Abstract
We have used functional co-reconstitution of purified sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA) with phospholamban (PLB), its inhibitor in the heart, to test the hypothesis that loss-of-function (LOF) PLB mutants (PLBM) can compete with wild-type PLB (PLBW) to relieve SERCA inhibition. Co-reconstitution at varying PLB/SERCA ratios was conducted using synthetic PLBW, gain-of-function mutant I40A, or loss-of-function (LOF) mutants S16E (phosphorylation mimic) or L31A. Inhibitory potency was defined as the fractional increase in KCa, measured from the Ca2+-dependence of ATPase activity. At saturating levels of these PLB variants, the inhibitory potency of I40A was about three times that of PLBW, while the potency of each of the LOF PLBM was about one third that of PLBW. However, there was no significant variation in the apparent SERCA affinity for these four PLB variants. When SERCA was co-reconstituted with mixtures of PLBW and LOF PLBM, inhibitory potency was reduced relative to that of PLBW alone. Furthermore, FRET between donor-labeled SERCA and acceptor-labeled PLBW was decreased by both (unlabeled) LOF PLBM. These results show that LOF PLBM can compete both physically and functionally with PLBW, provide a rational explanation for the success of S16E-based gene therapy in animal models of heart failure, and establish a powerful platform for designing and testing more effective PLBM targeted for gene therapy of heart failure in humans.
Keywords: Ca2+-ATPase, SERCA, fluorescence resonance energy transfer, FRET
1. Introduction
Muscle relaxation occurs when the sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA) hydrolyzes ATP and pumps Ca2+ from the sarcoplasm back into the SR against its concentration gradient. In the heart, SERCA activity is regulated by phospholamban (PLB), a single-span membrane protein that functions to inhibit SERCA by decreasing its apparent Ca-affinity (increasing KCa) [1]. PLB inhibition of SERCA is relieved physiologically either by micromolar Ca2+ or by phosphorylation of PLB at Ser 16 by PKA [2–4], and can also be relieved by a number of point mutations [5], including S16E (a cytoplasmic domain mutation that partially mimics phosphorylation [6]) and L31A (a transmembrane domain mutation [5]).
Insufficient SERCA activity is a hallmark of heart failure (HF), which is a leading cause of hospitalization and death in most parts of the world [7], and overexpression of SERCA, using recombinant AAV vectors, has been shown to relieve heart failure in clinical trials [8]. HF is associated with an increased ratio of PLB to SERCA [9], so the inhibitory interaction between SERCA and PLB has become an attractive therapeutic target [2]. Indeed, interfering with the SERCA-PLB interaction in HF animal models can result in improved cardiac function [10–14]. However, complete ablation of PLB can lead to premature death in humans [15], suggesting that a more subtle approach is needed.
Relief of PLB-dependent SERCA inhibition, whether by micromolar Ca2+, PLB phosphorylation, or functional mutation in PLB, has long been thought to require dissociation of the PLB-SERCA complex (Fig. 1A), a hypothesis supported primarily by cross-linking studies [16, 17]. However, this hypothesis is inconsistent with measurements of fluorescence resonance energy transfer (FRET) from SERCA to PLB that demonstrated no Ca2+-dependence of SERCA-PLB affinity [18] and with intra-PLB FRET and electron paramagnetic resonance (EPR) studies that showed no SERCA-PLB dissociation by either Ca2+ or phosphorylation of PLB at S16 [19, 20], suggesting that PLB remains bound upon SERCA activation (Fig. 1B). In addition, several loss-of-function (LOF, with less inhibitory potency than WT) PLB mutants have been shown to retain at least some SERCA binding affinity [6, 21, 22]. These results suggest that it might be feasible to identify a LOF PLB mutant (denoted PLBM below) with sufficient SERCA affinity to compete with WT (denoted PLBW below) for SERCA binding, and that such a mutant would be a useful therapeutic reagent. Intriguingly, in vivo cardiac rAAV delivery of a gene corresponding to S16E, a pseudophosphorylated PLB mutant, prevents HF occurrence or progression in small and large animal models [12, 14, 23]. To understand the molecular basis of the therapeutic effectiveness of S16E, we previously studied its structural dynamics, showing that the S16E mutation does not abolish SERCA binding, but it partially unfolds the cytoplasmic domain of PLB (detected by EPR and NMR) [6, 22], almost as much as is caused by phosphorylation at S16 [20, 22, 24]. We suggest that S16E can relieve SERCA inhibition by competing with PLBW for SERCA binding.
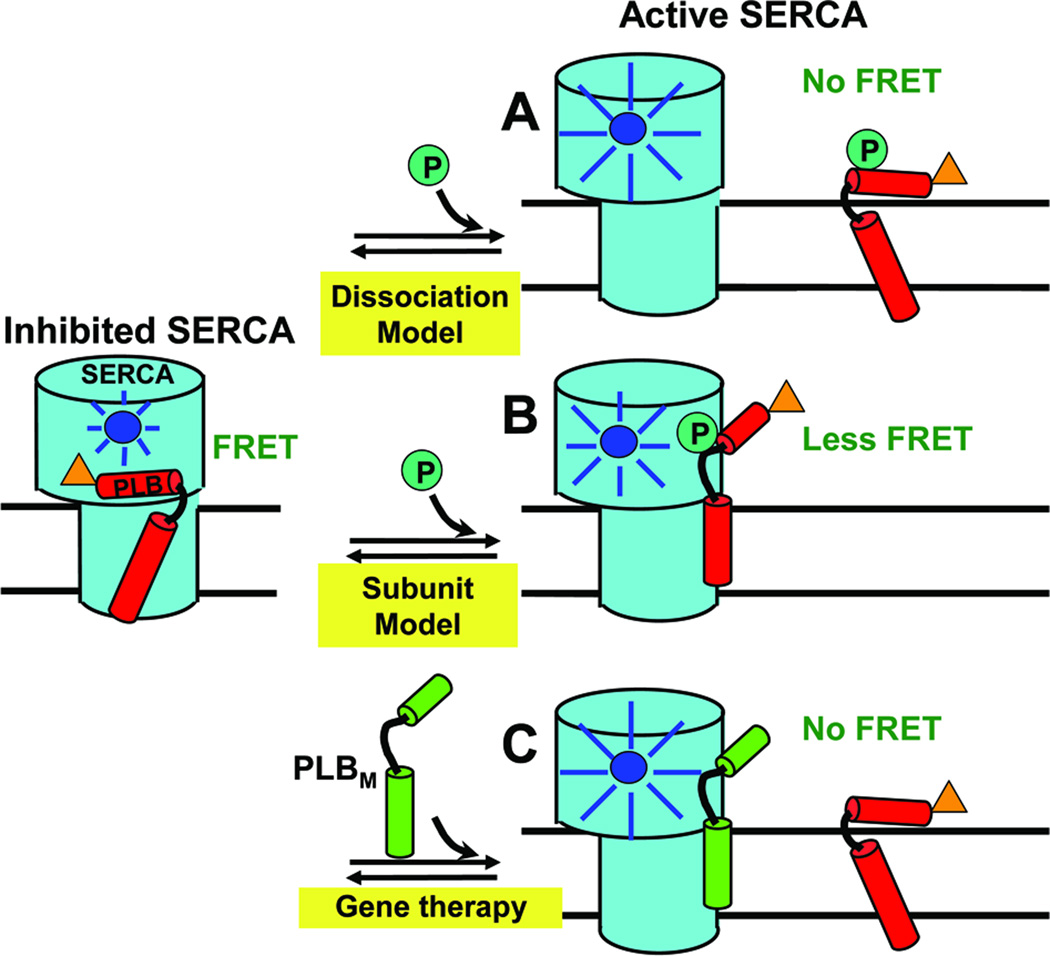
Fig. 1. Models for relief of SERCA inhibition.
Left: PLBW binding to SERCA is detected when the fluorescence of donor (blue) on SERCA is quenched by the acceptor (orange) on PLB via FRET. (A) In the Dissociation Model, loss of function (e.g., due to phosphorylation) requires dissociation of the SERCA-PLB complex, which would completely eliminate FRET. (B) In the Subunit Model, inhibition can be relieved by a structural rearrangement, without dissociation of the SERCA-PLB complex. (C) For HF gene therapy applications, if a LOF mutant PLBM has affinity for SERCA comparable to that of PLBW, it can compete with PLBW to increase SERCA function. These hypotheses can be tested by FRET.
In the present study, we test this hypothesis directly and quantitatively by performing both FRET and functional assays on reconstituted membranes containing donor-labeled SERCA, acceptor-labeled PLBW, and unlabeled S16E. We ask whether this LOF mutant of PLB can compete with the native WT for SERCA binding, which should reduce both FRET and inhibition (Fig. 1C). We use a similar approach to evaluate the LOF PLB mutant L31A [5] as a phosphorylatable alternative to S16E. The results have important implications for future efforts in gene therapy.
2. Materials and Methods
2.1. SERCA Purification and Labeling
SERCA was purified [25] and labeled with IAEDANS [18] as described previously. To determine the dye concentration in labeled samples, the absorbance (ε334nm = 6100 M-1 cm-1) [26], was taken after treatment with 0.1M NaOH and 1% SDS. Samples of AEDANS-SERCA were flash-frozen and stored in the dark at −80°C until further usage.
2.2. Synthesis, Purification, and Labeling of PLB Mutants
PLB was assembled on Fmoc-Leu-PEG-PS resin by Fmoc chemistry using a PE Biosystems Pioneer™ peptide synthesis system, as previously reported [18]. The N-terminal amino group of unlabeled PLBs was acetylated using acetic anhydride. For FRET, PLBW was labeled at the N-terminus with the non-fluorescent acceptor Dabcyl-SE (denoted Dab-PLBW). Peptide composition and concentration were confirmed by MALDI-TOF and amino acid analysis, and samples were stored in methanol at −20°C.
2.3. Co-reconstitution of SERCA and PLB
SERCA and PLB were co-reconstituted, as previously described [27–29], at 700 lipids/SERCA and various PLB/SERCA ratios. Each sample contained 40µg of SERCA and varying amounts of PLB to obtain molar ratios of 0–20 PLB to SERCA. Ca2+-ATPase activity and FRET measurements were performed immediately after co-reconstitution.
2.4. Ca2+-ATPase Functional Measurements
Functional and FRET measurements were carried out at 25°C. ATPase activity was measured as a function of [Ca2+] in 96-well microtiter plates [28, 30]. The time-dependence of absorbance at 340 nm was measured in a SpectraMaxPlus™ microplate reader (Molecular Devices, Sunnyvale, CA). Data were fitted using the Hill function:
| Equation 1 |
where V is the initial ATPase rate and n is the Hill coefficient. The inhibitory effect of each PLB variant was indicated by the observed increase in the apparent Ca2+ dissociation constant KCa, measured relative to SERCA reconstituted in the absence of PLB.
Based on KCa measured as above, we define inhibitory potency, P, as the % increase in KCa (decrease in apparent Ca2+ affinity) caused by PLB:
| Equation 2 |
where n = PLB/SERCA.
To determine the apparent affinity of each PLB variant for SERCA, P(n) data in Fig. 2B were fitted using the specific binding function:
| Equation 3 |
where Kd is the apparent dissociation constant.
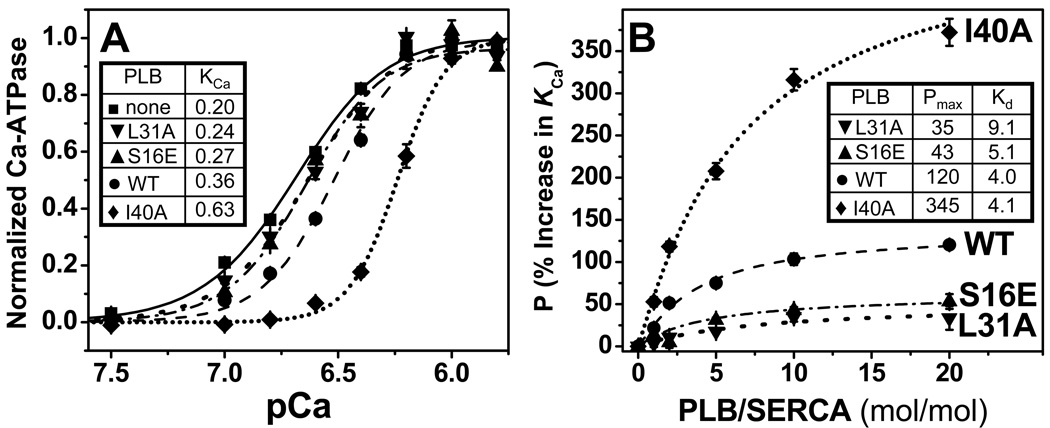
Fig. 2. Effect of PLB mutants on the apparent Ca2+-affinity of SERCA.
(A) Ca2+ dependence of ATPase activity of SERCA reconstituted in lipid in the absence of PLB or in the presence of 5 moles of PLB per mole of SERCA. Activity is normalized relative to Vmax, along with fits using the Hill function (Equation 1), with best-fit KCa (µM) values in inset table. (B) Dependence of PLB inhibitory potency (P, Equation 2) on PLB/SERCA, along with fits to the data using Equation 3, with best-fit Pmax (%) and Kd (PLB/1000 lipids) values in inset table. Each plotted symbol represents mean ± SEM from at least three measurements.
We assume that the effects of a mixture of PLBW with a LOF PLBM (Fig. 3) on the SERCA KCa depends on the relative potencies and affinities of the PLB variants competing for a single inhibitory binding site on SERCA:
| Equation 4 |
where w = PLBW/SERCA; m = PLBM/SERCA; A = Kd(PLBW)/Kd(PLBM).
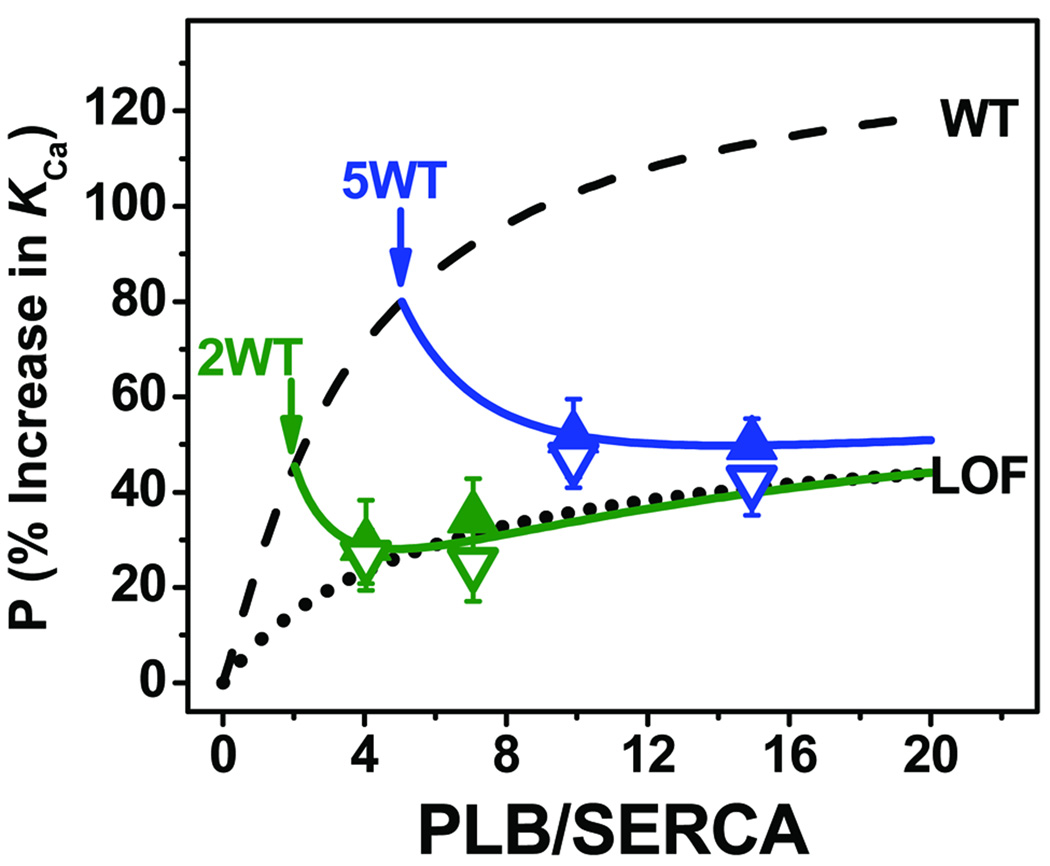
Fig. 3. Functional competition.
Effects of LOF PLBM (S16E solid triangles, L31A open triangles) on inhibitory potency in the presence of 2 (green) and 5 (blue) PLBW/SERCA. Dashed curve: PLBW alone (Fig. 2B). Dotted curve: averaged PLBM profile (Fig. 2B). Solid curves represent fits to the competition data using Equation 4. Each plotted point is the mean ± SEM from at least three measurements.
2.5. Fluorescence Resonance Energy Transfer (FRET) Measurements
Fluorescence emission spectra were acquired using a Gemini EM microplate fluorimeter (Molecular Devices, Sunnyvale, CA) with excitation at 350 nm from a Xenon flash lamp (1 Joule/flash). Samples were plated in triplicate (75 µL per well) on 384-well, black wall, optical bottom well plates (Nalge Nunc International, Rochester, NY). Emission spectra were recorded in triplicate, from 420 nm to 600 nm, with a 2 nm step size, and a 420 nm long-pass emission filter. The fractional decrease in the integrated fluorescence emission of the donor (AEDANS-SERCA) caused by the presence of an acceptor (Dab-PLBW) is defined as the FRET efficiency E = 1 – (FD+A/FD), where FD+A and FD are the fluorescence intensities in the presence and absence of acceptor. If the acceptor-labeled PLB binds to a single specific binding site with a dissociation constant Kd, then
| Equation 5 |
where Emax is the limiting value of E for the SERCA-PLB complex at saturation, and [PLB] and Kd are in 2-dimensional units, PLB per 1000 lipids [18]. For convenience, titration curves are plotted below with units of PLB/SERCA on the abscissa, but the units are converted to PLB per 1000 lipids to calculate meaningful Kd values. In the competition experiments, when AEDANS-SERCA is co-reconstituted with Dab-PLBW and unlabeled PLBM, E depends on the relative affinities of the co-existing PLBs competing for binding to SERCA:
| Equation 6 |
where w, m, A, E are as defined as in Equation 4. Equation 6 is simpler than Equation 4 because PLBM is unlabeled, so E(m) = 0.
3. Results
3.1. Effects of Synthetic PLB and its Mutants on SERCA KCa
The effects of synthetic PLB variants on KCa, the apparent Ca2+-affinity of SERCA, were determined in co-reconstituted samples of well-defined lipid/SERCA, and PLB/SERCA. At the approximately physiological level of 5PLB/SERCA [31], all PLB variants decrease the apparent Ca2+ affinity of SERCA (KCa values in Fig. 2A). This pattern of potency is consistent with previous results using recombinant PLB variants [5, 6, 28, 32, 33]; I40A inhibits much more than PLBW, while S16E and L31A inhibit much less. While I40A is predominantly monomeric, the other three PLB variants have been shown to be primarily pentameric, [5, 34, 35], so loss of inhibitory function is not due to differences in PLB oligomeric stability.
To further characterize the inhibitory potencies of these PLB mutants, we performed co-reconstitution varying PLB/SERCA from 0 to 20 (maintaining 700 lipids/SERCA), thus probing the normal physiological range (PLB/SERCA ≤ 5), as well as the pathophysiological range (PLB/SERCA > 5) that is often present in failing myocardium [36, 37]. Potency profiles are given in Fig. 2B, along with fits to Equation 3. As expected, the saturating potency, Pmax, of I40A (345 ± 3%) is about 3 times that of PLBW (120 ± 6%) [5, 28, 32], whereas both LOF PLBM have Pmax values about 3 times less than that of PLBW (43 ± 7% for S16E, 35 ± 10% for L31A). Surprisingly, the apparent affinities of PLBW and I40A for SERCA obtained from fitting their potency profiles in Fig. 2B are not significantly different: Kd values (PLB/1000 lipids) are 4.1 ± 0.8 (PLBW), 4.0 ± 0.4 (I40A), 5.1 ± 1.1 (S16E), and 9.1 ± 4.9 (L31A). We conclude that the differences in potency displayed by these PLB variants reflect mainly the intrinsic potency of the SERCA-PLB complex at saturation, Pmax, not the binding affinity, 1/Kd (Equation 3).
3.2. Functional Competition between PLBW and LOF PLBM
To determine whether LOF PLBM can attenuate the inhibition of SERCA induced by PLBW, we co-reconstituted SERCA with mixtures of PLBW and each of the LOF PLBM (S16E and L31A). In Fig. 3, triangles illustrate the measured potencies of SERCA/PLBW/PLBM mixtures at 1/2/2, 1/2/5, 1/5/5, and 1/5/10. Functional competition data are displayed relative to the inhibition profile of PLBW from Fig. 2B (as upper bound, Fig. 3 dashed line) and an average of LOF PLBM profiles from Fig. 2B (as lower bound, Fig. 3 dotted line). For each sample containing w PLBW and m PLBM mixtures, the increase in KCa is significantly smaller than for the corresponding reference samples containing only w PLBW. This is the relevant effect for gene therapy applications. These experimental data, reflecting the functional competition of PLBM with PLBW for SERCA binding (Fig. 3 symbols), were fitted using Equation 4, yielding apparent Kd values (PLBM/1000 lipid) of 3.2 ± 0.5 for S16E and 3.5 ± 0.6 for L31A. These are similar to the Kd values measured directly from the functional potency profiles of these PLBM (Fig. 2B, Equation 3).
3.3. FRET measurement of competition between PLBW and LOF PLBM
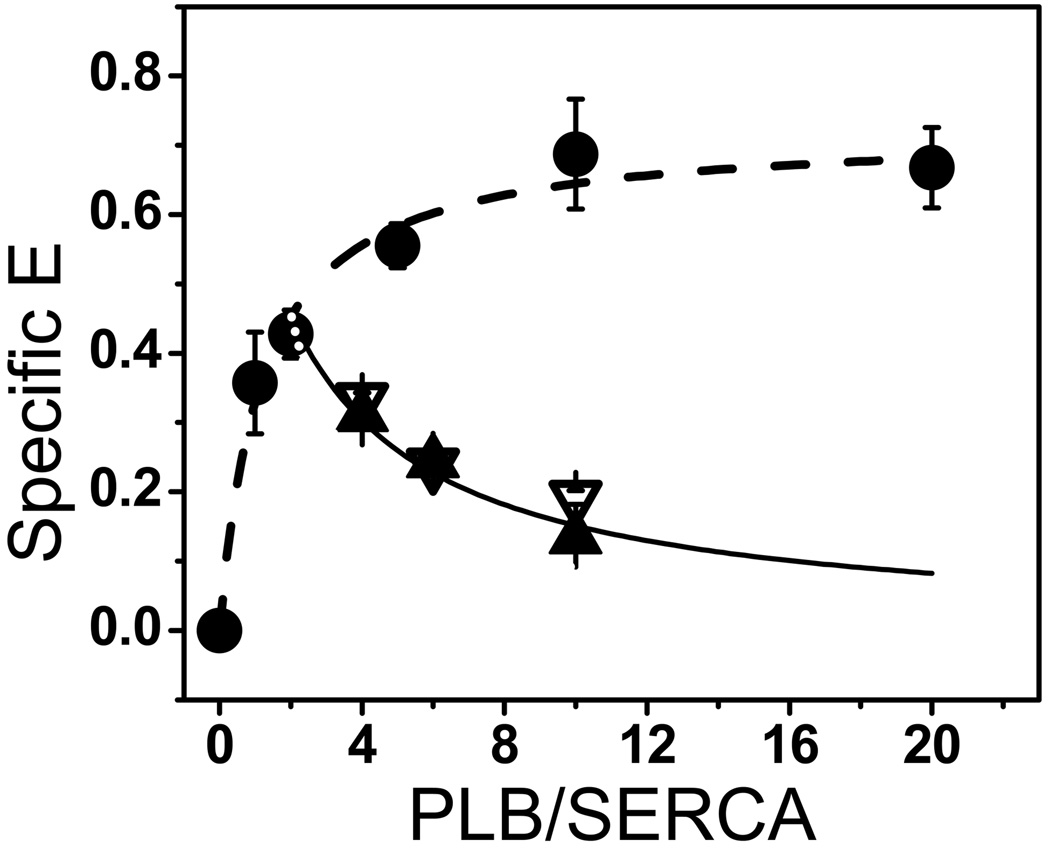
We measured FRET between AEDANS-SERCA and Dab-PLBW co-reconstituted in lipid over a range of PLB/SERCA. Lipids/SERCA was fixed at 700. The resulting FRET profile (Fig. 4, circles) allows a direct determination of PLB’s binding affinity for SERCA, as previously shown [18]. The dashed line represents the best fit to Equation 5, giving Kd = 1.2 ± 0.3 PLB/1000 lipids. This affinity is significantly tighter than indicated by the functional profile (Fig. 2B, PLBW), suggesting that some binding occurs without inhibition. To determine whether PLBM and PLBW physically compete for SERCA binding, we co-reconstituted AEDANS-SERCA, Dabcyl-PLBW, and unlabeled LOF PLBM at SERCA/PLBW/PLBM = 1/2/m (m= 2, 4, 8). As in the case of functional competition, FRET is decreased by both PLBM (Fig. 4, triangles are below circles), indicating competition with PLBW for binding to SERCA. Thus, both unlabeled LOF mutants PLBM compete physically with labeled PLBW (Fig. 4). This FRET competition data was fitted using Equation 6, yielding Kd values (PLB/1000 lipids) for the two PLBM binding to SERCA that were indistinguishable: 1.8 ± 0.5 for S16E and 2.2 ± 0.8 for L31A.
Fig. 4. FRET Competition.
The dependence of FRET E(w) on the ratio w of Dab-PLBW to AEDANS-SERCA (circles) was fitted using Equation 3 (dashed curve), giving Kd = 1.2 ± 0.3 PLB/1000 lipids. FRET competition data, E(w+m), are shown for w = 2 Dab-PLBW and m = 2, 4, or 8 unlabeled PLBM (S16E closed triangles, L31A open triangles). Solid curve shows the best fit of the averaged PLBM data to Equation 6, giving Kd = 2.0 ± 0.9 PLB/1000 lipids. Each plotted point represents the mean ± SEM from at least three measurements.
4. Discussion
By varying the molar ratio of PLB/SERCA and measuring the Ca2+-dependence of SERCA activity, we found that the potency profiles, P(n), of all four PLB variants are characterized by indistinguishable affinities (1/Kd) (Fig. 2A), despite saturating potencies (Pmax) that vary by a factor of 10 (I40A≫PLBW>S16E>L31A) (Fig. 2B). We conclude that, for these PLB variants, the inhibition in the SERCA-PLB complex at saturation (Pmax) is essentially independent of affinity. This result suggests that the two LOF PLBM should compete effectively with PLBW for SERCA binding, thus relieving SERCA inhibition. The functional competition data (Fig. 3) supports this hypothesis – both S16E and L31A compete effectively with PLBW to decrease the net potency (shift in KCa). Indeed, the Kd values of S16E and L31A calculated from the functional competition measurements (3.2 ± 0.5 for S16E and 3.5 ± 0.6 from L31A, Fig. 3) are even slightly smaller (greater affinity) than calculated from the potency profiles (5.1 ± 1.1 for S16E and 9.1 ± 4.9 for L31A Fig. 2B). The similar SERCA affinity of PLBW and LOF PLBM is further supported by the FRET data, which demonstrate physical competition between these PLB variants (Fig. 4).
These results (1) provide a rational explanation for the success of S16E-based gene therapy in animal models of heart failure [12, 14, 23], (2) further support the notion that decreased potency of PLBM is distinct from binding affinity, and (3) demonstrate convenient, rapid methods for in vitro testing future PLBM candidates for HF therapies. Indeed, the L31A mutant may already be superior to S16E as a therapeutic candidate, because it can be phosphorylated by PKA at Ser 16, thus providing a level of adrenergic response that S16E lacks. Although the current collection of PLB variants does not show significant variation in Kd, the observation that Pmax is uncoupled from Kd supports the hypothesis that more LOF PLBs can be designed that have either (a) a lower Pmax than S16E or L31A, without decreasing affinity, or (b) a higher affinity without increasing Pmax. Indeed, previous studies with PLB mutants show that insight into structure-function correlations, gained from EPR and NMR analysis of PLB structural dynamics and function, can be used effectively to guide the PLBM engineering process [6, 22].
HIGHLIGHTS.
Explanation for success of S16E-based gene therapy in heart failure animal models.
Decreased potency of PLB mutants is distinct from binding affinity.
In vitro testing of future PLB mutant candidates for heart failure therapies.
Acknowledgements
We thank Gianluigi Veglia for helpful discussions, Cory I. Paterson and Zhiwen Zhang for excellent technical assistance, and Octavian Cornea for preparing the manuscript for publication.
This work was supported, in part, by a grant to D.D.T. (NIH GM27906). E.L.L. was supported by NIH Chemical Biology Training Grant (NIH GM870008), followed by a predoctoral fellowship from the American Heart Association (Midwest Affiliate 0815604G).
Abbreviations
- Ca2+
divalent calcium ion
- Dabcyl-SE
4-((4-(dimethylamino)phenyl)azo)-benzoic acid succinimidyl ester
- Fmoc
9-fluorenylmethyloxycarbonyl
- FRET
fluorescence resonance energy transfer
- IAEDANS
5-((((2-iodoacetyl)amino)ethyl)amino) naphthalene-1-sulfonic acid
- Kd
dissociation constant
- LOF
loss of function
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy
- NaOH
Sodium hydroxide
- pCa
−log[Ca2+]
- PKA
protein kinase A
- PEG-PS
polyethylene glycol–polystyrene (graft support)
- pKCa
−log(KCa), calcium concentration at half-maximal ATPase activity
- PLB
phospholamban
- SDS
Sodium dodecyl-sulfate
- SERCA
Sarco-endoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cantilina T, Sagara Y, Inesi G, Jones LR. Comparative studies of cardiac and skeletal sarcoplasmic reticulum ATPases. Effect of a phospholamban antibody on enzyme activation by Ca2+ J Biol Chem. 1993;268:17018–17025. [PubMed] [Google Scholar]
- 2.Chien KR, Ross J, Jr, Hoshijima M. Calcium and heart failure: the cycle game. Nat Med. 2003;9:508–509. doi: 10.1038/nm0503-508. [DOI] [PubMed] [Google Scholar]
- 3.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 4.Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986;261:13333–13341. [PubMed] [Google Scholar]
- 5.Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban inhibitory function is activated by depolymerization. J Biol Chem. 1997;272:15061–15064. doi: 10.1074/jbc.272.24.15061. [DOI] [PubMed] [Google Scholar]
- 6.Ha KN, Traaseth NJ, Verardi R, Zamoon J, Cembran A, Karim CB, Thomas DD, Veglia G. Controlling the inhibition of the sarcoplasmic Ca2+-ATPase by tuning phospholamban structural dynamics. J Biol Chem. 2007;282:37205–37214. doi: 10.1074/jbc.M704056200. [DOI] [PubMed] [Google Scholar]
- 7.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 2010;10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nature Reviews. 2003;4:666–678. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 10.Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone ME, Wang Y, Ross J, Jr, Kranias EG, Giles WR, Chien KR. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 11.del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J., Jr Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113:727–736. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer M, Belke DD, Trost SU, Swanson E, Dieterle T, Scott B, Cary SP, Ho P, Bluhm WF, McDonough PM, Silverman GJ, Dillmann WH. A recombinant antibody increases cardiac contractility by mimicking phospholamban phosphorylation. Faseb J. 2004;18:1312–1314. doi: 10.1096/fj.03-1231fje. [DOI] [PubMed] [Google Scholar]
- 14.Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, Wang Y, Ross J, Jr, Chien KR. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 15.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW, 2nd, MacLennan DH, Kremastinos DT, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James P, Inui M, Tada M, Chiesi M, Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989;342:90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Akin BL, Jones LR. Mechanism of reversal of phospholamban inhibition of the cardiac Ca2+-ATPase by protein kinase A and by anti-phospholamban monoclonal antibody 2D12. J Biol Chem. 2007;282:20968–20976. doi: 10.1074/jbc.M703516200. [DOI] [PubMed] [Google Scholar]
- 18.Mueller B, Karim CB, Negrashov IV, Kutchai H, Thomas DD. Direct detection of phospholamban and sarcoplasmic reticulum Ca-ATPase interaction in membranes using fluorescence resonance energy transfer. Biochemistry. 2004;43:8754–8765. doi: 10.1021/bi049732k. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Bigelow DJ, Squier TC. Conformational changes within the cytosolic portion of phospholamban upon release of Ca-ATPase inhibition. Biochemistry. 2004;43:3870–3879. doi: 10.1021/bi036183u. [DOI] [PubMed] [Google Scholar]
- 20.Karim CB, Zhang Z, Howard EC, Torgersen KD, Thomas DD. Phosphorylation-dependent conformational switch in spin-labeled phospholamban bound to SERCA. J Mol Biol. 2006;358:1032–1040. doi: 10.1016/j.jmb.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Stokes DL, Jones LR. Role of leucine 31 of phospholamban in structural and functional interactions with the Ca2+ pump of cardiac sarcoplasmic reticulum. J Biol Chem. 2005;280:10530–10539. doi: 10.1074/jbc.M414007200. [DOI] [PubMed] [Google Scholar]
- 22.Gustavsson M, Traaseth NJ, Karim CB, Lockamy EL, Thomas DD, Veglia G. Lipid-Mediated Folding/Unfolding of Phospholamban as a Regulatory Mechanism for the Sarcoplasmic Reticulum Ca(2+)-ATPase. J Mol Biol. 2011 doi: 10.1016/j.jmb.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye DM, Preovolos A, Marshall T, Byrne M, Hoshijima M, Hajjar R, Mariani JA, Pepe S, Chien KR, Power JM. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 24.Traaseth NJ, Thomas DD, Veglia G. Effects of Ser16 phosphorylation on the allosteric transitions of phospholamban/Ca(2+)-ATPase complex. J Mol Biol. 2006;358:1041–1050. doi: 10.1016/j.jmb.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 25.Stokes DL, Green NM. Three-dimensional crystals of CaATPase from sarcoplasmic reticulum. Symmetry and molecular packing. Biophys J. 1990;57:1–14. doi: 10.1016/S0006-3495(90)82501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderkooi JM, Ierokomas A, Nakamura H, Martonosi A. Fluorescence energy transfer between Ca2+ transport ATPase molecules in artificial membranes. Biochemistry. 1977;16:1262–1267. doi: 10.1021/bi00626a003. [DOI] [PubMed] [Google Scholar]
- 27.Reddy LG, Autry JM, Jones LR, Thomas DD. Co-reconstitution of phospholamban mutants with the Ca-ATPase reveals dependence of inhibitory function on phospholamban structure. J Biol Chem. 1999;274:7649–7655. doi: 10.1074/jbc.274.12.7649. [DOI] [PubMed] [Google Scholar]
- 28.Reddy LG, Cornea RL, Winters DL, McKenna E, Thomas DD. Defining the molecular components of calcium transport regulation in a reconstituted membrane system. Biochemistry. 2003;42:4585–4592. doi: 10.1021/bi026995a. [DOI] [PubMed] [Google Scholar]
- 29.Reddy LG, Jones LR, Pace RC, Stokes DL. Purified, reconstituted cardiac Ca2+-ATPase is regulated by phospholamban but not by direct phosphorylation with Ca2+/calmodulin- dependent protein kinase. J Biol Chem. 1996;271:14964–14970. doi: 10.1074/jbc.271.25.14964. [DOI] [PubMed] [Google Scholar]
- 30.Karim CB, Paterlini MG, Reddy LG, Hunter GW, Barany G, Thomas DD. Role of cysteine residues in structural stability and function of a transmembrane helix bundle. J Biol Chem. 2001;276:38814–38819. doi: 10.1074/jbc.M104006200. [DOI] [PubMed] [Google Scholar]
- 31.Ferrington D, Moewe P, Yao Q, Bigelow D. Comparison of the stoichiomtery of phospholamban to SERCA2a in cardiac and skeletal muscle. Biophys J. 1998;74:356–361. [Google Scholar]
- 32.Cornea RL, Autry JM, Chen Z, Jones LR. Reexamination of the role of the leucine/isoleucine zipper residues of phospholamban in inhibition of the Ca2+ pump of cardiac sarcoplasmic reticulum. J Biol Chem. 2000;275:41487–41494. doi: 10.1074/jbc.M008195200. [DOI] [PubMed] [Google Scholar]
- 33.Zvaritch E, Backx PH, Jirik F, Kimura Y, de Leon S, Schmidt AG, Hoit BD, Lester JW, Kranias EG, MacLennan DH. The transgenic expression of highly inhibitory monomeric forms of phospholamban in mouse heart impairs cardiac contractility. J Biol Chem. 2000;275:14985–14991. doi: 10.1074/jbc.275.20.14985. [DOI] [PubMed] [Google Scholar]
- 34.Toyofuku T, Kurzydlowski K, Tada M, MacLennan DH. Amino acids Glu2 to Ile18 in the cytoplasmic domain of phospholamban are essential for functional association with the Ca(2+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1994;269:3088–3094. [PubMed] [Google Scholar]
- 35.Simmerman HKB, Kobayashi YM, Autry JM, Jones LR. A Leucine Zipper Stabilizes the Pentameric Membrane Domain of Phospholamban and Forms a Coiled-coil Pore Structure. J. Biol. Chem. 1996;271:5941–5946. doi: 10.1074/jbc.271.10.5941. [DOI] [PubMed] [Google Scholar]
- 36.Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–784. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- 37.Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol. 2001;33:1345–1353. doi: 10.1006/jmcc.2001.1394. [DOI] [PubMed] [Google Scholar]