Abstract
Objective
To examine associations between autonomic nervous system and adrenocortical reactivity to laboratory stressors and buccal cell telomere length (BTL) in children.
Methods
The study sample comprised 78 five- and six-year-old children from a longitudinal cohort study of kindergarten social hierarchies, biological responses to adversity, and child health. Buccal cell samples and reactivity measures were collected in the spring of the kindergarten year. BTL was measured by realtime PCR, as the telomere-to-single copy gene (T/S) ratio. Parents provided demographic information; parents and teachers reported children’s internalizing and externalizing behavior problems. Components of children’s autonomic (heart rate (HR), respiratory sinus arrhythmia (RSA), pre-ejection period (PEP)) and adrenocortical (salivary cortisol) responses were monitored during standardized laboratory challenges. We examined relations between reactivity, internalizing and externalizing behavior, and BTL, adjusted for age, race, and gender.
Results
Heart rate and cortisol reactivity were inversely related to BTL, PEP was positively related to BTL, and RSA was unrelated. Internalizing behaviors were also inversely related to BTL (standardized β=−0.33, p=0.004). Split at the median of reactivity parameters, children with high sympathetic activation (decreasing PEP) and high parasympathetic withdrawal (decreasing RSA) did not differ with regard to BTL. However, children with both this profile and high cortisol reactivity (N=12) had significantly shorter BTL (0.80 vs. 1.00, χ2=7.6, p=0.006), compared with other children.
Conclusions
Autonomic and adrenocortical reactivity in combination were associated with shorter buccal cell telomere length in children. These data suggest that psychophysiological processes may influence, and that BTL may be a useful marker of, early biological aging.
Keywords: autonomic reactivity, adrenocortical reactivity, buccal cell telomeres, internalizing, stress, children
Introduction
Telomeres are the protective ends of chromosomal DNA; short length leads to cell senescence. Blood cell telomere length has frequently been used to measure biological aging and may underlie cardiovascular aging. In adulthood, chronic stress has been associated with shorter telomere length (1), and shorter telomeres have been associated with heart disease (2, 3) and more rapid cardiovascular disease mortality (4, 5). Telomere length reflects genetic, cellular, and environmental factors and is thought to decline throughout life (6); longer telomeres may confer a survival advantage (7, 8).
As a generalized measure of biological aging, telomeres could be helpful in establishing the early impact of risk factors for health outcomes, including heart disease. In fact, differences in telomere length among individuals may materialize early in life. Previous researchers have demonstrated that telomere length shortens rapidly during early childhood, with significant shortening by age 4 years, concurrent with children’s rapid growth (9, 10). There are, however, obstacles to measuring telomeres in children. Collection of blood samples, which is generally acceptable in adults, is relatively invasive for children. However, while leukocyte telomere length is an increasingly well-established measure of cellular aging, telomeres can be measured using DNA from any cell type, and buccal epithelial (oral mucosa) cells may provide an alternative source for assessment of telomere length. There is evidence that the rate of attrition of telomeric base pairs differs by organ tissue (11), and that the rate of attrition of buccal cell telomere length (BTL) may differ from that of leukocyte telomere length (12). However, BTL has been used to predict health outcomes including oral, lung and bladder cancers (13, 14) and Alzheimer’s Disease (15). Telomere length in other types of epithelial cells has also predicted age-related disease (16).
To establish the potential usefulness of BTL as a marker of biological aging in children, we wanted to evaluate its possible associations with a biologically well-characterized, trait-like variable (17) with potential ramifications for aging over the life course. We employed profiles of autonomic nervous system (ANS) and hypothalamic-pituitary-adrenocortical (HPA) axis responses to stress to accomplish this goal.
Autonomic nervous system (ANS) and hypothalamic-pituitary-adrenocortical (HPA) axis responses to stress and adversity take place in a coordinated and temporally sequenced manner that maximizes the organism’s capacity for survival and recovery. Complementary ANS and HPA responses produce a shift to a state of biological and behavioral preparedness, increasing heart rate and blood pressure, mobilizing cellular nutrients, preferentially redirecting energy resources and perfusion to the brain, and inducing behavioral states of vigilance and fear (18-22). Autonomic reactivity, occurring over seconds of time employs the sympathetic nervous system (SNS), and the parasympathetic nervous system (PNS), which counters SNS activation by down-regulating activity within the same set of target end organs. The HPA axis, involving the sequential secretion of corticotropin releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), activates the adrenal cortex, triggering secretion of cortisol, the principal human glucocorticoid regulating blood pressure, glucose metabolism, and immune competence. The HPA response, occurring over minutes of time, also serves to temper and control sympathetic activation.
The coordinated, counterbalancing roles of the SNS, PNS and HPA axis in the integrated stress response can be indexed using a variety of accessible and peripheral physiologic measures. SNS activation can be assessed using the cardiac cycle pre-ejection period (PEP), a measure reflecting the timed length of the period of isovolumetric contraction, while PNS withdrawal can be measured as a diminution in the magnitude of respiratory sinus arrhythmia (RSA), the portion of heart rate variability driven by the respiratory cycle. HPA activation can be ascertained using salivary cortisol, which reliably reflects the concentration of free cortisol in plasma. Further, the concurrent, coordinated or dysregulated activation of these systems can be described in reactivity profiles reflecting joint patterns of SNS, PNS and HPA responses to challenge (23-25). A maximally vigorous response profile might invoke the combination of high ANS reactivity (decreasing PEP by SNS activation and decreasing RSA by PNS withdrawal) and high HPA activation (increasing salivary cortisol).
Therefore, in this study, we examined associations between such profiles of stress reactivity and BTL. We hypothesized that children who are most “reactive” to stress, i.e. those characterized by high sympathetic activation, parasympathetic withdrawal, and/or high cortisol response, and especially those characterized by all three, may exhibit more accelerated aging of mitotic cells in the first few years of life and that this will be reflected in shorter buccal cell telomere length. Additionally, because highly “stress reactive” children tend to have more internalizing symptoms (26-30), we hypothesized that children with higher internalizing behaviors would have shorter BTL. We examined associations of externalizing behaviors with BTL to evaluate whether relationships were specific to internalizing vs. any behavioral problems.
MATERIALS AND METHODS
Study Population
The study sample included 78 kindergarten children in the San Francisco Bay area who were recruited from a longitudinal study of social dominance status, biological responses to adversity, and mental and physical health (Peers and Wellness Study (PAWS)). These children derived from a single cohort of 141 children entering kindergarten in 2005, the third of three cohorts of kindergarten children participating in the study (n=338). Other details of data collection have previously been reported (31, 32).
The final study sample (n = 78) consisted of children who had parental written permission to participate, who provided buccal cell samples, who had complete information on ethnicity, age, gender, and reactivity measures, and who were not taking human growth hormone or exogenous glucocorticoids, medications known to alter salivary cortisol levels (33). Because our hypothesis about temperament and BTL was subsidiary to the primary hypothesis, we did not require complete data on internalizing or externalizing, and these analyses included 73 children.
The sample was ethnically diverse (13% African-American, 15% Asian, 6% Hispanic, 45% White, 21% multiracial or other) but limited to children who spoke English and whose parents spoke English or Spanish. School districts were chosen to ensure a wide socioeconomic range of students. However, despite variation in income, parents in the PAWS study were more educated than the general population; more than 50% of mothers were college graduates, compared with <30% of the general population (34).
Data Collection
Data for the study were collected in the fall (time 1) and spring (time 2) during the kindergarten year. Data on age and body mass index (kg/m2) were collected in the fall, with information on height and weight collected by nurse practitioners. Data on reactivity, internalizing symptoms, externalizing symptoms, and BTL were collected in the spring. Parents’ informed consent and children’s assent were obtained prior to the start of data collection, and all study procedures were approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley and the Committee on Human Research at the University of California, San Francisco.
Telomere length
We measured telomere length in buccal (cheek) cells that were collected specifically for this study. Buccal cells were collected by a nurse practitioner at the end of the children’s kindergarten year by brushing the oral mucosa, using 10 strokes with a sterile nylon bristle cytology brush. A separate brush was used for each cheek in order to maximize the amount of DNA obtained. Samples were processed and DNA purified using the Puregene DNA isolation kid (Gentra Systems, now QIAGEN) for analyzing DNA with buccal cells (35).
Telomere length values were measured from DNA by a quantitative PCR assay that determines the ratio of telomere repeat copy number to single-copy gene copy number (T/S) ratio in experimental samples as compared with a reference DNA sample. Higher T/S ratio signifies longer telomeres (36). We repeated the assays for five samples and found a coefficient of variation of 9.1%. BTL assays were conducted blinded to other participant information.
Internalizing and externalizing symptoms
Parents and teachers completed the internalizing and externalizing scales from the MacArthur Health and Behavior Questionnaire (27, 37). The internalizing behavior problems scale assesses children’s symptoms of depression and anxiety. The externalizing behavior problems scale reflects children’s aggressive or impulsive behaviors. Respondents were asked to report on the child’s behavior within the past six months, based on a three-point Likert scale ranging from “never or not true” to “often or very true.” Higher scores on both forms indicate higher levels of symptoms. The Berkeley Puppet Interview (BPI) is a semi-structured interview measure of young children’s perceptions of their family environment, school context, relationships with teachers, social skills and behaviors, and internalizing and externalizing symptomatology (38, 39). The scores from the three reporters were combined using principal components analysis (PCA) (40), yielding a ‘core’ score that reflected concurrent impressions of internalizing and externalizing symptomatology by teacher, parent and child.
Reactivity protocol
A 20-minute reactivity protocol was completed by children in a separate, quiet room at their elementary school. The child completed four sets of paired tasks (one control condition and one challenge condition each). The challenge tasks were designed to elicit autonomic responses to challenges across social (41), cognitive (42), sensory (43), and emotional (44) domains, geared for 4-6 year old children (45, 46). The protocol included four tasks: 1) a structured child interview taken from the Gesell School Readiness Screening Test (41) and administered by an unknown examiner, 2) a digit span recitation task from the Kaufman Assessment Battery for Children (42), 3) a drop of lemon juice placed on the child’s tongue, and 4) an emotion-evoking videotape, chosen to elicit fear (47). Roughly half the entire cohort showed a decrease in PEP or RSA for each task. Since the challenges involved some degree of potentially confounding psychomotor activity, each challenge task was preceded by a non-challenging “control task” that paralleled the psychomotor demands of that challenge task (32). Before and after the set of four challenges, children were read a two-minute calming story to procure resting physiologic measures. Other details have previously been reported (31, 32).
Autonomic nervous system reactivity
Children’s autonomic nervous system reactivity was assessed using changes in RSA, PEP, and heart rate reactivity (HR) in response to the series of challenges. Four spot electrodes (two current, two impedance) were placed in the standard tetrapolar configuration on the child’s neck and chest, and electrocardiograph (ECG) electrodes were placed on the right clavicle and lower left rib. ANS measures (HR, RSA, and PEP) were monitored continuously during the protocol. Data were acquired using the Biopac MP150 (Biopac Systems, Santa Barbara, CA) interfaced to a PC-based computer. RSA refers to fluctuations in heart rate related to the respiratory cycle and gated by efferent fibers of the vagus nerve. RSA is an index of the PNS’s capacity to regulate responses to positive and negative environmental demands (48, 49). RSA was estimated as the natural logarithm of the variance of the heart period within the frequency bandpass associated with respiration at this age (i.e., 0.15 to 0.80 Hz) (50, 51). PEP time intervals were calculated based on the time in milliseconds from the ECG Q-wave (corresponding to the onset of ventricular depolarization) to the B-point of the dZ/dt waveform (corresponding to the onset of left ventricular ejection (52)). Heart rate is an integrative, multiply-determined physiologic parameter under the influence of autonomic and other biologic factors. HR was ascertained from interbeat interval data acquired using an electrocardiograph (ECG) digitized at 500 Hz, analyzed with the detection algorithm of Berntson and colleagues (1990) (53). Other details have previously been reported (31, 32, 45, 46, 54).
ANS reactivity responses during each of the control tasks were used as baseline reference values. Mean RSA, PEP, and HR magnitudes for each of four tasks were calculated for one-minute intervals and averaged within each task (54). The four difference scores were then averaged within each measure of ANS reactivity. Reactivity scores were computed as ANS activity during the challenge task minus that during the paired control task. Negative RSA and PEP difference scores reflect autonomic reactivity via PNS withdrawal and SNS activation, respectively. HR difference scores measure heart rate acceleration and provide an integrated measure of sympathetic and parasympathetic reactivity. Henceforth, the abbreviations RSA, PEP, and HR signify reactivity or change.
Adrenocortical (HPA-axis) reactivity
At the beginning (baseline) and end of the reactivity protocol, saliva was collected by instructing the child to chew on a cotton roll for 20 to 30 seconds. Wet cotton rolls were deposited in salivette tubes and stored at −7° C until assayed. Salivary cortisol samples were assayed using a commercial immunoassay with chemiluminescence detection (Cortisol Luminescence Immunoassay; IBL-Hamburg, Hamburg, Germany). Other details have previously been reported (55). Given that cortisol levels in saliva reach their peak approximately 15 to 20 minutes following stressor onset, cortisol values collected at the beginning of the session were considered baseline reference values. The average session lasted 27 minutes; cortisol values collected at the end of the session were considered a measure of reactivity to a novel, mildly stressful context. Slightly more than a third of the cohort showed an increase in cortisol We created standardized difference scores by subtracting pre-protocol, baseline values from post-protocol cortisol values. Positive cortisol reactivity values indicated a stress response.
Data Preparation
Reactivity scores were standardized to a mean of 0 and standard deviation of 1. We subsequently created autonomic reactivity profiles (56, 57) from the cross-classification of positive (>0) and negative (<=0) difference scores for PEP and RSA. Children in the four cross-tabular cells were classified as showing coactivation, coinhibition, reciprocal parasympathetic activation, or reciprocal sympathetic activation (24, 58). Theoretically, the “most reactive” children, with regard to ANS response, should be those characterized by reciprocal sympathetic activation (sympathetic activation accompanied by parasympathetic withdrawal).
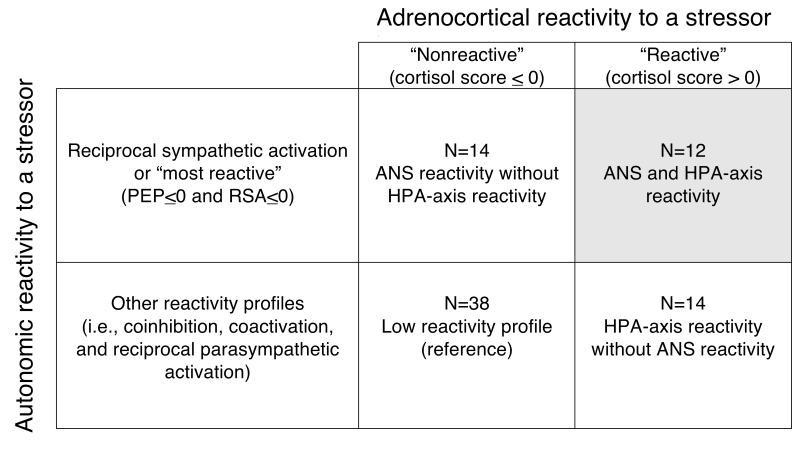
However, because a maximally reactive response to stress should provoke both ANS and adrenocortical responses, we additionally computed a reactivity profile based on the cross-classification of reciprocal sympathetic activation (PEP≤0 and RSA≤0, yes or no) and high vs. low (standardized cortisol reactivity score >0 vs. ≤0) cortisol response (Figure 1).
Figure 1.
Cross-classification of autonomic nervous system (ANS) reactivity and adrenocortical reactivity in the Peers and Wellness Study, n = 78. PEP = preejection period; RSA = respiratory sinus arrhythmia; HPA = hypothalamic-pituitary-adrenal.
Statistical analyses
We used t-tests to compare the children in the telomeres sub-sample with the entire cohort. We evaluated the distribution of BTL with univariate analyses (SAS PROC UNIVARIATE). We computed the Pearson correlation between age and BTL.
Using analysis of covariance, we regressed potential confounding variables against categories of reactivity adjusted for continuous age (Table 1).
Table 1.
Selected characteristics by category of reactivity among participants in the PAWS study, n=78.
| Autonomic nervous system and adrenocortical reactivity profile | ||||
|---|---|---|---|---|
| Low reactivity profile |
ANS reactivity without HPA- axis reactivity |
HPA-axis reactivity without ANS reactivity |
ANS and HPA- axis reactivity or “Most reactive” |
|
| N | 38 | 14 | 14 | 12 |
| Gender (% female) * | 60.5 | 58.3 | 48.8 | 66.8 |
| Ethnicity (%) | ||||
| White | 44.9 | 37.9 | 40.9 | 57.6 |
| African-American | 15.8 | 7.8 | 13.6 | 8.4 |
| Asian | 18.3 | 18.1 | 10.3 | 8.9 |
| Hispanic/Latino | 5.3 | 7.2 | 7.0 | 0.0 |
| Multi-ethnic/other | 15.8 | 28.9 | 28.2 | 25.0 |
| Height (cm) | 115.9 | 115.7 | 114.6 | 118.2 |
| Body mass index (kg/m2) | 16.7 | 15.5 | 16.4 | 15.5 |
| Internalizing score‡† | 0.01 a | −0.64 b | −0.04 | −0.00 |
| Externalizing score‡ | −0.23 | −0.58 | −0.13 | −0.13 |
| Mothers>college graduates (%)† | 71.2 a | 81.6 | 82.8 | 99.4 b |
| Child reactivity | ||||
| HR score † ‡ | −0.3 a | 0.6 b | 0.4 b | 1.1 b |
| PEP score † ‡ | 0.3 a | −0.8 b | 0.0 a | −1.4 b |
| RSA score † ‡ | 0.2 a | −0.3 b | 0.3 a | −0.3 b |
| Cortisol difference score ‡† | −2.5 a | −1.8 a | 1.8 b | 1.6 b |
All variables age-adjusted
Different letters indicate significant (p<0.05) differences between groups.
Standardized scores
Analyses of subcomponents of ANS and HPA-axis stress reactivity
We evaluated possible outliers. The cortisol reactivity measure was left-skewed due to two influential outliers for linear cortisol reactivity and BTL. We evaluated linear associations with and without these points. Through histograms and univariate analyses, we verified that other telomere and reactivity measures were approximately normally distributed.
We regressed standardized BTL scores separately against continuous HR, PEP, RSA, and cortisol difference scores, adjusted for age (continuous), race, and gender. To overcome issues with influential outliers for cortisol, we additionally regressed BTL against a dichotomous cortisol variable, defined as higher vs. lower than median cortisol difference scores.
Analyses of high stress reactive profiles
Using generalized linear models (SAS PROC GENMOD), we regressed standardized BTL values against linear reactivity variables and then categories of autonomic reactivity profiles based on information on RSA and PEP, with children characterized by reciprocal parasympathetic activation or as the “least reactive” (i.e., low sympathetic response and high, unchanging parasympathetic tone) as the reference. Because of the non-ordinal nature of categorical reactivity variables, we used contrast statements to compare children who were defined to be the “most reactive” to other groups of children and evaluated significance with Wald chi-square (χ2) tests.
We additionally evaluated the combination of ANS and adrenocortical reactivity by regressing BTL against the cross-classification of high and low cortisol reactivity and high and low ANS reactivity (defined here as presence of reciprocal sympathetic activation) (Figure 1). The reference group was children with low cortisol responses who were not characterized by reciprocal sympathetic activation.
Analyses of internalizing and externalizing symptoms
We regressed standardized BTL score against continuous internalizing and externalizing symptoms scores, adjusted for age (continuous), race, and gender.
Approach to covariate adjustment
We sought to ensure parsimonious models and to avoid inappropriate adjustment. All models were adjusted for demographic characteristics including age, race (White (reference), African-American, Hispanic, Asian, other), and gender. Additional adjustment for maternal education had little notable influence on associations, so reported findings did not include it.
We considered models adjusted for body mass index (BMI). However, adjustment for BMI would be inappropriate if genes for reactivity and body mass are autosomally linked or alternatively, if they are pleiotropic traits. Kagan and colleagues reported that reactive children tended to have characteristics including blue eyes, light hair, and thinner faces and bodies (17). In our data, reactivity was inversely associated with BMI, consistent with Kagan’s work, suggesting that adjustment may inappropriately constrain variability in reactivity variables of interest. Though we report BMI-adjusted findings, primary models were not adjusted for BMI.
All analyses were conducted using SAS 9.2.
RESULTS
Children in the telomere sub-study (N=78 of 141) were similar to the rest of the same-grade cohort from the PAWS study with regard to age, gender, and continuous reactivity scores including PEP, RSA, and cortisol difference scores. However, those in the sub-study were more likely to be white (46% vs. 26%, p < 0.001), to have mothers who were college graduates (55% vs. 32%, p=0.008), and to have higher heart rate reactivity scores (score=2.6 vs. 1.7, p=0.03), compared with those from the full cohort.
We collected sufficient DNA to obtain telomere length for each child (10ng-1μg). Buccal cell telomere length (BTL) as measured by T/S ratio, ranged from 0.33 to 1.59 with a mean=0.97, median=0.96, and standard deviation=0.26. The distribution of BTL appeared to be approximately normal. Within the sample, age was weakly inversely associated with BTL (r=−0.20, p=0.08).
Analyses of subcomponents of ANS and HPA stress reactivity
Using Spearman correlations, PEP reactivity was positively correlated with RSA (r=0.29, p=0.01), BMI (r=0.33, p=0.004), and BTL (r=0.23, p=0.04) and inversely correlated with HR (r=−0.40, p=0.0003). RSA reactivity was also inversely related to HR reactivity (r=−0.48, p<0.0001). HR reactivity was positively related to cortisol (r=0.27, p=0.02) and inversely related to BMI (r=−0.29, p=0.01). Besides HR reactivity, cortisol was not significantly related to other variables.
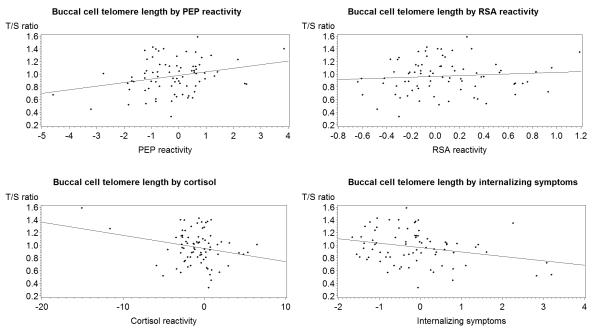
In linear regression models, adjusted for age, gender, and race, HR reactivity was inversely related (standardized β=−0.22, p=0.03), PEP change was positively related (standardized β=0.24, p=0.02), and RSA change was not associated (standardized β=0.11, p=0.29) with BTL (Figure 2). An inverse association of the linear cortisol reactivity score with BTL became nonsignificant when two outliers were removed. However, while children with cortisol reactivity to the stressor had significantly shorter BTL than those who were nonreactive (0.90 vs. 1.01, χ2=4.2, p=0.04), exclusion of the same two children with two outlier values had no material influence on the results from analysis of the dichotomous variable.
Figure 2.
Standardized reactivity difference scores* and buccal cell telomere length (BTL) in the Peers and Wellness Study, n = 78. * Heart rate reactivity was inversely related to BTL. Externalizing symptoms were not related to BTL. PEP = preejection period; T/S = telomere repeat copy number to single-copy gene copy number; ANS = autonomic nervous system; RSA = respiratory sinus arrhythmia.
Adjustment for BMI diminished linear associations of HR and PEP with BTL which became nonsignificant but did not diminish associations between dichotomous cortisol and BTL.
Analyses of stress reactivity profiles
Descriptive differences between reactivity profile groups
Of the four groups, the mothers of the “least reactive” children, those not characterized by either reciprocal sympathetic activation or with HPA-axis reactivity, were less likely to have a college degree than the mothers of those children characterized as “most reactive”. Children with ANS reactivity but without HPA-axis reactivity had lower internalizing scores than children characterized as having a low reactivity profile. Other than expected differences in measures of reactivity, there were no other significant differences between groups. Differences were significant (p<0.05) (table 1).
BTL differences between reactivity profile groups
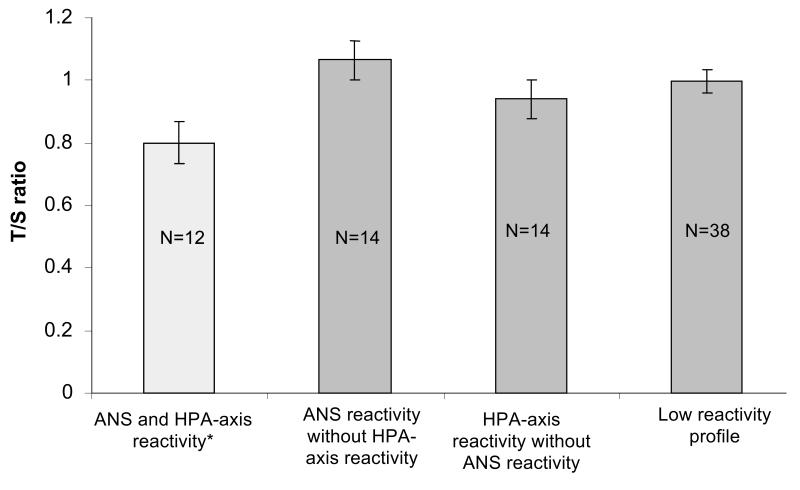
Reactivity categories based on the combination of PEP and RSA were not significantly associated with BTL (Likelihood χ2=2.5, df=3, p=0.48). However, children who showed simultaneous activation of systems—who were characterized by reciprocal sympathetic activation and demonstrated cortisol reactivity to the stressor—had shorter BTL than other groups (T/S ratio=0.80 vs. 1.00, χ2=7.5, p=0.006) (Figure 3) (Likelihood χ2=9.2, df=3, p=0.03 for group difference). Adjustment for maternal education and BMI did not substantially alter these associations.
Figure 3.
Reactivity profiles and buccal cell telomere length. * Children with reciprocal sympathetic activation and high cortisol response differed from other reactivity profiles (T/S ratio, 0.80 versus 1.00; χ2 = 7.5, p = .0006). T/S = telomere repeat copy number to single-copy gene copy number; ANS = autonomic nervous system; HPA = hypothalamic-pituitary-adrenal.
Analyses of internalizing and externalizing symptoms
Internalizing symptoms were inversely associated (standardized β=−0.33, p=0.004), but externalizing symptoms were not associated with BTL (standardized β=−0.11, p=0.38). Adjustment for BMI did not substantially attenuate associations between internalizing symptoms and BTL. We considered a final model adjusted both for the cross-classified measure of ANS and adrenocortical reactivity and internalizing symptoms. Simultaneous adjustment did not substantively influence either the association of reactivity or internalizing with BTL; each was independently associated with the outcome.
DISCUSSION
Consistent with our hypotheses, autonomic and adrenocortical reactivity in response to a series of standardized laboratory challenges was inversely associated with buccal cell telomere length (BTL) in this sample of 78 kindergarten children. In particular, children with the combination of higher sympathetic reactivity, greater parasympathetic withdrawal, and higher cortisol response to an acute stressor had the shortest BTLs. As predicted, internalizing but not externalizing symptoms were also inversely related to BTL. These findings suggest the utility of buccal cells to assess telomere length in children and demonstrate that factors early in childhood may already be influencing the aging process. These findings are novel and need replication.
The findings are also consistent with a growing literature linking stress arousal in adults to shortened telomeres. Leukocyte telomere length has been linked to greater levels of urinary stress hormones (cortisol and catecholamine) (59, 60). Parasympathetic withdrawal to an acute stressor has been associated with low levels of telomerase, an enzyme shown to help maintain telomere length (59). There are few biomarkers of health in children since the appearance of chronic disease generally occurs many decades later. Although researchers have suggested that differences in cardiovascular trajectories materialize early in the lifespan, with the exception of one study of cardiovascular risk factors in 6-7 year olds (61), most studies of youth have examined outcomes in later childhood and adolescence. These, moreover, have primarily evaluated risk factors for disease including body mass index and blood pressure, which have uncertain trajectories given the long potential horizon to the development of chronic disease. Thus, due to methodological and technological limitations, it has been difficult to demonstrate the impact of risk factors on biological aging early in life. If BTL measures early biological aging or disease risk, it has great potential utility in research on human development and aging. To our knowledge, this is the first study to examine associations of childhood stress reactivity and telomere length.
The mechanism through which reactivity to stress might contribute to telomere attrition in children is unknown. Early in life, there is rapid shortening of leukocyte telomeres, with greater declines than in any other period of life (10). The fact that adverse events early in life are predictive of health outcomes throughout life (62) suggests that this could be a critical point in the lifecourse during which childhood exposure to adverse levels of stress could augment early biological aging. However, no studies have examined whether stress or psychosocial risk factors might accelerate this early decline. In adults, chronic stress and greater perceptions of stress appear to contribute to telomere shortening (1). The mechanisms of stress-induced shortening are unknown, but may involve increased exposure to stress hormones, inflammation, and oxidative stress (59, 63, 64). Mechanisms linking stress reactivity and telomere length (65) in children are likely to be similar to those in adults, though particular cardiovascular risk factors unique to early childhood such as low birth weight, “catch-up growth”, prenatal exposures or epigenetic influences may promote telomere attrition through unique pathways not yet delineated (6).
Strengths of this study included a normative sample of five- and six-year-old children, reactivity measures with controls for psychomotor activity, and blinded examinations of telomere length in children, using noninvasive means. Also, our method employing cheek swabs rather than a mouthwash rinse, which may produce a high proportion of leukocytes (66), could have generated a larger proportion of buccal epithelial cells.
Study limitations included a relatively small sample size and a measure of telomere length from only one cell type (buccal cells), at only one time point. Future research will ideally enable exploration of reactivity with longitudinal change in telomeres. The children who participated in the sub-study were of higher socioeconomic status (SES) than study participants generally. Furthermore, the entire PAWS sample was more educated than the general population, in keeping with the demography of the East San Francisco Bay Area. Results may thus not generalize to children of extremely low socioeconomic status. Nevertheless, given the level of stress experienced by children from low socioeconomic environments (67), it is likely that a sample with a greater representation of children of low SES might have even larger variability in telomere length. Previous work suggests that both children of low SES and high SES may be more reactive than children of middle SES (20, 68). Thus, it is possible that the findings in this study underestimate the association between reactivity and telomere length.
Methodologically, it would be preferable to be able to compare associations with both leukocyte and buccal cell TL, since leukocyte DNA has been more often used in research. Indeed, Thomas and colleagues found no correlation between absolute leukocyte TL and BTL (15), calling into question the utility of BTL. However, a more recent article by Gadalla and colleagues found a high correlation between relative measures of BTL and blood TL (r=0.74, p<0.0001) (69). In any case, both BTL and blood/leukocyte TL measures were predictive of age-related disease outcomes in each of the Thomas and Gadalla articles, (Alzheimer’s Disease in the former and dyskeratosis congenita in the latter) suggesting at a minimum that BTL may be useful means of providing relative rankings for TL, if not absolute length, and as such may be useful for differentiating risk in children. Buccal cell DNA has also been successfully used to measure DNA methylation, another aging-related, epigenetic modification in DNA, like BTL (70). Further research is needed to clarify the importance of TL in different cell types for disease prediction.
Other limitations include a lack of information on birthweight, paternal age at birth, parental health, or sibship characteristics. Most notably, paternal age at birth has been positively related to leukocyte telomere length in offspring (71). However, a recent study reported paternal age to be positively related to externalizing but not internalizing behaviors (72), suggesting paternal age is unlikely to explain our findings.
We noted a relatively large coefficient of variation in reliability testing of the telomere assay—up to a third of the total variation. Nonetheless, associations for internalizing symptoms, HR, PEP, and the dichotomous reactivity measure defined by ANS and cortisol response with BTL approached significance even when effect sizes were reduced by a third. The general consistency in the pattern of associations between reactivity and internalizing symptoms with BTL provides reassurance as to the integrity of these findings, notable because of the small sample size.
To summarize, high reactivity was associated with shorter buccal cell telomere length in children. This study is a first step toward examining BTL in a psychosocial context and in children. Although in need of replication, the association of reactivity with buccal cell telomere length suggests that children with greater sensitivity to social stressors and adversities may prove more susceptible to aging-related biological changes (62). BTL may also serve as a marker of health in studies in children, although more data are needed to establish the clinical significance of BTL in childhood and subsequently to adult health.
Acknowledgments
The first author thanks the Robert Wood Johnson Foundation’s Health & Society Scholars Program and the MacArthur Network for its financial support.
ABBREVIATIONS
- ACTH
adrenocorticotropic hormone
- ANS
autonomic nervous system
- BMI
body mass index
- BPI
Berkeley Puppet Interview
- BTL
buccal cell telomere length
- CRH
corticotropin releasing hormone
- DNA
deoxyribonucleic acid
- ECG
electrocardiograph
- HPA
hypothalamic-pituitary-adrenocortical
- HR
heart rate (reactivity)
- PAWS
Peers and Wellness Study
- PCA
principal components analysis
- PEP
pre-ejection period
- PNS
parasympathetic nervous system
- RSA
respiratory sinus arrhythmia
- SES
socioeconomic status
- SNS
sympathetic nervous system
- T/S RATIO
ratio of telomere repeat copy number to single-copy gene copy number
Footnotes
Disclosures: Elizabeth Blackburn, Jue Lin, and Elissa Epel own Telome Health, Incorporated, which was created to apply telomere health diagnostics to aspects of medical and clinical care.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zee RY, Michaud SE, Germer S, Ridker PM. Association of shorter mean telomere length with risk of incident myocardial infarction: A prospective, nested case-control approach. Clin Chim Acta. 2009;403:139–41. doi: 10.1016/j.cca.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 4.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 5.Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2009 doi: 10.18632/aging.100007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epel ES. Telomeres in a life-span perspective: a new “psychobiomarker”? Current Directions in Psychological Science. 2009;18:6–10. [Google Scholar]
- 7.Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–74. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 8.Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, Christiansen L, Vaupel JW, Aviv A, Christensen K. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeichner SL, Palumbo P, Feng Y, Xiao X, Gee D, Sleasman J, Goodenow M, Biggar R, Dimitrov D. Rapid telomere shortening in children. Blood. 1999;93:2824–30. [PubMed] [Google Scholar]
- 10.Frenck RW, Jr., Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–10. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura K, Izumiyama-Shimomura N, Sawabe M, Arai T, Aoyagi Y, Fujiwara M, Tsuchiya E, Kobayashi Y, Kato M, Oshimura M, Sasajima K, Nakachi K, Takubo K. Comparative analysis of telomere lengths and erosion with age in human epidermis and lingual epithelium. J Invest Dermatol. 2002;119:1014–9. doi: 10.1046/j.1523-1747.2002.19523.x. [DOI] [PubMed] [Google Scholar]
- 12.Leach NT, Rehder C, Jensen K, Holt S, Jackson-Cook C. Human chromosomes with shorter telomeres and large heterochromatin regions have a higher frequency of acquired somatic cell aneuploidy. Mech Ageing Dev. 2004;125:563–73. doi: 10.1016/j.mad.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26:1263–71. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 14.Kemp RA, Reinders DM, Turic B. Detection of lung cancer by automated sputum cytometry. J Thorac Oncol. 2007;2:993–1000. doi: 10.1097/JTO.0b013e318158d488. [DOI] [PubMed] [Google Scholar]
- 15.Thomas P, NJ OC, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev. 2008;129:183–90. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA, 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105:13051–6. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagan J, Snidman N. The Long Shadow of Temperament. Belknap Press; Cambridge, MA: 2004. [Google Scholar]
- 18.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinol Rev. 1984;5:25–43. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 19.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 20.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 22.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 23.Allen MT, Matthews KA, Sherman FS. Cardiovascular reactivity to stress and left ventricular mass in youth. Hypertension. 1997;30:782–7. doi: 10.1161/01.hyp.30.4.782. [DOI] [PubMed] [Google Scholar]
- 24.Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev. 1991;98:459–87. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- 25.Manuck SB, Kasprowicz AL, Muldoon MF. Behaviorally-evoked cardiovascular reactivity and hypertension: Conceptual issues and potential associations. Ann Behav Med. 1990;12:17–29. [Google Scholar]
- 26.Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: advantages of a multisystem approach. J Dev Behav Pediatr. 2002;23:102–13. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. Br J Psychiatry. 2001;179:144–50. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- 28.Monk C, Kovelenko P, Ellman LM, Sloan RP, Bagiella E, Gorman JM, Pine DS. Enhanced stress reactivity in paediatric anxiety disorders: implications for future cardiovascular health. Int J Neuropsychopharmacol. 2001;4:199–206. doi: 10.1017/S146114570100236X. [DOI] [PubMed] [Google Scholar]
- 29.Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. J Abnorm Psychol. 1994;103:267–76. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- 30.Kagan J, Snidman N, Zentner M, Peterson E. Infant temperament and anxious symptoms in school age children. Dev Psychopathol. 1999;11:209–24. doi: 10.1017/s0954579499002023. [DOI] [PubMed] [Google Scholar]
- 31.Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev. 2010;81:270–89. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bush NR, Alkon A, Obradovic J, Stamperdahl J, Boyce WT. Deconstructing psychobiological reactivity: the contributions of psychomotor activity and stress response in five year old children. 2009. unpublished data.
- 33.Masharani U, Shiboski S, Eisner MD, Katz PP, Janson SL, Granger DA, Blanc PD. Impact of exogenous glucocorticoid use on salivary cortisol measurements among adults with asthma and rhinitis. Psychoneuroendocrinology. 2005;30:744–52. doi: 10.1016/j.psyneuen.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Bauman KJ, Graf NL. Educational attainment, 2000: US Census Brief. U.S. Census Bureau; Washington, D.C.: 2003. [Google Scholar]
- 35.Gentra Systems I . Puregene DNA Purification Kit: DNA purification from 1 buccal brush. vol. 2007. Gentra Systems; 2004. [Google Scholar]
- 36.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: developing the Macarthur health and Behavior Questionnaire. J Am Acad Child Adolesc Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Ablow JC, Measelle JR. The Berkeley Puppet Interview (BPI): interviewing and coding system manuals. 1993. Unpublished.
- 39.Measelle JR, Ablow JC, Cowan PA, Cowan CP. Assessing young children’s views of their academic, social, and emotional lives: an evaluation of the self-perception scales of the Berkeley Puppet Interview. Child Dev. 1998;69:1556–76. [PubMed] [Google Scholar]
- 40.Kraemer HC, Measelle JR, Ablow JC, Essex MJ, Boyce WT, Kupfer DJ. A new approach to integrating data from multiple informants in psychiatric assessment and research: mixing and matching contexts and perspectives. Am J Psychiatry. 2003;160:1566–77. doi: 10.1176/appi.ajp.160.9.1566. [DOI] [PubMed] [Google Scholar]
- 41.Carlson RD. Gesell School Readiness Test. In: Keyser D, Sweetland R, editors. Test Critiques. Test Corporation of America; Kansas City, KS: 1985. [Google Scholar]
- 42.Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children. American Guidance Service; Circle Pines, MN: 1983. [Google Scholar]
- 43.Kagan J, Snidman N. Temperamental factors in human development. Am Psychol. 1991;46:856–62. doi: 10.1037//0003-066x.46.8.856. [DOI] [PubMed] [Google Scholar]
- 44.Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: a longitudinal study. Child Dev. 1995;66:1360–84. [PubMed] [Google Scholar]
- 45.Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Dev Psychobiol. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- 46.Boyce WT, Chesney M, Alkon A, Tschann JM, Adams S, Chesterman B, Cohen F, Kaiser P, Folkman S, Wara D. Psychobiologic reactivity to stress and childhood respiratory illnesses: results of two prospective studies. Psychosom Med. 1995;57:411–22. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Eisenberg N, Fabes RA, Bustamante D, Mathy RM, Miller PA, Lindholm E. Differentiation of vicariously induced emotional reactions in children. Dev Psychol. 1988;24:237–46. [Google Scholar]
- 48.Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol. 2007;74:174–84. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev Psychobiol. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 51.Rudolph CD, Rudolph AM, Hostetter MK, Lister GL, Siegel NJ. Rudolph’s Pediatrics. 21st ed McGraw Hill Medical; New York: 2003. [Google Scholar]
- 52.Kelsey RM, Guethlein W. An evaluation of the ensemble averaged impedance cardiogram. Psychophysiology. 1990;27:24–33. doi: 10.1111/j.1469-8986.1990.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 53.Berntson GG, Quigley KS, Jang JF, Boysen ST. An approach to artifact identification: application to heart period data. Psychophysiology. 1990;27:586–98. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 54.Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: the psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–9. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- 55.Obradovic J, Bush NR, Stamperdahl J, Adler N, Boyce WT. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2009 doi: 10.1111/j.1467-8624.2009.01394.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: empirical perspectives and conceptual implications. Psychol Bull. 1993;114:296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- 57.Alkon A, Lippert S, Vujan N, Rodriquez ME, Boyce WT, Eskenazi B. The ontogeny of autonomic measures in 6- and 12-month-old infants. Dev Psychobiol. 2006;48:197–208. doi: 10.1002/dev.20129. [DOI] [PubMed] [Google Scholar]
- 58.Salomon K, Matthews KA, Allen MT. Patterns of sympathetic and parasympathetic reactivity in a sample of children and adolescents. Psychophysiology. 2000;37:842–9. [PubMed] [Google Scholar]
- 59.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–87. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, Sandler DP. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–60. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Treiber FA, Turner JR, Davis H, Thompson W, Levy M, Strong WB. Young children’s cardiovascular stress responses predict resting cardiovascular functioning 2 1/2 years later. J Cardiovasc Risk. 1996;3:95–100. [PubMed] [Google Scholar]
- 62.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. Jama. 2009;301:2252–9. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 63.Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91:635–40. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 64.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 65.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 66.Osswald K, Mittas A, Glei M, Pool-Zobel BL. New revival of an old biomarker: characterisation of buccal cells and determination of genetic damage in the isolated fraction of viable leucocytes. Mutat Res. 2003;544:321–9. doi: 10.1016/j.mrrev.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–80. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 68.Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17:303–28. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- 69.Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany NY) 2010;2:867–74. doi: 10.18632/aging.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Essex MJ, Boyce WT, Hertzman C, Lam L, Armstrong JM, Neumann SMA, Kobor MS. Epigenetic vestiges of early developmental adversity: Childhood stress exposure and DNA methylation in adolescence. 2011. unpublished data. [DOI] [PMC free article] [PubMed]
- 71.Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, Cupples A, Hunkin JL, Gardner JP, Lu X, Cao X, Sastrasinh M, Province MA, Hunt SC, Christensen K, Levy D, Spector TD, Aviv A. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4:e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saha S, Barnett AG, Buka SL, McGrath JJ. Maternal age and paternal age are associated with distinct childhood behavioural outcomes in a general population birth cohort. Schizophr Res. 2009;115:130–5. doi: 10.1016/j.schres.2009.09.012. [DOI] [PubMed] [Google Scholar]