Abstract
Aniline exposure is associated with toxicity to the spleen which is characterized by splenomegaly, hyperplasia, fibrosis, and a variety of sarcomas on chronic exposure in rats. However, mechanisms by which aniline elicits splenotoxic responses are not well understood. Earlier we have shown that aniline exposure leads to increased nitration of proteins in the spleen. However, nitrated proteins remain to be characterized. Therefore, in the current study using proteomic approaches, we focused on characterizing the nitrated proteins in the spleen of aniline-exposed rats. Aniline exposure led to increased tyrosine nitration of proteins, as determined by 2D Western blotting with anti-3-nitrotyrosine specific antibody, compared to the controls. The analyzed nitrated proteins were found in the molecular weight range of 27.7 to 123.6 kDa. A total of 37 nitrated proteins were identified in aniline-treated and control spleens. Among them, 25 were found only in aniline-treated rats, 11 were present in both aniline-treated and control rats, while one was found in controls only. The nitrated proteins identified mainly represent skeletal proteins, chaperones, ferric iron transporter, enzymes, nucleic acids binding protein, and signaling and protein synthesis pathways. Furthermore, aniline exposure led to significantly increased iNOS mRNA and protein expression in the spleen, suggesting its role in increased reactive nitrogen species formation and contribution to increased nitrated proteins. The identified nitrated proteins provide a global map to further investigate alterations in their structural and functional properties, which will lead to a better understanding of the role of protein nitration in aniline-mediated splenic toxicity.
Keywords: Aniline, Reactive nitrogen species, Nitrated proteins, Proteomics, Splenic toxicity
Introduction
Aniline is among the most extensively used industrial chemicals with an annual production of over one billion pounds in the United States (Di Girolamo et al., 2009). Aniline exposure, besides inducing methemoglobinemia, hemolysis and hemolytic anemia (Jenkins et al., 1972; Harrison and Jollows, 1987; Khan et al., 1997; Pauluhn, 2004), is also associated with toxic responses in the spleen, which are characterized by splenomegaly, increased erythropoietic activity, hyperpigmentation, hyperplasia, fibrosis, and a variety of primary sarcomas of the spleen after chronic exposure in rats (Goodman et al., 1984; Weinberger et al., 1985; Bus and Popp, 1987; Khan et al., 1993, 1997, 1999a, 1999b, 2006; Pauluhn, 2004; Ma et al., 2008). Despite well-documented splenotoxic effects and tumorigenic responses resulting from aniline exposure (Bus and Popp, 1987; Khan et al., 2006), the molecular mechanisms by which aniline exerts its toxic effects in the spleen are not known. Earlier, we have shown that aniline exposure is associated with the release and accumulation of iron in the spleen, leading to oxidative damage through enhanced production of reactive oxygen/nitrogen species (ROS/RNS) (Khan et al., 1997, 1999a, 1999b, 2003a, 2003b; Ma et al., 2008; Wang et al., 2010). The excessive production of oxidants could attack proteins, nucleic acids and lipids, leading to structural and functional changes (Monteiro et al., 2008; Abello et al., 2009). In fact, our earlier studies have shown that aniline exposure results in protein oxidation, lipid peroxidation and DNA oxidation (Khan et al., 1997, 1999a, 2003a; Wu et al., 2005; Ma et al., 2008). Moreover, our studies have also shown an increased nitration of protein tyrosines (nitrotyrosine formation) in the spleen of aniline-exposed rats (Khan et al., 2003b). However, the identity and characteristics of nitrated proteins remain unknown.
Nitration of protein tyrosines is one of the post-translational covalent modifications. Under physiological conditions, very little nitrated proteins are present, but are increased under oxidative/nitrosative stress. The RNS such as nitrogen dioxide (•NO2) and peroxynitrite anion (ONOO−) are secondary reactive intermediates which are more toxic than primary oxidant nitric oxide radical (•NO), and are produced by the reaction of •NO with other oxidants such as superoxide radicals (O2•−), hydrogen peroxide (H2O2), and/or transition metals (Radi, 2004). Inducible nitric oxide sythase (iNOS) is one of the major enzymes that catalyzes •NO formation (Pacher et al., 2007). An unbalanced, high rate of •NO formation, catalyzed by iNOS, could lead to increased protein nitration (Greenacre et al., 2001; Radi Rafael, 2004). Therefore, increased expression of iNOS could also be a potential contributor to nitrosative stress in aniline-induced splenic toxicity, leading to increased nitration of protein tyrosines in the spleen (Khan et al., 2003b).
RNS-mediated protein tyrosine nitration could alter their properties leading to their inactivation, increased degradation, perturbed tyrosine phosphorylation and even generation of an autoimmune response (Monteiro et al., 2008; Abello et al., 2009). Nitrated proteins have been detected in several human diseases, including cancer, neurodegenerative disorders, multiple sclerosis, diabetes, coronary artery disease and inflammation (Miyagi et al., 2002; Dalle-Donne et al., 2005; 2006), suggesting their potential involvement in disease pathogenesis. Hence, it is important to identify nitrated proteins for an in-depth understanding of the mechanism of aniline-induced splenic toxicity.
Proteomic approaches have been widely used to identify protein modifications as a result of oxidative stress. The current study, using proteomics combined with 2D Western blotting, was focused on identifying and characterizing nitrated proteins in the spleens of aniline-treated vs. control rats. We identified 25 nitrated proteins which were prevalent only in aniline-exposed rats, whereas 11 nitrated proteins were found in both aniline-treated and control groups. Our data also show increased expression of iNOS mRNA and protein in the spleen following aniline exposure and provide a mechanism by which increased nitrated proteins could be formed.
Materials and Methods
Materials
Precast immobilized DryStrips (pH 3–11NL, 11 cm) and IPG buffer (pH 3–11NL) were purchased from GE Healthcare (Uppsala, Sweden). 2D protein extraction buffer-III was purchased from GE Healthcare (Piscataway, NJ). SDS-tris-glycine gradient gel (10–20%), coomassie blue G250 stain solution, PVDF membrane and fat-free milk were purchased from Bio-Rad (Hercules, CA). Specific goat anti-3-nitrotyrosine (3-NT) polyclonal antibody and HRP-conjugated donkey anti-goat IgG were purchased from Oxford Biomedical Research (Oxford, MI). Amersham ECL Plus Western blotting detection kit was purchased from GE Healthcare (Buckinghamshire, UK). The other chemicals were of analytical grade and purchased from Sigma Aldrich (St. Louis, MO).
Animals and treatment
Male Sprague-Dawley rats (~200 g), obtained from Harlan Sprague-Dawley (Indianapolis, IN), were housed in wire-bottom cages over adsorbent paper with free access to tap water and Purina lab chow and maintained in a controlled-environment in animal room (temperature, 22 °C; relative humidity, 50%; photoperiod, 12-h light/dark cycle) for 7 days prior to the treatment. The experiments were performed in accordance with the guideline of National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of University of Texas Medical Branch at Galveston, TX.
Rats were divided into two groups of six each, one group received 0.5 mmol/kg/day aniline hydrochloride (97%; Aldrich, Milwaukee, WI) via drinking water (pH ~6.8), while the other group received water only to serve as control. The drinking pattern of aniline-treated and control rats was similar. Choice of dose and duration of exposure were based on earlier studies (Khan et al., 1993, 1999a, 1999b, 2003a, 2006; Wang et al., 2005, 2011; Ma et al., 2008). After 30 days of treatment, the rats were euthanized under nembutal (sodium pentobarbital) anesthesia. The spleens were removed immediately, blotted, weighed, and stored at −80 °C until further analysis. A potion of the spleen was snap-frozen in liquid nitrogen and stored at −80 °C for RNA isolation (Wang et al., 2011).
Protein extraction
Spleen tissues from control and aniline-treated rats were first washed with ice cold phosphate buffered saline (PBS) and then homogenized in a buffer [PBS pH 7.4, 1% Igepal-CA630 (NP-40), 1X protease inhibitors, 10 mM DTT, 10 mM EDTA] on ice and then centrifuged at 750 g for 20 min at 4 °C to remove the cell debris. The top fraction was transferred to new tubes and centrifuged at 20,000 g for 20 min at 4 °C. The supernatant was collected and mixed with 1% streptomysin sulfate to remove DNA contamination (Blackburn et al.,1999; Fraction #1), while the pellet was dissolved in a buffer containing 6M urea, 1% NP-40, 20 mM Tris.Cl pH 7.4, and 10 mM EDTA (Fraction #2). The mixture of fractions #1 and #2 represented the total protein, and was subjected to further analysis.
Detection of nitrated proteins by 2D-Gel and Western blot
Three hundred µg of total protein was added in 200 µl of 2-D protein extraction buffer-III containing 100 mM DTT, trace amount of bromophenol blue and 1% (v/v) IPG buffer pH 3–11NL, and incubated at 21 °C for 1 hr. The proteins were then rehydrated to the DryStrip (11 cm, pH3–11 NL) overnight at the same temperature. For each sample, IPG strips were used in duplicate. For the first dimension, isoelectric focusing (IEF) was performed at 20 °C on EttanIPGphor3 (GE Healthcare, Sweden) in the following steps: 200 V for 30 min., 500 V for 1 hr, 1000 V for 1.5 hrs, 8000 V for 2.5 hrs and 8000 V for 24000 Vhr. The strips were then equilibrated for 1 hr in equilibration buffer (Tris-HCl 50 mM pH 8.8, urea 6 M, DTT 100 mM, SDS 2%, glycerol 20%). After rinsing two times with SDS-PAGE running buffer, the strips were loaded on to 10–20% SDS-tris-glycine gradient gel (13.3 × 8.7 cm) and were then run at 150 V for 2 hrs at room temperature in the 2nd dimension. Following the electrophoresis, one of the duplicate gels of each sample was stained with coomassie blue G250 (CBB G250), while the other one was used for Western blotting by transferring the proteins to PVDF membrane as described previously (Benndorf and Babel, 2002). The PVDF membrane was blocked with 5% fat-free milk in TBST (pH 7.4) for 1 hr at room temperature and then incubated with anti-3NT IgG (1:4000 dilution in TBST containing 5% fat-free milk, pH 7.4) at 4 °C overnight. The membrane was washed and incubated with HRP conjugated secondary antibody (1:8000 dilution in TBST containing 5% fat-free milk) for 45 min at room temperature. The signal was visualized by enhanced chemiluminescent detection.
SameSpots analysis of protein expression variation
2D gel images (CBB G250 stained) were acquired by 2D Proteomic Imaging System (ProXPRESS, PerkinElmer, Inc.). The scanning resolution of CCD camera (software, ProScan V4.0.0.10) was 100 µm with white light and the exposure time is 600 msec. The 2D gel images were then analyzed by the software Progenesis SameSpots (nonlinear dynamics, version 4.0) which has been judged to be much improved in reproducibility and objectivity compared to previous generations of 2D gel analysis software (Silva et al., 2009). To match the same spot (same protein) between overlapped gel images, one of the six gels was chosen as reference. With the help of manually drawn and automatically added vectors from each image to the reference, images were aligned at the pixel level. The program then performed automatic spot detection and background subtraction. The software assigns the same spot on every gel in the analysis of identical shape (spot outline) and spot number. Spot volumes were normalized to those of the reference gel to obtain normalized volumes that are comparable across gels. Protein expression fold changes between controls and aniline-treated rats were determined as described below in the statistical analysis.
Trypsin digestion and MALDI TOF/TOF MS analysis
The nitrated protein spots were manually picked up from the 2D gel. The protein was digested with trypsin (0.1 µg per spot, Promega) in 10 µl of 25 mM ammonium bicarbonate, pH 8.0, for 6 hrs at 37°C. One µl of digested sample solution was used for MALDI TOF/TOF MS. The data was collected by using an Applied Biosystems 4800 MALDI TOF/TOF proteomics analyzer. The instrument was operated in a positive ion reflection mode with mass range from 850 to 3000 Da. The focus mass was set at 1700 Da. For MS data, 2000–4000 laser shots were acquired and averaged from each sample spot. Following MALDI MS analysis, MALDI MS/MS was performed on several (5~10) abundant ions from each sample spot. A 1 kV positive ion MS/MS method was used to acquire data under post-source decay (PSD) conditions. The instrument precursor selection window was +/− 3 Da. For MS/MS data, 2000 laser shots were acquired and averaged from each sample spot. Automatic external calibration was performed using reference fragment masses 175.120, 480.257, 684.347, 1056.475, and 1441.635 (from precursor mass 1570.700).
Proteins identification
Applied Biosystems GPS Explorer™ (Version 3.6) software was employed for searching the respective protein database using both MS and MS/MS spectral data for protein identification. Protein match probabilities were determined by using MASCOT scores, and a score of more than 61 was considered significant (p<0.05). MS peak filtering included the following parameters: mass range 800 Da to 4000 Da, minimum S/N filter = 10, mass exclusion list tolerance = 0.5 Da, and mass exclusion list (for some trypsin and keratin-containing compounds) included masses 842.51, 870.45, 1045.56, 1179.60, 1277.71, 1475.79, and 2211.1. For MS/MS peak filtering, the minimum S/N filter = 10. The mass data was matched to the NCBI protein database. Precursor tolerance was set at 0.2 Da; MS/MS fragment tolerance was set at 0.3 Da; mass = monoisotopic; and peptide charges were only considered as +1.
Real time PCR for iNOS mRNA
RNA isolation
Total splenic RNA was isolated with RiboPure kit (Ambion, Austin, TX) as per manufacturer’s instructions. To eliminate gemomic DNA contamination, isolated RNA was treated with RNase free DNase I (DNA free kit, Ambion). The total RNA concentration was determined by measurement of the absorbance of 260 nm. RNA integrity was verified electrophoretically by ethidium bromide stain and A260/280 ratio.
Real time PCR
Real-time PCR was performed essentially as described earlier (Wang et al., 2005, 2008, 2010; Ma et al., 2008). First-strand cDNA was prepared from total RNA by using the SuperScript first-strand synthesis kit (Invitrogen, Carlsbad, CA) as per the manufacturer's instructions. The template sequence of iNOS was used from NCBI GenBank (accession No. U03699). Oligonucleotide primers were designed by OligoPerfert Designer (Invitrogen, Calsbad, CA), and synthesized by Sigma Genosys (St. Louis, MO). The primer sequences are as follows: 5’-GTGGCTGTGGTCACCTATCG (forward) and 5’-ACTGACACTCCGCACAAAGC (reverse). Real-time PCR was performed a two-step cycling protocol (denaturation and annealing/extension), and was carried out by a Mastercycler Realplex (Eppendorf, Westbury, NY). The primers of 18S (QuantumRNA Universal 18S standards; Ambion, Austin, TX) was used as the internal control. For each cDNA sample, the parallel of triplicate reactions were performed for the detection of 18S and rat iNOS. The reaction samples in a final volume of 25 µl contained 2 µl cDNA templates, 2 µl primer pair, 12.5 µl iQ SYBR Green Supermix, and 8.5 µl water. Amplification conditions were identical for all reactions: 95°C for 2 min for template denaturation and hot-start before PCR cycling. A typical cycling protocol consisted of three stages, 15 s at 95°C for denaturation, 30 s at 60°C for annealing, and 30 s at 72°C for extension and an additional 20 s hold for fluorescent signal acquisition. To avoid nonspecific signal from primer dimers, the fluorescence signal was detected at 2°C below the melting temperature (Tm) of individual amplicons and above the Tm of the primer dimmers. A total of 40 cycles were performed. Quantitation of PCR was done by using the comparative CT method as described in User Bulletin No. 2 of Applied Biosystems (Foster City, CA) and reported as fold difference relative to the calibrator cDNA. The fold changes in rat iNOS cDNA (target gene) relative to the 18S endogenous control were determined as fold change = 2−ΔΔCT, where ΔΔCT = (CT aniline – CT 18S) − (CT control – CT 18S).
Western blot detection of iNOS protein expression
The Western blot was performed as described earlier (Khan et al., 2006; Wang et al., 2010). Briefly, 50 µg of total proteins extracted from the spleens of control or aniline-treated rats were subjected to 10% SDS-PAGE and transferred to PVDF membranes. Membrane was blocked with non-fat dry milk buffer (5%, w/v, in TBST, pH 7.4) and probed with specific anti-iNOS antibody (rabbit anti-rat, Abcam Inc., Cambridge, MA). After washing with wash buffer, the membrane was incubated with HRP-conjugated anti-rabbit secondary antibody and the signal was detected by a chemiluminescence using an ECL Plus kit following the manufacturer’s instructions. Blots were quantitated by densitometry and normalized with the actin signal which was used as a loading control (Moon et al., 2001).
Statistical analysis
The normalized volumes of same protein spots in 2D gels of control and aniline-treated mice were analyzed by Student’s t-test to compare the differences in protein expression. Similarly, iNOS protein and mRNA expression in the two groups were also compared using Student’s t-test. p values ≤ 0.05 were considered statistically significant.
Results
Nitrated protein spots identified by 2D Western blotting
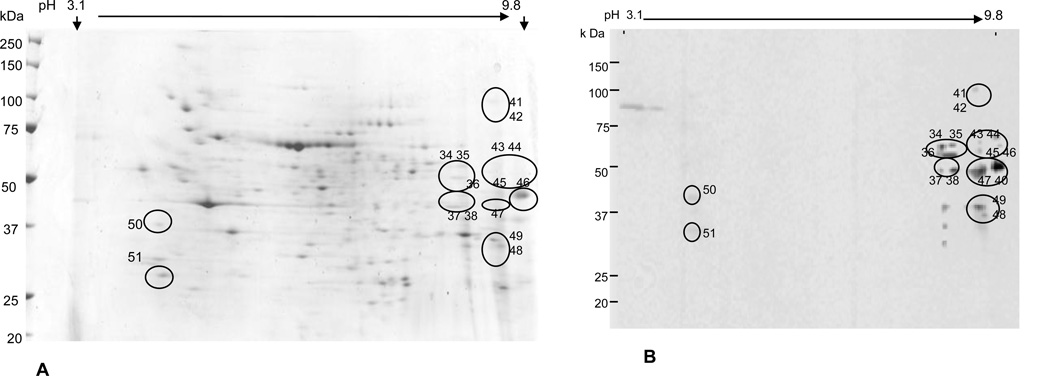
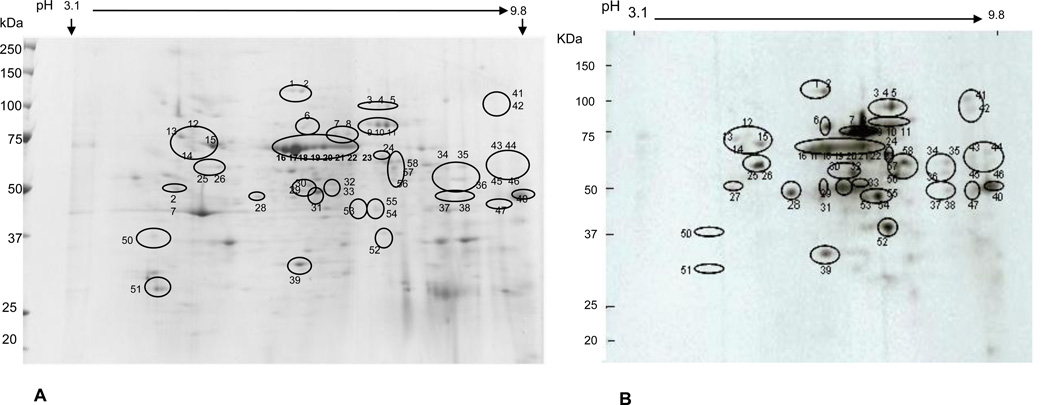
In order to identify nitrated proteins, 2D gels for each sample were run in duplicate, one for CBB G250 stain and the other one for Western blot analysis. The 2D gel protein profile of a representative control sample is shown in Fig. 1A, while Fig. 1B is the corresponding Western blot map of nitrated proteins of the same sample. The nitrated proteins shown in black circles (Fig. 1B) were matched to the 2D gel protein profiles in Fig. 1A. Similarly, the 2D gel protein profile of splenic extract from an aniline-treated rat is shown in Fig. 2A, whereas Fig. 2B shows the corresponding Western blot map of nitrated protein spots of the same sample. The black circled spots (Fig. 2B) were also matched to the 2D gel protein profiles in Fig. 2A. As shown in the figures, the nitrated proteins spots were found in the pI range of pH 4.63 to 9.37 and molecular weights of 27.7 to 123.6 kDa. The 2D gel protein profiles of randomly chosen three controls and three aniline-treated rats matched well when analyzed by using Samespots program (data not shown). Among the three analyzed controls, we identified 14 nitrated protein spots, 12 of these spots were also found in aniline-treated protein extracts. The protein extracts from aniline-treated rats showed remarkably increased protein nitration, which was evident from greater number of spots for nitrated proteins. We identified a total of 53 nitrated protein spots among the three aniline-treated samples, out of which 41 were found only in the aniline-treated protein extracts (Fig. 2B). The nitrated protein spots (same protein spots present in at least two samples) were picked up and subjected to MALDI TOF/TOF MS/MS analyses.
Fig. 1.
2D gel profile of splenic proteins of a control rat (CBB G250 stained; Fig. 1A). The protein spots for nitrated proteins, identified by Western blot, are shown in black circles (Fig. 1B), and were also matched to the 2D gel protein profile (Fig.1A). The numbers used for the spots are the same as in Tables 2 and 3.
Fig. 2.
2D gel profile of splenic proteins of an aniline-treated rat (CBB G250 stained; Fig. 2A). The protein spots for nitrated proteins, identified by Western blot, are shown in black circles (Fig. 2B), and were also matched to the 2D gel protein profile (Fig. 2A). The numbers used for the spots are the same as in Tables 1, 2 and 3.
Identified nitrated proteins in the spleen
Combination of MALDI TOF/TOF MS and MS/MS and protein database search enabled us to further identify the nitrated proteins. Probability-based MASCOT score was used to evaluate the identifications. The MASCOT score of more than 61 represents the statistical confidence >95% (p<0.05). A total of 37 nitrated proteins were identified (MASCOT score > 61), of which 25 were found in aniline treated spleen extracts only (Table 1), 11 were found in both control and aniline-treated rats, and one was found in controls only (Table 2). The nitrated proteins represented skeletal proteins, vinculin (No. 1, 2), ezrin (No. 6), moesin (No. 7, 8), ARP3 (No. 28), tropomyson (No. 50); chaperone and stress proteins, HSP90 protein 1 beta (No. 12), HSP70 protein 5 (GRP78; No. 13), HSP 8 (No. 15), T-complex protein 1 theta (TCP1 theta; No. 26); ferric iron transporter, transferrin (No. 9, 10, 11); protein transporter, selenium binding protein 2 (No. 32); plasma protein, albumin (No. 20, 21, 22, 23) and albumin precursor (No. 16, 17, 18, 19), fibrinogen, B beta polypeptide (No. 57); enzymes, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase (ATIC; No. 24), ATP synthase (No. 27), aldehyde dehydrogenase (ALDH; No. 29, 30), enolase 1 alpha (No. 31), acetyl-coenzymeA acyltransferase 2 (No. 36), aldolase A (No. 37), glutamate oxaloacetate transaminase 2 (No. 38, 40, 48), 14-3-3 protein zeta/delta (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide; No. 51), long-chain specific acyl-CoA dehydrogenase mitochondrial precursor (No. 53), glutamine synthetase (No. 54), isocitrate dehydrogenase 1 (No. 55), glutamate dehydrogenase l, (mitochondria precursor; No. 56) ; signaling pathway proteins, angiomotin (No. 33), annexin A3 (No. 39), annexin A1 (lipocortin I; No. 52), eukaryotic translation initiation factor 2 subunit 3 (No. 45) and lymphocyte cytosolic protein 1 (L-plastin; No. 14); nucleic acids binding protein, heterogeneous nuclear ribonucleoprotein A3 isoform c (No. 49) and protein synthesis factor, eukaryotic translation elongation factor 2 (No.3, 4, 5), putative eukaryotic translation elongation factor 1e (No. 44) and 1f (No. 47).
Table 1.
Summary of nitrated proteins present in aniline-treated rats.
| Spots no. *a |
Protein Name | Accession no. *b | MW.(Da) | Identified Peptides no. |
Identified Peptides Coverage % |
MASCOT score *c |
Theo. pI |
|---|---|---|---|---|---|---|---|
| 1 | Vinculin (Metavinculin) | gi|157822133 | 123555.8 | 14 | 14 | 302 | 5.54 |
| 2 | Vinculin, isoform CRA_b | gi|149031250 | 116542.3 | 23 | 22 | 458 | 5.83 |
| 3 | Eukaryotic translation elongation factor | gi|8393296 | 95222.9 | 5 | 5 | 70 | 6.41 |
| 4 | Eukaryotic translation elongation factor | gi|8393296 | 95222.9 | 9 | 10 | 366 | 6.41 |
| 5 | Eukaryotic translation elongation factor | gi|8393296 | 95222.9 | 12 | 13 | 381 | 6.41 |
| 6 | Ezrin | gi|17902245 | 69362.6 | 6 | 9 | 152 | 5.83 |
| 7 | Moesin, isoform CRA_a | gi|149042266 | 67784.9 | 37 | 55 | 795 | 6.12 |
| 8 | Moesin | gi|149042266 | 67696.8 | 31 | 42 | 768 | 6.16 |
| 9 | Transferrin | gi|61556986 | 76314.5 | 8 | 12 | 386 | 6.94 |
| 10 | Transferrin | gi|61556986 | 76345.6 | 14 | 21 | 755 | 7.14 |
| 11 | Transferrin | gi|61556986 | 75809.4 | 13 | 21 | 542 | 7.57 |
| 12 | Heat shock 90kDa protein 1, beta | gi|40556608 | 83289.1 | 14 | 22 | 383 | 4.97 |
| 13 | Heat shock 70kD protein 5 | gi|25742763 | 72302.4 | 33 | 54 | 1170 | 5.07 |
| 14 | Lymphocyte cytosolic protein 1 | gi|58865656 | 70077.7 | 27 | 44 | 845 | 5.15 |
| 15 | Heat shock protein 8 | gi|178847300 | 70827.2 | 28 | 53 | 960 | 5.37 |
| 16 | Serum albumin precursor | gi|124028612 | 68686.1 | 8 | 14 | 836 | 6.09 |
| 17 | Serum albumin precursor | gi|124028612 | 68686.1 | 18 | 27 | 987 | 6.09 |
| 18 | Serum albumin precursor | gi|124028612 | 68686.1 | 18 | 25 | 780 | 6.09 |
| 19 | Serum albumin precursor | gi|124028612 | 68686.1 | 21 | 29 | 866 | 6.09 |
| 20 | Albumin | gi|124028612 | 68686.1 | 21 | 27 | 872 | 6.09 |
| 21 | Albumin | gi|124028612 | 68686.1 | 21 | 27 | 931 | 6.09 |
| 22 | Albumin | gi|124028612 | 68686.1 | 18 | 32 | 812 | 6.09 |
| 23 | Albumin | gi|124028612 | 68686.1 | 13 | 31 | 101 | 6.09 |
| 24 | 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase | gi|2541906 | 64168.2 | 26 | 51 | 627 | 6.69 |
| 25 | APEG-1 protein | gi|1457952 | 12660.3 | 8 | 61 | 59 | 8.54 |
| 26 | T-complex protein 1 subunit theta | gi|34867525 | 59550.5 | 19 | 28 | 183 | 5.38 |
| 27 | ATP synthase /F1-ATPase |
gi|149029718 /gi|6729935 |
56309.5 51320.8 |
23 22 |
51 | 1080 1070 |
4.95 4.97 |
| 28 | ARP3 (actin-related protein 3 homolog) | gi|161728791 | 47327 | 15 | 34 | 465 | 5.61 |
| 29 | Mitochondrial aldehyde dehydrogenase | gi|25990263 | 53295.9 | 6 | 14 | 108 | 5.7 |
| 30 | Mitochondrial aldehyde dehydrogenase | gi|25990263 | 53295.9 | 15 | 30 | 583 | 5.7 |
| 31 | Enolase 1, alpha | gi|158186649 | 47086.2 | 17 | 50 | 903 | 6.16 |
| 32 | Selenium binding protein | gi|18266692 | 52498.4 | 15 | 35 | 235 | 6.1 |
| 33 | Angiomotin | gi|109510553 | 116787.9 | 21 | 63 | 55 | 7.06 |
| 39 | Annexin A3 (Annexin-3) | gi|122065130 | 36340.6 | 22 | 62 | 564 | 5.96 |
| 52 | Lipocortin I (Annex A1) | gi|235879 | 38794.9 | 11 | 34 | 401 | 6.97 |
| 53 | Long-chain specific acyl-CoA dehydrogenase, mitochondrial precursor | gi|6978431 | 47842.4 | 2 | 5 | 124 | 7.63 |
| 54 | Gln synthetase | gi|228136 | 40411.4 | 5 | 10 | 139 | 6.38 |
| 55 | Isocitrate dehydrogenase 1 | gi|89573967 | 42628.3 | 7 | 19 | 133 | 5.98 |
| 56 | Glutamate dehydrogenase 1, mitochondrial precursor | gi|6980956 | 61377.3 | 4 | 7 | 169 | 8.05 |
| 57 | Fibrinogen, B beta polypeptide | gi|149048261 | 27423 | 2 | 7 | 71 | 8.71 |
| 58 | Protein Vav 3 oncogene (predicted) | gi|149025734 | 69319.3 | 10 | 16 | 57 | 8.98 |
Spot numbers are the same as shown in the 2D gel protein profiles of Fig. 2;
The accession numbers in NCBI database;
A MASCOT score >61 was considered significant (p<0.05) for proteins identified from data base.
Table 2.
Summary of nitrated proteins present in both control and aniline-treated rats.
| Spots no. *a |
Protein Name | Accession no. *b |
MW. Da | Identified Peptides no. |
Identified Peptides coverage % |
MASCOT score *c |
Theo. pI |
|---|---|---|---|---|---|---|---|
| 34 | PREDICTED: similar to Talin-1 | gi|189181726 | 269504.1 | 8 | 2 | 62 | 5.86 |
| 35 | Elongation factor-1 alpha | gi|28460696 | 50118.1 | 10 | 25 | 328 | 9.1 |
| 36 | Acetyl-Coenzyme A acyltransferase 2 | gi|149027157 | 38410.7 | 8 | 36 | 114 | 8.64 |
| 37 | Aldolase A | gi|202837 | 39235.3 | 17 | 45 | 452 | 8.31 |
| 38 | Glutamate oxaloacetate transaminase 2 | gi|6980972 | 44279.3 | 8 | 23 | 181 | 8.52 |
| 40 | Glutamate oxaloacetate transaminase 2 | gi|6980972 | 47284.1 | 10 | 23 | 234 | 9.13 |
| 41 | NonO/p54nrb homolog | gi|109477262 | 65950.5 | 4 | 8 | 204 | 9.37 |
| 42 | NonO/p54nrb homolog | gi|109477262 | 65950.5 | 3 | 5 | 87 | 9.37 |
| 43 | 1700019E19Rik protein (predicted), isoform | gi|149025230 | 23537.6 | 4 | 13 | 49 | 4.57 |
| 44 | rCG25445, isoform CRA_e(putative eukaryotic translation elongation factor 1e) | gi|149019083 | 37741.7 | 4 | 13 | 102 | 9.06 |
| 45 | Eukaryotic translation initiation factor 2, subunit 3, structural gene X-linked | gi|189217906 | 51047.2 | 4 | 8 | 131 | 8.66 |
| 46 | Adenosine deaminase, RNA-specific, isoform CRA_a | gi|149048040 | 70067.5 | 5 | 13 | 41 | 8.73 |
| 47 | rCG25445, isoform CRA_f (putative eukaryotic translation elongation factor 1f) | gi|149019084 | 31915.8 | 6 | 22 | 261 | 9.29 |
| 50 | Tropomyosin beta chain | gi|66730475 | 32937.6 | 13 | 33 | 411 | 4.63 |
| 51 | Tyrosine 3-monooxygenase/tryptophan5- monooxygenase activation protein, zeta | gi|6756041 | 27753.7 | 12 | 51 | 571 | 4.73 |
| 48 | Aspartate aminotransferase, mitochondrial | gi|6980972 | 47284.1 | 10 | 24 | 304 | 9.13 |
| 49 # | Heterogeneous nuclear ribonucleoprotein A3 isoform c | gi|157277969 | 37063.2 | 6 | 20 | 299 | 8.46 |
Protein expression variation in 2D gel profiles
The normalized mean intensities of same protein spots in 2D gels of aniline-treated group were compared with the controls by Progenesis SameSpots (version 4.0). The changes in protein expression is summarized in Table 3. Among a total of 37 nitrated proteins, only the expression of T-complex protein 1 theta (No. 26), ARP3 (actin-related protein 3 homolog, No. 28) and aspartate aminotransferase (No. 48) changed significantly (p<0.05). The expression of the other proteins showed no significant difference between the controls and aniline-treated groups.
Table 3.
Summary of protein expression variation of 37 nitrated Proteins in 2D protein profile of aniline-treated vs. control rats.
| Spots No. *a |
Protein Name | Significance Expression Variation (p value, *b) |
Expression Variation of fold change *c |
Spots No. *a |
Protein Name | Significance Expression Variation (p value, *b) |
Expression Variation of fold change *c |
|---|---|---|---|---|---|---|---|
| 1 | Vinculin (Metavinculin) | 0..539 | 1.1 | 31 | enolase 1, alpha | 0.44 | 1.1 |
| 2 | vinculin, isoform CRA_b | 0..542 | 1.1 | 32 | selenium binding protein 2 | 0.59 | 1.2 |
| 3 | eukaryotic translation elongation factor 2 | 0.188 | 2.1 | 33 | Angiomotin | 0.363 | 1.2 |
| 4 | eukaryotic translation elongation factor 2 | 0.052 | 1.4 | 39 | Annexin A3 (Annexin-3) | 0.589 | 1.2 |
| 5 | eukaryotic translation elongation factor 2 | 0..58 | 1.2 | 52 | lipocortin I (Annex A1) | 0.117 | 1.9 |
| 6 | Ezrin | 0.763 | 1.0 | 53 | long-chain specific acyl-CoA dehydrogenase | 0.34 | 1.3 |
| 7 | moesin, isoform CRA_a | 0.068 | 1.9 | 54 | Gln synthetase | 0.171 | 1.5 |
| 8 | moesin | 0.145 | 1.5 | 55 | isocitrate dehydrogenase 1 | 0.171 | 1.5 |
| 9 | transferrin | 0.601 | 1.3 | 56 | glutamate dehydrogenase 1, mitochondrial precursor | 0.831 | 1.0 |
| 10 | transferrin | 0..558 | 1.2 | 57 | fibrinogen, B beta polypeptide, | 0.114 | 1.3 |
| 11 | transferrin | 0.73 | 1.0 | 58 | protein Vav 3 oncogene | 0.893 | 1.0 |
| 12 | Heat shock 90kDa protein 1, beta | 0.116 | 1.2 | Spots No.*d | Protein Name | Significance Expression Variation (p value, *b) | Expression Variation of fold change *c |
| 13 | heat shock 70kD protein 5 | 0.263 | 1.2 | ||||
| 14 | lymphocyte cytosolic protein 1 | 0.151 | 1.4 | 34 | PREDICTED: similar to Talin-1 | 0.168 | 1.7 |
| 15 | heat shock protein 8 | 0.743 | 1.0 | 35 | elongation factor-1 alpha | 0.426 | 1.2 |
| 16 | Serum albumin precursor | 0.72 | 1.0 | 36 | acetyl-Coenzyme A acyltransferase 2 | 0.066 | 1.2 |
| 17 | Serum albumin precursor | 0.766 | 1.0 | 37 | aldolase A | 0.207 | 1.6 |
| 18 | Serum albumin precursor | 0.051 | 1.1 | 38 | glutamate oxaloacetate transaminase 2 | 0.752 | 1.2 |
| 19 | Serum albumin precursor | 0.79 | 1.0 | 40 | glutamate oxaloacetate transaminase 2 | 0.483 | 1.1 |
| 20 | Albumin | 0.196 | 1.3 | 41 | NonO/p54nrb homolog | 0.522 | 1.2 |
| 21 | Albumin | 0.155 | 1.4 | 42 | NonO/p54nrb homolog | 0.522 | 1.2 |
| 22 | Albumin | 0.869 | 1.0 | 43 | 1700019E19Rik protein (predicted), isoform | 0.739 | 1.2 |
| 23 | Albumin | 0.433 | 1.2 | 44 | rCG25445, isoform CRA_e | 0.562 | 1.1 |
| 24 | 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferas /IMP cyclohydrolase | 0.287 | 1.4 | 45 | eukaryotic translation initiation factor 2, subunit 3,. | 0.256 | 2.0 |
| 25 | APEG-1 protein | 1.2 | 46 | adenosine deaminase, RNAspecific, isoform CRA_a | 0.823 | 1.1 | |
| 26 | T-complex protein 1 subunit theta | 0.006 | 1.1 | 47 | rCG25445, isoform CRA_f | 0.578 | 1.4 |
| 27 | ATP synthase | 0.261 | 1.2 | 50 | tropomyosin beta chain | 0.807 | 1.1 |
| 28 | ARP3, actin-related protein 3 | 0.016 | 1.4 | 51 | tyrosine 3 monooxygenase/tryptophan5- monooxygenase activation protein, zeta | 0.238 | 1.2 |
| 29 | mitochondrial aldehyde dehydrogenase | 0.227 | 1.2 | 48 | aspartate aminotransferase | 0.021 | 3.8 |
| 30 | mitochondrial aldehyde dehydrogenase | 0.155 | 1.3 | 49 *e | heterogeneous nuclear ribonucleoprotein A3 isoform c | 0.214 | 1.8 |
Nitrated proteins found only in aniline-treated rats.
The significance of protein expression variation between two groups were analyzed by student t-test, and p<0.05 was considered significant. The protein spots No. 26, 28 and 48 show significant difference.
Protein expression fold changes between the two groups were analyzed on CBB G250 stained protein spots in 2D gels.
Nitrated proteins found in both control and aniline treated rats.
Nitrated proteins found only in control rats.
Up-regulation of iNOS expression in the spleens of aniline-treated rats
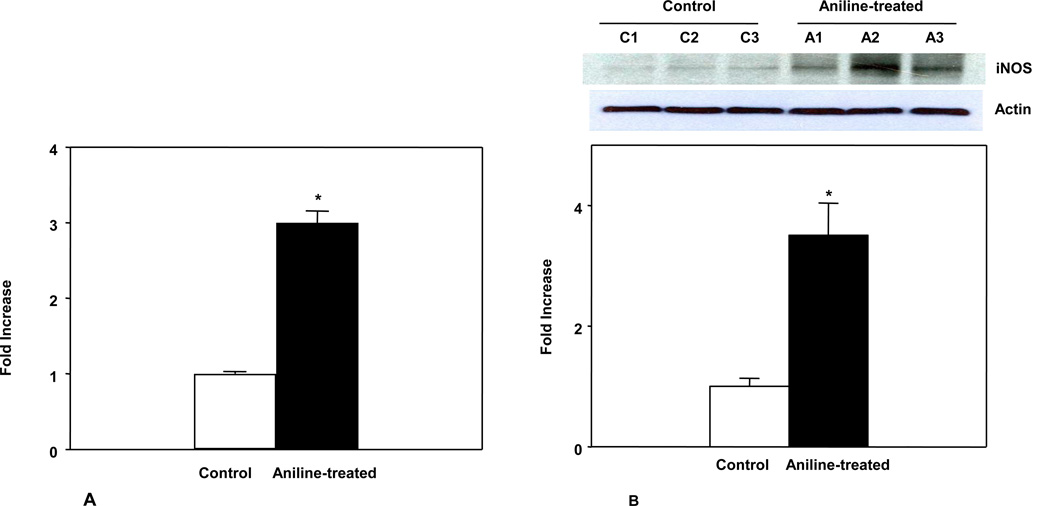
iNOS catalyzes formation of prooxidant •NO. Thus, it could be a major enzyme contributing to nitrosative stress in the spleen. Therefore, we analyzed both mRNA and protein expression of iNOS using real time PCR and Western blot, respectively. As shown in Fig. 3A and 3B, aniline exposure resulted in significant up-regulation of iNOS mRNA and protein expression. The iNOS mRNA and protein expression increased by 3.0 fold (p<0.05) and 3.5 fold (p<0.05), respectively. The results support that increased iNOS expression contributes to increased protein tyrosine nitration following aniline exposure as observed in proteomic analysis.
Fig. 3.
iNOS mRNA expression in the spleens of control and aniline-treated rats (A), and iNOS protein, as analyzed by Western blot, in the spleens of control and aniline-treated rats (B). Values are mean ± SD. * p<0.05.
Discussion
In the current study, we combined proteomic and Western blot approaches for the identification of nitrated proteins in the spleen. Aniline exposure led to formation of twenty five nitrated proteins which were found only in aniline-exposed rats. The results thus support the earlier findings that aniline exposure elicits increased tyrosine nitration of proteins (Khan et al., 2003). We also found eleven nitrated proteins which were common in both control and aniline-treated rats. The findings serve as a reference for nitrated proteins under normal conditions and provide a global map of the modified proteins in the spleen under nitrosative stress following aniline exposure. We also observed that both mRNA and protein levels of iNOS were elevated in spleens of the aniline-treated rats, as compared to controls. These findings suggest that aniline-induced up-regulation of iNOS in the spleen contributes to increased protein nitration.
In this study, vinculin (a skeletal protein) was one of the identified nitrated proteins in the spleen following aniline exposure. Overexpression of vinculin in tumor cells lead to suppression of the tumorigenicity (Rodriguez Fernandez et al., 1992). The function of vinculin is also associated with cell apoptosis; knocking down expression of vinculin makes the cells resistant to apoptosis. The tyrosine residue (Tyr822) plays a key role in maintaining normal function of vinculin. Vinculin mutation (Tyr822Phe) resulted in the loss of its normal functional role in cell apoptosis (Ziegler et al., 2006). Therefore, nitration of tyrosine residues of vinculin could perturb its normal function since tyrosine nitration changes the structure and interferes in the tyrosine phosphorylation. Recently, nitrated vinculin was also detected in patients with amyotrophic lateral sclerosis (ALS) and mouse models of familial ALS (Nardo, et al., 2009). It was suggested that nitration of vinculin may play a role in the disease pathogenesis. In addition, the other skeletal proteins, such as ezrin, moesin and ARP3 were also found to be nitrated following aniline exposure in this study. Functional defects in these proteins cause abnormal cellular functions and/or are associated with diseases such as cancer (Crepaldi et al., 1997; Kanski et al., 2005; Hong et al., 2007; Wegner et al., 2008; Zheng et al., 2008; Brambilla et al., 2009; Shaffer et al., 2010). Therefore, further studies on the nitrated skeletal proteins, which were evident following aniline exposure, will open a new avenue for understanding their roles in the splenic toxicity and/or splenic function.
Among the identified nitrated chaperone proteins following aniline exposure, Hsp90 is an evolutionarily conserved molecular chaperone involved in the folding, stabilization, activation and assembly of the partner proteins which include kinases, transcription factors, signaling molecules, and many other proteins (Hahn, 2009). The nitration of Hsp90 has been reported to decrease its chaperone activity in vitro (Crow et al., 1998; Ye et al., 2007), and nitrated Hsp90 was also detected in many diseases such as ALS (Basso et al., 2009), coronary heart diseases in human (Paier et al., 2009), and hypertensive pulmonary arteries of fetal lambs (Konduri et al., 2006). These findings thus support a potential link among aniline-induced nitration of Hsp90, its functional loss and involvement in splenic toxicity. The other chaperones, Hsp70 protein 5 (GRP78, BIP), (Li et al., 2006; Fu et al., 2006; Lee, 2007; Gonzalez-Gronow et al., 2009), (Hsc70) (Rajapandi et al., 2000; Sultana et al., 2007; Fu et al., 2009) and T-complex protein 1 (Pucciarelli et al., 2006) have also been reported to be involved in many cellular activities. The consequences of nitration on these proteins, as observed in this study, are not known so far. Illustrating the role of these proteins in mediating the toxicity of aniline deserves further investigation.
Transferrin is an iron tansportor which primarily functions in binding and transporting free iron in a redox-inactive form. The two tyrosine residues, Tyr-95 and Tyr-188, are present in the iron binding active site (Gomme and McCann, 2005), and nitration of the two tyrosines of apotransferrin abolishes the binding activity in vitro (Tsao et al., 1974). Increased nitration of transferrin tyrosines in our study thus provides a potential mechanism for iron accumulation and exacerbated oxidative damage in the spleen following aniline exposure in our earlier studies (Khan et al., 1999b). Nitrated transferrin has also been found in patients with lung cancer (Pignatelli et al., 2001), acute respiratory distress syndrome (ARDS) (Gole et al., 2000) and aging rat cardiac tissues (Kanski et al., 2005). It would be interesting to establish a cause-and-effect relationship of transferrin nitration and iron-overload related pathologies.
Our data also showed increased nitration of selenium binding protein 2 (SBP2) which is a protein necessary for incorporation of selenium into the selenoproteins (Duntas, 2006). It is believed that SBP2 plays a role in fibrosis and in the anti-carcinogenesis (Mattow et al., 1986; Milner, 1986; Chu et al., 2004). To our knowledge, this is first report for aniline-induced SBP2 tyrosine nitration. Further investigation of the perturbation in the function of the nitrated SBP2 could present new avenue for a clear understanding of its contributions to the splenic fibrosis and/or fibrosarcomas.
Fibrinogen is a soluble plasma protein which plays a major role in clotting. The nitrated form of fibrinogen has been shown to polymerize faster than native protein both in vitro and in vivo (Vadseth et al., 2004; Peluffo et al., 2007; Parastatidis et al., 2008). Nitrated fibrinogen was also found to be increased in lung cancer patient and coronary artery diseases (Pignatelli et al., 2001; Peluffo and Radi, 2007). These findings support that nitrated fibrinogen may contribute to the vascular diseases, including vascular congestion, fibrosis and sarcomas of the spleen and following aniline exposure (Bus and Popp, 1987; Khan et al., 1999a, 1999b).
Among the nitrated proteins, the enzyme enolase is a key enzyme which catalyzes the conversation of 2-phosphoglycerate to phosphoenolpyruvate in the glycolysis pathway. Growing evidences also show enolase is a plasminogen receptor on the surface of variety of hematopoetic, epithelial and endothelial cells, and a heat shock protein, and also plays a role in cancer, inflammation and autoimmune disorders (Pancholi et al., 2001). It is also a target protein for oxidation. The nitration of alpha-enolase causes its inactivation as found in cardiac proteins of diabetic rats (Lu et al., 2010). These findings thus implicate a possible role for nitrated enolase in the pathological process.
ATP synthase/ ATPase play a key role in biological energy metabolism, and ATP is crucial for functioning of all living cells. ATP synthase/ATPase activity has been shown to be inhibited by tyrosine nitration (Dorgan et al., 1981; Lam et al., 2009; Fujisawa et al., 2009; Haynes et al., 2010), suggestting that nitration of ATPase in aniline-exposed spleen would contribute to an energy metabolic disorder. In addition, nitration of the other enzymes, such as glutamine synthetase, isocitrate dehydrogenase, glutamate dehydrogenase, ATIC, ALDH and long-chain specific acyl-coA dehydrogenase, as observed in this study, could also result in their dysfunctions, resulting in the enzymes’ inactivity (Smith et al., 1970; Berlett et al., 1996; Ma et al., 2000; Lee et al., 2003; Gorg et al., 2007; Boccalatte et al., 2009; Bhattacharjee et al., 2009).
The signaling pathway protein, Annexin A1, plays multiple roles in inflammation pathways, cell proliferation, regulation of cell death signaling, phagocytic clearance of apoptosing cells and, more importantly, in carcinogenesis (Lim et al., 2007). Annexin A1 is also a selective surface marker of the vascular endothelium in several solid tumors (Oh et al., 2004; Gerke et al., 2005). The other Annexin family member, Annexin A3, is also associated with many cancers (Köllermann, et al., 2008; Liu et al., 2008; Yan et al., 2010; Jung et al., 2010). The nitration of Annexin A1/A3 have been found in lung cancer (Masri et al., 2005), which imply the involvement of nitrated Annexin A1/A3 in the pathogenesis. Although the functional consequences of Annexin A1/A3 nitration are unknown, nitration of these proteins, as observed in this study, necessitates further studies to explore their contribution in the pathogenesis of splenic carcinoma following aniline exposure. Similar to other nitrated proteins, nitration of eEF2, angiomotin, lymphocyte cytosolic protein 1 and serum albumin, as observed in this study, also necessitates further studies to illustrate the significance of nitration of these proteins in aniline-induced splenic toxicity.
In conclusion, our study shows that aniline exposure results in significantly increased formation of nitrated protein in the spleen. Twenty five nitrated proteins were identified only in aniline-treated rats. The nitrated proteins represented skeletal proteins, chaperones, ferric iron transporter, protein transporter, enzymes, signaling pathways and protein synthesis. Our data also show that eleven nitrated proteins were present in both control and aniline-treated rats. Most of the identified nitrated proteins did not show significant differences in protein expression variation as compared to the controls, suggesting tyrosine modifications at the post-translational level. The nitrosative modification of proteins in the current study not only support our earlier findings (Khan et al., 2003) but also suggest a potential role of nitrosative stress in the splenic toxicity of aniline. Understanding the functional consequences of nitration of the proteins will be helpful in elucidating the role of nitrosative stress in the splenic toxicity.
Highlights.
Proteomic approaches are used to identify nitrated proteins in the spleen of aniline exposed rats.
Twenty five nitrated proteins were identified only in the spleen of aniline-treated rats in the range of 27.7 to 123.6 kDa.
Aniline exposure led to significantly increased iNOS mRNA and protein expression in the spleen.
Results are highly supportive for a role of nitrated proteins/nitrosative stress in splenic toxicity of aniline.
Acknowledgments
The work was supported By Grant ES006476 from the National Institute of Environmental Health Sciences (NIEHS), NIH, and it contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. Authors also thank Dr. Robert D. English in the UTMB Mass Spectrometry Core of Biomolecular Resource Facility (Supported by NHLBI Proteomics Center Grant N01-HV-00245 to Dr. A. Kurosky) for assistance with MALDI TOF/TOF MS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there is no conflict of interest.
References
- Abello N, Kerstjens HA, Postma DS, Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J. Proteome Res. 2009;8:3222–3238. doi: 10.1021/pr900039c. [DOI] [PubMed] [Google Scholar]
- Basso M, Samengo G, Nardo G, Massignan T, D'Alessandro G, Tartari S, Cantoni L, arino M, Cheroni C, De BS, Giordana MT, Strong MJ, Estevez AG, Salmona M, Bendotti C, Bonetto V. Characterization of detergent-insoluble proteins in ALS indicates a causal link between nitrative stress and aggregation in pathogenesis. PLoS ONE. 2009;4:e8130. doi: 10.1371/journal.pone.0008130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf D, Babel W. Assimilatory detoxification of herbicides by Delftia acidovorans MC1: induction of two chlorocatechol 1, 2-dioxygenases as a response to chemostress. Microbiology. 2002;148:2883–2888. doi: 10.1099/00221287-148-9-2883. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Friguet B, Yim MB, Chock PB, Stadtman ER. Peroxynitrite-mediated nitration of tyrosine residues in Escberichiu coli glutamine synthetase mimics adenylation: relevance to signal transduction. Proc. Natl. Acad. Sci. USA. 1996;93:1776–1780. doi: 10.1073/pnas.93.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Majumdar U, Maity D, Sarkar TS, Goswami AM, Sahoo R, Ghosh S. In vivo protein tyrosine nitration in S. cerevisiae: identification of tyrosine-nitrated proteins in mitochondria. Biochem. Biophys. Res. Commun. 2009;388:612–617. doi: 10.1016/j.bbrc.2009.08.077. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Doe WF, Buffinton GD. Protein carbonyl formation on mucosal proteins in vitro and in dextran sulfate-induced colitis. Free Radic. Biol. Med. 1999;27:262–270. doi: 10.1016/s0891-5849(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Boccalatte FE, Voena C, Riganti C, Bosia A, D'Amico L, Riera L, Cheng M, Ruggeri B, Jensen ON, Goss VL, Lee K, Nardone J, Rush J, Polakiewicz RD, Comb MJ, Chiarle R, Inghirami G. The enzymatic activity of 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase is enhanced by NPM-ALK: new insights in ALK-mediated pathogenesis and the treatment of ALCL. Blood. 2009;113:2776–2790. doi: 10.1182/blood-2008-06-161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla D, Fais S. The Janus-faced role of ezrin in "linking" cells to either normal or metastatic phenotype. Int. J. Cancer. 2009;25:2239–2245. doi: 10.1002/ijc.24734. [DOI] [PubMed] [Google Scholar]
- Bus JS, Popp JA. Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally related compounds. Food Chem. Toxicol. 1987;25:619–626. doi: 10.1016/0278-6915(87)90024-x. [DOI] [PubMed] [Google Scholar]
- Chu R, Lim H, Brumfield L, Liu H, Herring C, Ulintz P, Reddy JK, Davison M. Protein profiling of mouse livers with peroxisome proliferator-activated receptor alpha ctivation. Mol. Cell. Biol. 2004;24:6288–6297. doi: 10.1128/MCB.24.14.6288-6297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JP, Sampson JB, Beckman JS. In: Frontiers in Cerebrovascular Disease: Mechanisms, Diagnosis, and Treatment. Robertson J, Nowak T, editors. Armonk, NY: Futura Publishing Company, Inc.; 1998. pp. 315–337. [Google Scholar]
- Dalle-Done I, Rossi R, Colombo R, Giustarni D, Milzani A. Biomarkers of xidative damage in human disease. Clin. Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Scaloni A, Giustarini D, Cavarra E, Tell G, Lungarella G, Colombo R, Rossi R, Milzani A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectrom Rev. 2005;24:55–99. doi: 10.1002/mas.20006. [DOI] [PubMed] [Google Scholar]
- Di Girolamo F, Campanella L, Samperi R, Bachi A. Mass spectrometric dentification of hemoglobin modifications induced by nitrosobenzene. Ecotoxicol. Environ. Saf. 2009;72:1601–1608. doi: 10.1016/j.ecoenv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Dorgan LJ, Schuster SM. The effect of nitration and D2O on the kinetics of beef heart mitochondrial adenosine triphosphatase. J. Biol. Chem. 1981;256:3910–3916. [PubMed] [Google Scholar]
- Duntas LH. The role of selenium in thyroid autoimmunity and cancer. Thyroid. 2006;6:455–460. doi: 10.1089/thy.2006.16.455. [DOI] [PubMed] [Google Scholar]
- Fries DM, Paxinou E, Themistocleous M, Swanberg E, Griendling KK, Salvemini D, Slot JW, Heijnen HF, Hazen SL, Ischiropoulos HJ. Biol. Chem. 2003;278:22901–22907. doi: 10.1074/jbc.M210806200. [DOI] [PubMed] [Google Scholar]
- Fu QY, Gao YQ. Screening of AMP-activated protein kinase alpha2 subunit interacting proteins by bacterial two-hybrid system. Mol. Biol. Rep. 2007;36:337–344. doi: 10.1007/s11033-007-9184-1. [DOI] [PubMed] [Google Scholar]
- Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol. Ther. 2006;5:741–744. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Kato K, Giulivi C. Nitration of tyrosine residues 368 and 345 in the β-subunit elicits FoF1-ATPase activity loss. J. Biochem. 2009;423:219–231. doi: 10.1042/BJ20090594. [DOI] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2 signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF, III, Finkel B, Lanken PN. Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am. J. Physiol. 2000;278:L961–L967. doi: 10.1152/ajplung.2000.278.5.L961. [DOI] [PubMed] [Google Scholar]
- Gomme PT, McCann KB, Bertolini J. Transferrin: structure, function and potential therapeutic actions. Drug Discov. Today. 2005;10:267–273. doi: 10.1016/S1359-6446(04)03333-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gronow M, Selim MA, Papalas J, and Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid. Redox Signal. 2009;11:2299–2306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- Goodman DG, Ward JM, Reichardt WD. Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4’-sulfonyldianiline, or D & C red No. 9. J. Natl. Cancer Inst. 1984;63:37–122. [PubMed] [Google Scholar]
- Görg B, Qvartskhava N, Voss P, Grune T, Häussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- Greenacre SA, Ischiropoulos H. Tyrosine nitration: localization, quantitation, consequences for protein function and signal transduction. Free Radic. Res. 2001;34(l):541–581. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- Hahn JS. The Hsp90 chaperone machinery: from structure to drug development. BMB Rep. 2009;42:623–630. doi: 10.5483/bmbrep.2009.42.10.623. [DOI] [PubMed] [Google Scholar]
- Harrison JH, Jr, Jollow DJ. Contribution of aniline metabolites to aniline induced methemoglobinemia. Mol. Pharmacol. 1987;32:423–431. [PubMed] [Google Scholar]
- Haynes V, Traaseth NJ, Elfering S, Fujisawa Y, Giulivi C. Nitration of specific tyrosines in FoF1 ATP synthase and activity loss in aging. Am. J. Physiol. Metab. 2010;298:E978–E987. doi: 10.1152/ajpendo.00739.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Gokulrangan G, Schoneich C. Proteomic analysis of age dependent nitration of rat cardiac proteins by solution isoelectric focusing coupled to nanoHPLC tandem massometry. Exp. Gerontol. 2007;42:639–651. doi: 10.1016/j.exger.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins FP, Robinson JA, Gellatly JBM, Salmond GWA. The no-effect dose of aniline in human subjects and a comparison of aniline toxicity in man and the rat. Food Cosmet. Toxicol. 1972;10:671–679. doi: 10.1016/s0015-6264(72)80147-0. [DOI] [PubMed] [Google Scholar]
- Jung EJ, Moon HG, Park ST, Cho BI, Lee SM, Jeong CY, Ju YT, Jeong SH, Lee YJ, Choi SK, Ha WS, Lee JS, Kang KR, Hong SC. Decreased annexin A3 expression correlates with tumor progression in papillary thyroid cancer. Proteomics Clin. Appl. 2010;4:528–537. doi: 10.1002/prca.200900063. [DOI] [PubMed] [Google Scholar]
- Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3- nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H371–H38. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- Kanski J, Hong SJ, Schoneich C. Proteomic analysis of protein nitration in aging skeletal muscle identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. J. Biol. Chem. 2005;280:24261–24266. doi: 10.1074/jbc.M501773200. [DOI] [PubMed] [Google Scholar]
- Khan MF, Gu Y, Alcock NW, Boor PJ, Ansari GAS. Oxidative Stress in splenotoxicity of aniline. Fundam. Appl. Toxicol. 1997;35:22–30. doi: 10.1006/faat.1996.2259. [DOI] [PubMed] [Google Scholar]
- Khan MF, Kannan S, Wang J. Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2006;210:86–93. doi: 10.1016/j.taap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Khan MF, Kaphalia BS, Boor PJ, Ansari GAS. Subchronic toxicity of aniline hydrochloride in rats. Arch. Environ. Contam. Toxicol. 1993;24:368–374. doi: 10.1007/BF01128736. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Alcock NW, Boor PJ, Ansari GAS. Iron exacerbates aniline-associated splenic toxicity. J. Toxicol. Environ. Health. 1999b;57:173–184. doi: 10.1080/009841099157746. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Ansari GAS, Boor PJ. Malondialdehyde-protein adducts in the spleens of aniline-treated rats: Immunohistochemical detection and localization. J. Toxicol. Environ. Health, Part A. 2003a;66:93–102. doi: 10.1080/15287390306464. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Boor PJ, Ansari GAS. Oxidative modification of proteins and lipids in aniline-induced splenic toxicity. Toxicol. Sci. 1999a;48:134–140. doi: 10.1093/toxsci/48.1.134. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Boor PJ, Ansari GAS. Nitrotyrosine formation in splenic toxicity of aniline. Toxicology. 2003b;194:95–102. doi: 10.1016/j.tox.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Köllermann J, Schlomm T, Bang H, Schwall GP, von Eichel-Streiber C, Simon RS, Schostak M, Huland H, Berg W, Sauter G, Klocker H, Schrattenholz A. Expression and prognostic relevance of annexin A3 in prostate cancer. Eur. Urol. 2008;54:1314–1323. doi: 10.1016/j.eururo.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Konduri GG, Bakhutashvili I, Eis A, Pritchard KA. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1812–H1820. doi: 10.1152/ajpheart.00425.2006. [DOI] [PubMed] [Google Scholar]
- Lam PY, Yin F, Hamilton RT, Boveris A, Cadenas E. Elevated neuronal nitric oxide synthase expression during ageing and mitochondrial energy production. Free Radic. Res. 2009;43:431–439. doi: 10.1080/10715760902849813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yang ES, Park JW. Inactivation of NADP+-dependent Isocitrate Dehydrogenase by Peroxynitrite, implication for cytotoxicity and alcohol-induced liver injury. J. Biol. Chem. 2003;278:51360–51371. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr. Mol. Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- Liu YF, Xiao ZQ, Li MX, Li MY, Zhang P, Li C, Li F, Chen YH, Yi H, Yao HX, Chen ZC. Quantitative proteome analysis reveals annexin A3 as a novel biomarker in lung adenocarcinoma. J. Pathol. 2009;217:54–64. doi: 10.1002/path.2429. [DOI] [PubMed] [Google Scholar]
- Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol. Cell. 2000;92:305–316. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- Lu N, Zhang Y, Li H, Gao Z. Oxidative and nitrative modifications of alpha-enolase in cardiac proteins from diabetic rats. Free Radic. Biol. Med. 2010;48:873–881. doi: 10.1016/j.freeradbiomed.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Ma H, Wang J, Abdel-Rahman SZ, Boor PJ, Khan MF. Oxidative DNA damage and its repair in rat spleen its repair in rat spleen following subchronic exposure to aniline. Toxicol. Appl. Pharmacol. 2008;233:247–253. doi: 10.1016/j.taap.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Wang J, Abdel-Rahman SZ, Hazra TK, Boor PJ, Khan MF. Induction of NEIL1 and NEIL2 DNA glycosylases in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2011;251:1–7. doi: 10.1016/j.taap.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Cools J, Marynen P, Cui X, Siebert R, Gesk S, Schlegelberger B, Peeters B, De Wolf-Peeters C, Wlodarska I, Morris SW. Inv(2)(p23q35) in anaplastic large-cell lymphoma induces constitutive anaplastic lymphoma kinase (ALK) tyrosine kinase activation by fusion to ATIC, an enzyme involved in purine nucleotide biosynthesis. Blood. 2000;95:2144–2149. [PubMed] [Google Scholar]
- Masri FA, Comhair SA, Koeck T, Stuehr DJ, Xu W, Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, Erzurum SC, Aulak KS. Abnormalities in nitric oxide and its derivatives in lung cancer. Am. J. Respir. Crit. Care Med. 2005;172:597–605. doi: 10.1164/rccm.200411-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattow J, Demuth I, Haeselbarth G, Jungblut PR. Klose Selenium-binding protein 2, the major hepatic target for acetaminophen, shows sex differences in protein abundance. J. Electrophoresis. 2006;27:1683–1691. doi: 10.1002/elps.200500703. [DOI] [PubMed] [Google Scholar]
- Milner JA. Inhibition of chemical carcinogenesis and tumorigenesis by selenium. Adv. Exp. Med. Biol. 1986;206:449–463. doi: 10.1007/978-1-4613-1835-4_32. [DOI] [PubMed] [Google Scholar]
- Miyagi M, Sakaguchi H, Darrow RM, Yan L, West KA, Aulak KS, Stuehr DJ, Hollyfield JG, Organisciak DT, Crabb JW. Evidence that light modulates protein nitration in rat retina. Mol. Cell. Proteomics. 2002;1:293–303. doi: 10.1074/mcp.m100034-mcp200. [DOI] [PubMed] [Google Scholar]
- Monteiro HP, Arai RJ, Travassos LR. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid. Redox Signal. 2008;10:843–890. doi: 10.1089/ars.2007.1853. [DOI] [PubMed] [Google Scholar]
- Moon SK, Thompson LJ, Madamanchi N, Ballinger S, Papaconstantinou J, Horaist C, Runge MS, Patterson C. Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2779–H2788. doi: 10.1152/ajpheart.2001.280.6.H2779. [DOI] [PubMed] [Google Scholar]
- Nardo G, Pozzi S, Mantovani S, Garbelli S, Marinou K, Basso M, Mora G, Bendotti C, Bonetto V. Nitroproteomics of peripheral blood mononuclear cells from patientsand a rat model of ALS. Antioxid. Redox Signal. 2009;11:1559–1567. doi: 10.1089/ars.2009.2548. [DOI] [PubMed] [Google Scholar]
- Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of theendothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paier A, Agewall S, Kublickiene K. Expression of heat shock proteins and nitrotyrosine in small arteries from patients with coronary heart disease. Heart Vessels. 2009;24:260–266. doi: 10.1007/s00380-008-1117-y. [DOI] [PubMed] [Google Scholar]
- Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell. Mol. Life Sci. 2001;58:902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parastatidis I, Thomson L, Burke A, Chernysh I, Nagaswami C, Visser J, Stamer S, Liebler DC, Koliakos G, Heijnen HF, Fitzgerald GA, Weisel JW, Ischiropoulos H. Fibrinogen beta-chain tyrosine nitration is a prothrombotic risk factor. J. Biol. Chem. 2008;283:33846–33853. doi: 10.1074/jbc.M805522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J. Subacute inhalation toxicity of aniline in rats: analysis of timedependence and concentration-dependence of hematotoxic and splenic effects. Toxicol. Sci. 2004;81:198–215. doi: 10.1093/toxsci/kfh187. [DOI] [PubMed] [Google Scholar]
- Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascularpathology. Cardiovasc. Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Pignatelli B, Li CQ, Boffetta P, Chen Q, Ahrens W, Nyberg F, Mukeria A, Bruske-Hohlfeld I, Fortes C, Constantinescu V, Ischiropoulos H, Ohshima H. Nitrated and oxidized plasma proteins in smokers and lung cancer patients. Cancer Res. 2001;61:778–784. [PubMed] [Google Scholar]
- Prieels JP, Dolmans M, Leonis J, Brew K. Nitration of tyrosyl residues in human alpha-lactalbumin. Effect on Lactose Synthase Specifier Activity. Eur. J. Biochem. 1975;60:533–539. doi: 10.1111/j.1432-1033.1975.tb21032.x. [DOI] [PubMed] [Google Scholar]
- Pucciarelli S, Parker SK, Detrich HW, III, Melki R. Characterization of the cytoplasmic chaperonin containing TCP-1 from the Antarctic fish Notothenia coriiceps. Extremophiles. 2006;10:537–549. doi: 10.1007/s00792-006-0528-x. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Nat. Acad. Sci. USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapandi T, Greene LE, Eisenberg E. The molecular chaperones hsp90 and hsc70 are both necessary and sufficient to activate hormone binding by glucocorticoid receptor. J. Biol. Chem. 2000;275:22597–22604. doi: 10.1074/jbc.M002035200. [DOI] [PubMed] [Google Scholar]
- Rodriguez Fernandez JL, Geiger B, Salomon D, Sabanay I, Zoller M, Ben Ze'ev A. Suppression of tumorigenicity in transformed cells after transfection with vinculin cDNA. J. Cell Biol. 1992;119:427–438. doi: 10.1083/jcb.119.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer FJ, Baker PR, Freeman BA. NO-dependent protein nitration: a cell-signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, Freedman BD, Burkhardt JK. Ezrin and moesin function together to promote T cell activation. J. Immunol. 2009;182:1021–1032. doi: 10.4049/jimmunol.182.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, O’Gorman M, Becker S, Auer G, Eklund A, Grunewald J, Wheelock A. In the Eye of the Beholder: Does the Master See the SameSpots as the Novice? J. Proteome. 2010;9:1522–1532. doi: 10.1021/pr9010298. [DOI] [PubMed] [Google Scholar]
- Smith EJ, Landon M, Piszkiewicz D, Brattin WJ, Langley TJ, Melamed MD. Bovine liver glutamate dehydrogenase: tentative amino acid sequence; identification of a reactive lysine; nitration of a specific tyrosine and loss of allosteric inhibition by guanosine triphosphate. Proc. Natl Acad. Sci. USA. 1970;67:724–730. doi: 10.1073/pnas.67.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Reed T, Perluigi M, Coccia R, Pierce WM, Butterfield DA. Proteomic identification of nitrated brain proteins in amnestic mild cognitive impairment: a regional study. J. Cell Mol. Med. 2007;11:839–851. doi: 10.1111/j.1582-4934.2007.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao D, Azari P, Philips J. On the structure of ovotransferrin, III. Nitration of iron-ovotransferrin and distribution of tyrosines involved in iron-binding activity. Biochemistry. 1974;13:408–413. doi: 10.1021/bi00700a003. [DOI] [PubMed] [Google Scholar]
- Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, Muzykantov V, Penn MS, Hazen SL, Weisel JW, Ischiropoulos H. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J. Biol. Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- Wang J, Kannan S, Li H, Khan MF. Cytokine gene expression and activation of NF-κB in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2005;203:36–44. doi: 10.1016/j.taap.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma H, Boor PJ, Ramanujam VMS, Ansari GAS, Khan MF. Up-egulation of heme oxygenase-1 in rat spleen after aniline exposure. Free Radic. Biol. Med. 2010;48:513–518. doi: 10.1016/j.freeradbiomed.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang G, Ansari GAS, Khan MF. Activation of oxidative stress-responsive signaling pathways in early splenotoxic response of aniline. Toxicol. Appl. Pharmacol. 2008;230:227–234. doi: 10.1016/j.taap.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang G, Ma H, Khan MF. Enhanced expression of cyclins and cyclin-dependent kinases in aniline-induced cell proliferation in rat spleen. Toxicol. Appl. Pharmacol. 2011;250:213–220. doi: 10.1016/j.taap.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Liu HR, Tao L, Liang F, Yan L, Zhao RR, Lopez BL, Christopher TA, Ma XL. Role of iNOS-derived reactive nitrogen species and resultant nitrative stress in leukocytes-induced cardiomyocyte apoptosis after myocardial leukocytes-induced cardiomyocyte apoptosis after myocardial ischemia/reperfusion. Apoptosis. 2007;12:1209–1217. doi: 10.1007/s10495-007-0055-y. [DOI] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J. Biol. Chem. 2008;283:15912–15920. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger MA, Albert RH, Montgomery SB. Splenotoxicity associated with splenic sarcomas in rats fed high doses of D & C Red No. 9 or aniline hydrochloride. J. Natl. Cancer Inst. 1985;75:681–690. [PubMed] [Google Scholar]
- Wu X, Kannan S, Khan MF. Iron release and oxidative DNA damage in spleen following aniline intoxication. J. Toxicol. Environ. Health, Part A. 2005;68:657–666. doi: 10.1080/15287390590921757. [DOI] [PubMed] [Google Scholar]
- Yan X, Yin J, Yao H, Mao N, Yang Y, Pan L. Increased Expression of Annexin A3 Is a Mechanism of Platinum Resistance in Ovarian Cancer. Cancer res. 2010;70:1616–1624. doi: 10.1158/0008-5472.CAN-09-3215. [DOI] [PubMed] [Google Scholar]
- Ye Y, Quijano C, Robinson KM, Ricart KC, Strayer AL, Sahawneh MA, Shacka JJ, Kirk M, Barnes S, Accavitti-Loper MA, Radi R, Beckman JS, Estevez AG. Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J. Biol. Chem. 2007;282:6324–6337. doi: 10.1074/jbc.M610800200. [DOI] [PubMed] [Google Scholar]
- Zheng HC, Zheng YS, Li XH, Takahashi H, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Arp2/3 overexpression contributed to pathogenesis growth and invasion of gastric carcinoma. Anticancer Res. 2008;28:2225–2232. [PubMed] [Google Scholar]
- Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]