allele was not significantly related to the proportion of deaths in the older cohort from Indianapolis or both age groups from Ibadan. In the 75 and older group from Indianapolis, 24 (36.4%) with an ApoE ε4 allele died compared to 45 (41.7%) without the allele (p = 0.4878). In Ibadan, the ApoE ε4 allele was not significantly related to the proportion of deaths in either age group. In the under 75 year olds, 48 (17.8%) subjects with the allele died compared to 79 (18.5%) without the allele who died (p = 0.8380). Similarly in those 75 years and over, 35 (31.5%) with the allele died compared to 43 (26.9%) without the allele (p = 0.4051). However, in the under age 75 group from Indianapolis, ApoE was significantly related to the proportion of deaths. In this group, 25 (34.3%) of subjects with an ApoE ε4 allele died compared to 19 (17.9%) without a ε4 allele (p = 0.0127). Cox proportional hazards models on survival time as a function of ApoE adjusting for baseline age, gender, education, and smoking history found results similar to those for the proportion of deaths in both age groups in both sites. The number of ApoE ε4 alleles was significantly associated with mortality risk in the under 75 year olds from Indianapolis (HR: 2.22; 95% CI: 1.34–3.66; p-value = 0.0019). No associations were found in either the older group from Indianapolis (HR: 0.76; 95% CI: 0.50–1.17; p-value = 0.2173), the under 75 year olds from Ibadan (HR: 1.00; 95% CI: 0.75–1.35; p-value = 0.9875), or the 75 years and older group from Ibadan (HR: 1.04; 95% CI: 0.78–1.40; p-value = 0.7782).
In order to investigate whether any effect of ApoE on mortality risk was acting through the various diseases, all models were run after adding medical history covariates to the models.
Table 3 shows the results from the Cox proportional hazards models with time dependent variables on each age group for Indianapolis and Ibadan. In the younger group from Indianapolis, the number of ApoE ε4 alleles significantly increased mortality risk with a hazard ratio of 2.00 and a 95% confidence interval of 1.19 to 3.35 (p = 0.0089). However, in the 75 and older group from Indianapolis, or in both age groups from the Ibadan cohort, the number of ε4 alleles was not significantly associated with mortality risk. Dementia was a significant (p < 0.05) covariate in both age groups from Ibadan and the older cohort from Indianapolis. Age was a significant covariate in the younger age group from Ibadan and the older age group from Indianapolis and marginally associated (p = 0.0514) with mortality in the older Nigerians. Gender was also significant for both Indianapolis groups. Education was only marginally significant in the 75 and older group from Indianapolis (p = 0.0971), while hypertension was significant in the under 75 groups from both sites. The medical covariates of stroke significantly (p = 0.0022) and heart disease marginally (p = 0.0950) increased mortality risk in the under 75-year group from Ibadan. Smoking history, cancer, and diabetes were not significant in any of models. These results with the medical covariates in the models were consistent with the earlier results without the medical covariates in all four groups. Furthermore, in Ibadan, 52.8% of the younger cohort and 26.6% of the older cohort had never been clinically assessed. We also ran the models on only those with a clinical assessment and found the results consistent with the original findings.
Table 3.
Results from Cox Proportional Hazards Model with Time Dependent Covariates on Indianapolis and Ibadan Subjects
| Variable | Indianapolis
|
Ibadan
|
||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | p-value | Hazard Ratio (95% Confidence Interval) | p-value | |
| Age < 75 | ||||
| Number of ApoE ε4 alleles | 2.00 (1.19–3.35) | 0.0089 | 1.04 (0.78–1.40) | 0.7782 |
| Age at baseline | 1.08 (0.96–1.22) | 0.2124 | 1.16 (1.09–1.24) | < 0.0001 |
| Smoking history | 0.98 (0.45–2.13) | 0.9499 | 1.23 (0.76–2.01) | 0.3987 |
| Education* | 0.97 (0.87–1.07) | 0.4898 | 1.04 (0.63–1.73) | 0.8842 |
| Hypertension | 2.49 (1.02–6.04) | 0.0442 | 0.60 (0.37–0.95) | 0.0312 |
| Male | 2.42 (1.15–5.07) | 0.0198 | 1.17 (0.75–1.82) | 0.5023 |
| Cancer | 1.69 (0.73–3.91) | 0.2227 | NA | NA |
| Diabetes | 1.67 (0.88–3.18) | 0.1180 | 1.27 (0.57–2.82) | 0.5571 |
| Heart disease | 0.83 (0.44–1.58) | 0.5754 | 1.38 (0.95–2.01) | 0.0950 |
| Stroke | 1.59 (0.79–3.19) | 0.1983 | 3.06 (1.49–6.25) | 0.0022 |
| Dementia | 1.88 (0.74–4.79) | 0.1884 | 4.28 (2.39–7.65) | < 0.0001 |
| Age 75+ | ||||
| Number of ApoE ε4 alleles | 0.71 (0.45–1.10) | 0.1238 | 1.21 (0.83–1.75) | 0.3274 |
| Age at baseline | 1.06 (1.01–1.12) | 0.0180 | 1.03 (1.00–1.07) | 0.0514 |
| Smoking history | 1.05 (0.63–1.77) | 0.8528 | 1.15 (0.70–1.89) | 0.5721 |
| Education * | 0.94 (0.88–1.01) | 0.0971 | 0.91 (0.49–1.67) | 0.7590 |
| Hypertension | 1.25 (0.73–2.15) | 0.4183 | 0.96 (0.51–1.81) | 0.9062 |
| Male | 2.33 (1.40–3.90) | 0.0012 | 1.48 (0.89–2.48) | 0.1320 |
| Cancer | 0.73 (0.38–1.41) | 0.3500 | NA | NA |
| Diabetes | 1.15 (0.67–1.96) | 0.6199 | 1.26 (0.44–3.60) | 0.6668 |
| Heart Disease | 1.41 (0.83–2.37) | 0.2010 | 1.18 (0.68–2.04) | 0.5510 |
| Stroke | 1.56 (0.82–2.96) | 0.1737 | 1.69 (0.63–4.54) | 0.3018 |
| Dementia | 2.34 (1.26–4.34) | 0.0069 | 3.56 (1.88–6.74) | < 0.0001 |
Mean years of education (SD) for Indianapolis; Number of subjects (%) with any schooling for Ibadan.
In both age groups from Indianapolis, the subjects who had ApoE genotyping were not significantly different (results not shown) in age, gender, smoking history, hypertension or rate of death than those who were clinically assessed but did not provide blood samples except for 0.7 year more of education in the young Indianapolis cohort with ApoE (p = 0.0436). In both age groups from Ibadan, subjects who had ApoE genotyping were not significantly different on any of these characteristics except rate of death than those who were available to provide blood samples but did not. In the older cohort from Ibadan, only 31.8% of those who gave blood died compared to 52.8% who did not (p = 0.0001).
4. Discussion
In our study, the possession of an ε4 allele of APOE significantly increased mortality risk in African Americans under the age of 75 years at study baseline but not for African Americans over 75 years of age at baseline. There was no association between Apoe ε4 and mortality risk in either age group for the Yoruba. All of these results were the same whether or not adjustments were made for medical covariates. In the Kaplan Meier plots in Figs 1 and 2, the associations are shown when unadjusted for covariates. It is noteworthy that in the Indianapolis plot, African Americans under the age of 75 years with ε4 alleles had a similar curve to those of African Americans over the age of 75 also with ε4 alleles. This suggests that the effect of ApoE is age related.
Previous studies on the relationship between age, ethnicity, and ApoE ε4-associated mortality risk have produced inconsistent results. These inconsistencies may be explained in part by the difference in prevalence of other diseases associated with increased mortality risk between the cohorts or the cognitive status of the study subjects. For example, Corder et al. found an association between ε4 and mortality risk in 85-year-old subjects with good cognition but not in younger subjects or 85-year-old subjects with poor cognition [1]. Corder defined poor cognition as those with a Mini-Mental State Examination score of 0 to 23 [1,3]. In our study 15.1% of the younger cohort and 27.0% of the older cohort developed dementia during the course of the project. Thus, our results cannot really be compared to those of Corder because of the difference in age groups and definition of poor cognition. In a multi ethnic study, Lee et al. reported that in African Americans the ε3/ε4 genotype was associated with the lowest mortality risk whereas the subjects with ε3/ε3 genotypes had the highest risk. These findings were unchanged after stratifying by age group [10]. In our study of the older African-Americans, subjects with an ε4 allele did have lower mortality risks than those without but this difference was not significant. However the subjects in our study reported higher rates of diabetes, hypertension and cancer than did those in the Lee study. For example, in our study 35.8% of the younger cohort and 24.7% of the older cohort reported having diabetes at some point during the study. In addition, 41.9% of the young cohort and 35.1% of the older cohort had heart disease. In Lee’s study on African-Americans, 16.3% had diabetes and 24.6% of the subjects had heart disease [10]. Thus, it might be difficult to compare our results with those of Lee because of the differences in health between the groups.
The reason for our finding of increased risk for mortality in the under 75 years of age African-Americans possessing the ε4 allele is unclear. It does not appear to be related to the allele’s effect on hypertension, stroke, heart disease, cancer and diabetes because we adjusted for these variables in our models and the results were consistent when removing these variables from the models as well. It may reflect the age effect of ApoE ε4 on Alzheimer’s disease that ApoE ε4 is a more powerful risk factor for AD in younger age groups [2]. However, we also adjusted for dementia in our models. It is not possible to rule out the suggestion by Corder et al. that there is an interaction between age, cognitive status, ApoE, and mortality risk [1].
This is the first study to report on the association of ApoE with mortality in Sub-Sahara Africa. The lack of association between ApoE and mortality is not surprising. In a previous study, Perkins et al. found that dementia was significantly associated with increased mortality at both Indianapolis and Ibadan [13]. However, there was no association between the possession of the ApoE ε4 allele and dementia in the Nigerians [12]. Furthermore, the Nigerians had fewer known mortality risk factors such as heart disease, stroke, hypertension and diabetes, than the African-Americans [4]. Less than 25% of the Yoruba had hypertension at baseline and only 3.9% of the younger Yoruba and 11.8% of the older Yoruba were found demented during the study.
The choice of cut-off on age groups does not seem to affect our results. As in other studies, age 75 was chosen as the cut-off. The median age of the cohort in Indianapolis was 74 years. In the Yoruba, the median age was 70 years but 75 years was chosen to be consistent with the African-Americans. We also ran the models with 70 as the cut-off and the results were similar.
There are several limitations to this study. There was a difference in the length of study time between the study sites. Indianapolis subjects were followed from entry into study in 1992 through early 2002. Ibadan subjects were followed from entry into study in 1992 through late 2000. In total, Ibadan subjects had 2 complete follow-ups and a contact session compared to Indianapolis’ 3 complete follow-ups. Thus, all analyses were performed within each population.
Another limitation is that not all of the subjects in the original study had ApoE genotyping. Indianapolis only had ApoE genotyping on clinically assessed subjects, while Ibadan genotyped subjects who had a screening interview regardless of whether they had a clinical assessment. However, within age groups we compared both the Indianapolis and Ibadan subjects who had ApoE genotyping with their counterparts who had the same opportunities to donate blood and only found a few minor differences in patient characteristics or rates of death. In the young cohort from Indianapolis, those who provided a blood sample went to school for 0.7 year more than those who did not. Since education is included in the models, this difference is unlikely to affect our conclusion. In the older cohort but not the younger one from Ibadan, only 31.8% of those who gave blood died compared to 52.8% who did not. Those who did not give blood samples were likely more sick and fragile than those who were genotyped. A final limitation on the genotyping was that not all of the subjects in Ibadan had been clinically assessed. However, the results from the models on only those with a clinical assessment were consistent with the original findings. Nevertheless, because of the differences in the length of follow-up and the collection of ApoE genotypes, at this time we should be cautious about making direct comparisons between the two sites. In our current ongoing study, we are attempting to collect blood samples from all subjects and then should be able to reexamine the association between ApoE and mortality in more subjects with longer follow-up times.
In summary, we find that the ApoE ε4 allele is a mortality risk factor in African-Americans age 65–75 years but not in older African-Americans. Our study is the first to report the lack of association of the ApoE ε4 allele with mortality risk in Sub Saharan Africa.
Acknowledgments
This research was supported by NIH grants R01 AG 09956, R01 AG 15813, and P30 AG 10133.
References
- 1.Corder EH, Lannfelt L, Viitanen M, Corder LS, Manton KG, Winblad B, Basun H. Apolipoprotein E genotype determines survival in the oldest old (85 years or older) who have good cognition. Archives of Neurology. 1996;53:418–422. doi: 10.1001/archneur.1996.00550050048022. [DOI] [PubMed] [Google Scholar]
- 2.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 3.Folstein MF, Folstein SE, McHugh PR. Mini Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Hendrie HC. Exploration of environmental and genetic risk factors for Alzheimer’s Disease: the value of cross-cultural studies. Current Directions in Psychological Science. 2001;10:98–101. [Google Scholar]
- 5.Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, Gao S, Evans RM, Ogunseyinde AO, Adeyinka AO, Musick BS, Hui SL. Incidence of dementia and alzheimer disease in two communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, USA. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 6.Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, Gureje O, Baiyewu O, Rodenberg CS, Musick BS, Farlow MR, Class CA, Brashear A, Burdine VE, Olawole S, Raji SO, Komolafe O. The prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. The American Journal of Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 7.Hixon JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hhal. Journal of Lipid Research. 1990;31:545–548. [PubMed] [Google Scholar]
- 8.Juva K, Verkkoniemi A, Viramo P, Polvikoski T, Kainulainen K, Kontula K, Sulkava R. APOE epsilon4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology. 2000;54:412–415. doi: 10.1212/wnl.54.2.412. [DOI] [PubMed] [Google Scholar]
- 9.Koivisto AM, Lempiainen P, Koivisto K, Helkala EL, Mykkanen L, Kuusisto J, Kervinen K, Kesaniemi YA, Laakso M, Soininen H. Apolipoprotein E phenotype alone does not influence survival in Alzheimer’s disease: a population-based longitudinal study. Neuroepidemiology. 2000;19:327–332. doi: 10.1159/000026272. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Tang MX, Schupf N, Stern Y, Jacobs DM, Tycko B, Mayeux R. Mortality and apolipoprotein E in Hispanic, African-American, and Caucasian Elders. American Journal of Medical Genetics. 2001;103:121–127. doi: 10.1002/ajmg.1528. [DOI] [PubMed] [Google Scholar]
- 11.Louhija J, Miettinen HE, Lontula K, Tikkanen MJ, Miettinen TA, Tilvis RS. Aging and genetic variation of plasma apolipoproteins: relative loss of the apolipoprotein E4 phenotype in centenarians. Arteriosclerosis Thrombosis. 1994;14:1084–1089. doi: 10.1161/01.atv.14.7.1084. [DOI] [PubMed] [Google Scholar]
- 12.Osuntokun BO, Sahota A, Ogunniyi AO, Gureje O, Baiyewu O, Adeyinka A, Oluwole SO, Komolafe O, Hall KS, Unverzagt FW, Hui SL, Yang M, Hendrie HC. Lack of an association between the E4 allele of ApoE and Alzheimer’s disease in elderly Nigerians. Annals of Neurology. 1995;38:463–465. doi: 10.1002/ana.410380319. [DOI] [PubMed] [Google Scholar]
- 13.Perkins AJ, Hui SL, Ogunniyi A, Gureje O, Baiyewu O, Unverzagt FW, Gao S, Hall KS, Musick BS, Hendrie HC. Risk of mortality for dementia in a developing country: the Yoruba in Nigeria. International Journal of Geriatric Psychiatry. 2002;17:566–573. doi: 10.1002/gps.643. [DOI] [PubMed] [Google Scholar]
- 14.Rea IM, McDowell I, McMaster D, Smye M, Stout R, Evans A MONICA group (Monitoring of Cardiovascular trends study group) Apolipoprotein E alleles in nonagenarian subjects in the Belfast Elderly Longitudinal Free-living Ageing Study (BELFAST) Mechanisms of Ageing and Development. 2001;122:1367–1372. doi: 10.1016/s0047-6374(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 15.SAS. SAS Institute. Cary, NC: [Google Scholar]
- 16.Schachter F, Faure-Delanef L, Guenot F, Rouger H, Froguel P, Lesueur-Ginot L, Cohen D. Genetic associations with human longevity at the APOE and ACE loci. Nature Genetics. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 17.Splus Version 6 for Windows. Insightful Corporation; Seattle, WA: [Google Scholar]
- 18.Tilvis RS, Strandberg TE, Juva K. Apolipoprotein E phenotypes, dementia and mortality in a prospective population sample. Journal of the American Geriatrics Society. 1998;46:712–715. doi: 10.1111/j.1532-5415.1998.tb03805.x. [DOI] [PubMed] [Google Scholar]