Abstract
Insomnia is a common clinical condition resulting in significant costs and morbidity. Previous models of insomnia focusing on psychological and behavioral processes are useful clinically, but lack neurobiological specificity. We propose an insomnia model based on basic and clinical neuroscience findings, and hypothesize that insomnia results from persistent activity in wake-promoting neural structures during NREM sleep. The simultaneous occurrence of sleeping and waking neural activity helps to explain clinical phenomenology and treatment effects in insomnia.
Introduction
Insomnia is defined as the symptom of difficulty falling asleep, repeated awakenings with difficulty returning to sleep, or sleep that is nonrestorative or poor in quality, often accompanied by the perception of short overall sleep duration. Insomnia is the most common sleep-related symptom, with point prevalence ranging from 20–50% in most epidemiologic studies. Insomnia can also be more narrowly defined as a syndrome or disorder, i.e., the complaint of insomnia accompanied by significant distress or daytime consequences such as irritability, impaired concentration, or fatigue. When defined in this way, the prevalence of insomnia disorder is approximately 5–15% in the adult population [1,2]. Implicit to either definition of insomnia is that the individual has adequate opportunity and circumstances for sleep, in order to distinguish it from sleep restriction/ deprivation, which have different causes and consequences. Insomnia is associated with consistent risk factors including female sex, increasing age, concurrent depressive and anxiety symptoms, and comorbid medical conditions [1]. Insomnia is often a chronic condition: Longitudinal studies indicate that approximately 2/3 of individuals have symptoms which persist for at least one year [3]. Although often considered a nuisance symptom in clinical practice, insomnia has repeatedly been shown to be a risk factor for subsequent mental disorders, increased health care costs, occupational and social dysfunction, and impaired quality of life [4].
Historical references to insomnia date back thousands of years, but scientific investigation of the etiology and pathogenesis of insomnia has developed slowly. Several factors may account for this, including the heterogeneity of insomnia symptoms, the absence of an objectively-defined phenotype, and the discrepancy between self-report and objective sleep measures using polysomnography (PSG). In addition, insomnia has not lent itself well to the development of animal models, and the absence of molecular or genetic biomarkers makes in vitro models infeasible. Most models of insomnia have been based upon heuristically useful psychological and behavioral constructs [5]. However, recent developments in sleep neuroscience [6,7] now make it possible to develop physiologically and neuroanatomically-based models which complement the original models. After briefly summarizing psychological-behavioral models of insomnia and relevant sleep neurobiology, we propose a testable neurobiological model of insomnia.
Psychological-Behavioral and Neurocognitive Models of Insomnia
The diathesis-stress model proposed by Spielman and colleagues [8], more commonly known as the “3-P” model, describes predisposing, precipitating, and perpetuating factors relevant to the development and maintenance of insomnia. Predisposing factors include genetic, physiological, or psychological diatheses that confer differential susceptibility to individuals. Precipitating factors include physiological, environmental, or psychological stressors which push an individual over a hypothetical insomnia threshold to produce acute symptoms. Perpetuating factors include behavioral, psychological, environmental, and physiological factors that prevent the individual from re-establishing normal sleep. Most attention has focused on behavioral patterns adopted in an attempt to relieve insomnia symptoms, but which inadvertently worsen those symptoms. For example, many individuals with insomnia attempt to compensate for reduced sleep by spending increased time in bed, which may have the unintended effect of further fragmenting sleep. The 3-P model is useful heuristically and clinically to identify potential treatment targets. It permits an integrated and flexible view of insomnia, including factors ranging from genetic/familial predisposition to medical/psychiatric illnesses and volitional behaviors. However, its very breadth can also be regarded as a limitation. The 3-P model applies equally well to a wide variety of psychological-behavioral conditions besides insomnia; it does not specify neurobiological substrates or mechanisms; and its predictions are general.
The stimulus control model proposed by Bootzin [9] is based on classical conditioning principles. Sleep is viewed, in part, a conditioned response to the stimulus of the sleep environment. In insomnia, the bed/ sleep environment instead become stimuli for increased arousal, frustration, and wakefulness. This model forms the basis of stimulus control therapy, which has shown consistent efficacy for chronic insomnia [10]. The therapy attempts to re-establish the sleep environment as a stimulus for sleep by restricting other activities in bed (e.g., lying awake, watching TV), and ensuring that sleep occurs only in bed. The stimulus control model is heuristically useful, is based on well-established principles, and logically leads to a treatment approach. Animal models based on classical conditioning, somewhat surprisingly, have not been exploited. Like the 3-P model, the stimulus control model does not specifically address the neurobiological substrate of insomnia.
Cognitive models of insomnia focus on thoughts, feelings, and beliefs that may interfere with sleep and lead to maladaptive behavioral patterns. Harvey [11] has proposed that insomnia results from inappropriate worry about poor sleep and its daytime effects, which leads to increased physiological and psychological arousal, selective scanning of the internal milieu and environment for threat cues, and the development of counter-productive “safety behaviors” designed to maximize sleep and minimize the consequences of insomnia. A strength of cognitive models is that they lead to hypotheses that can be tested with specific therapeutic interventions directed at distorted thoughts or maladaptive behaviors. Despite their clinical utility, however, there is limited evidence to date that cognitive interventions alone are efficacious for the treatment of insomnia, or that changes in cognitions are necessary for improvement. Another relative weakness is that it is difficult to know whether specific thought patterns precede or follow the development of insomnia. If maladaptive cognitions arise from insomnia, then improving sleep directly (for example, with medications) should also be efficacious. Like more behaviorally-oriented models, cognitive models do not propose specific neural substrates.
Another cognitively-based model, the Psychobiological Inhibition Model [12], addresses the role of selective attention in the development and maintenance of insomnia. Psychological and/or physiological stress is posited to lead to selective attention toward stressors, and inhibition of the “de-arousal” that normally accompanies sleep. Inappropriate arousal may then lead to selective attention to sleep-related cues (implicit or explicit) and increased explicit intention and effort to sleep, which further inhibit normal sleep-related de-arousal. The psychobiological inhibition model has good face validity and leads to specific, testable hypotheses regarding selective attention to sleep-related cues, which have received some support from empirical studies [13,14]. Limitations include the difficulty of measuring intention and effort in a comparative sense, and the lack of specification of neural substrates.
Finally, the neurocognitive model [15] builds on the diathesis-stress (3-P) model described above, but integrates neurobiological and neurophysiological observations. Specifically, the neurocognitive model proposes that insomnia leads to conditioned cortical arousal, manifest as increased high-frequency (beta and gamma) EEG activity during sleep. High-frequency EEG activity is thought to be associated with enhanced sensory processing, memory formation, and conscious perception. Thus, high-frequency EEG activity may underlie the phenomenon of “sleep state misperception” or “paradoxical insomnia,” in which subjective wakefulness is greater than that measured by concurrent PSG. Strengths of the neurocognitive model include good face validity and the incorporation of electrophysiological, neurocognitive, and clinical findings in chronic insomnia. For instance, high frequency EEG activity during non-rapid eye movement (NREM) sleep has been associated with subjective-objective sleep mismatch in some, if not all, studies [16–18]. The model is still relatively nonspecific with regard to neural structures or circuits involved.
Sleep Regulatory Mechanisms
Although psychological-behavioral models have considerable utility, they lack neurobiological specificity. A brief review of sleep-wake neurobiology is important to understand the model we subsequently propose.
Neural systems regulating sleep and wakefulness
Wakefulness, NREM and rapid eye movement (REM) sleep are regulated by the interaction of overlapping and redundant neural systems which utilize a variety of monoamine and protein neurotransmitters and neuromodulators [19,20]. Thus, no single brain region is uniquely necessary or sufficient for either sleep or wakefulness. Figure 1 shows a simplified circuit diagram of key sleep-wake regulatory structures. Wakefulness results from ascending activity in a number of brainstem and hypothalamic nuclei, comprising what had been referred to as the ascending reticular activation system. These nuclei include the noradrenergic locus coeruleus, the serotonergic raphe nuclei, cholinergic nuclei of the pedunculopontine tegmentum and the laterodorsal tegmentum, dopaminergic nuclei of the ventral tegmental area, and the histaminergic tuberomammillary nuclei of the posterior hypothalamus. Ascending projections from these nuclei project widely to the cerebral cortex. Cholinergic brainstem nuclei also project to thalamic nuclei, and from there to the cerebral cortex. Hypocretin/orexin-containing neurons in the peri-fornical area of the lateral hypothalamus (orexin) project to hypothalamic and brainstem arousal centers, and functionally reinforce their activity during wakefulness. The specific expression of wakefulness is further modified by input from the cerebral cortex and the limbic system, which influence voluntary behavior and affect [19,20].
Figure 1. Overview of Human Sleep Regulation.
This simplified circuit diagram outlines key neural structures and physiological processes that regulate sleep in humans. See text for details. Solid arrows indicate direct anatomic or physiologic pathways. Dotted arrows indicate indirect pathways. VLPO = Ventrolateral preoptic area. LHA = Lateral hypothalamus/perifornical area. LC = locus coeruleus. LDT = Laterodorsal pontine tegmentum. PPT = Pedunculopontine tegmentum. TMN = Tuberomamillary nucleus.
The onset of NREM sleep is accompanied by increased neuronal activity in the ventrolateral and median preoptic areas (VLPO, MnPO) of the anterior hypothalamus. Projections from the VLPO and MnPO inhibit activity of brainstem and posterior hypothalamic arousal centers. Thus, the lateral hypothalamus and VLPO/MnPO constitute a “sleep-wake state switching system” which regulates consolidated bouts of wakefulness and sleep. Specific cortical electroencephalographic (EEG) features of NREM sleep include sleep spindles, which arise from hyperpolarization of the reticular nucleus of the thalamus; delta waves, which result from cortical hyperpolarization; and slow delta oscillations, which reflect hyperpolarization of thalamo-cortical circuits [21].
Wakefulness and sleep are regulated by both circadian and homeostatic factors. Circadian factors refer to the influence of the approximately 24-hour biological clock, and homeostatic factors to the increase in sleep propensity as a function of accumulated wakefulness. The circadian timing system in humans and other mammals is governed by activity of the hypothalamic suprachiasmatic nuclei, which receive photic input from the retinohypothalamic tract, and project to the hypothalamus, thalamus and (indirectly) to brainstem arousal centers. One of the main functions of the circadian system is to appropriately time wakefulness and sleep. In humans, the circadian system promotes wakefulness during daylight hours, and sleep at night. Homeostatic sleep regulation is less well-localized to a specific brain region, but its time course and response to sleep deprivation parallel the accumulation of extracellular adenosine, particularly in the basal forebrain [22].
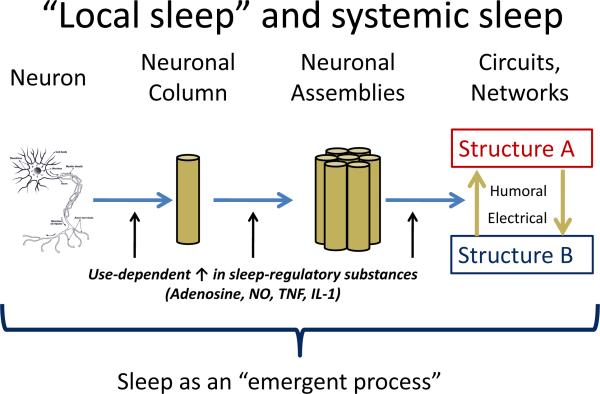
Local sleep
The model of sleep regulation described above views sleep as a centrally-regulated process in which a sleep-wake “switch” regulates the activity of downstream neuronal structures and circuits. However, a variety of evidence suggests that sleep, or sleep-like features, may be an intrinsic property of individual neurons and neuronal assemblies (Figure 2; reviewed in Kreuger [23]). Use-dependent activity in individual neurons, neuronal columns, and neuronal assemblies leads to an increase in sleep-regulatory substances including adenosine, tumor necrosis factor alpha (TNF-α), nitrous oxide, and interleukins (including IL-1, IL-6). These factors promote local sleep-like neuronal activity patterns, but can also affect activity in other regions via humoral and/or electrical signaling, thus leading to a propagation of sleep. Viewed in this way, sleep can be described as a local, use-dependent, and “bottom-up” process which ultimately triggers the activation (or de-activation) of central sleep-regulatory centers.
Figure 2. Conceptual overview of local sleep.
The concept of local sleep proposes that sleep-like activity is an inherent property of neurons and neural assemblies, mediated by the use-dependent accumulation of various sleep-regulatory substances. The occurrence of sleep behavior in the whole organisms depends on the proportion of neuronal assemblies in the sleep-like state, which can then influence central sleep regulatory structures. See text for details. Adapted from [23].
Experimental data support the concept of sleep as a local, use-dependent phenomenon. For instance, individual neuronal assemblies show spontaneous alterations in evoked response patterns suggesting wake-like and sleep-like states [24]; in vivo EEG slow wave activity increases in discrete neuronal assemblies and cortical regions corresponding to selective stimulation (e.g., twitching a single whisker in rats, applying a vibratory stimulus to one hand during wakefulness, or stimulating a particular brain region with transcranial magnetic stimulation during sleep or wakefulness in humans) [25–28]; and specific EEG waveforms during sleep show consistent, frequency-specific temporal and topographic characteristics [29,30].
The “central” and “local” models of sleep regulation are not mutually exclusive. For instance, one common model of homeostatic sleep regulation invokes local adenosine effects in the basal forebrain [22]. It also seems likely that the progressive increase in the proportion of “sleeping” neuronal assemblies ultimately triggers central sleep-wake regulatory centers that widely influence whole-brain activity.
The “local” sleep concept is useful for models of insomnia. Discrete lesions of sleep-regulatory centers such as the VLPO result in sleep patterns that do not bear a close resemblance to human insomnia [31]. The local sleep conceptualization suggests that “hyperarousal” in insomnia need not be viewed as a global construct, but may instead be viewed as a dysfunction, perhaps a use-dependent dysfunction, in specific neural circuits.
A Proposed Neurobiological Model of Insomnia
Our proposed neurobiological model of insomnia draws on previous psychological-behavioral models described in the first section of this paper, as well as the central and local neurobiological models of sleep regulation described in the second section. We hypothesize that insomnia is a disorder of sleep-wake regulation characterized by persistent wake-like activity in neural structures during NREM sleep, resulting in simultaneous and regionally-specific waking and sleeping neuronal activity patterns.
During NREM sleep defined by central and frontal EEG patterns, we hypothesize that regional neuronal activity and metabolism is greater in limbic and parietal cortices, thalamus, and hypothalamic-brainstem arousal centers in individuals with insomnia as compared to individuals with good sleep. The simultaneous occurrence of sleep-like and wake-like neural activity may help to explain clinical phenomenology and treatment effects in insomnia. For instance, individuals with insomnia may perceive wakefulness or persistent awareness of the environment despite the occurrence of cortical EEG patterns consistent with sleep. Relatively small increases in sleep time or decreases in wakefulness with treatment could be associated with large subjective improvements if treatment led to reduced local waking neural activity. The model is summarized and depicted graphically in Figures 3 and 4. Evidence for this model comes from animal models and human studies of brain activity patterns in insomnia.
Figure 3. Neurobiological Model of Insomnia, Part 1.
This figure depicts an overview of the Neurobiological Model of Insomnia with regard to existing conceptualizations of sleep-wake regulation. Panel A presents a simplified model of the bi-stable “sleep switch” proposed by Saper and others. Panel B presents a model of insomnia based on the work of Cano et al., using a rat model of transient insomnia. In this conceptualization, the “sleep switch” is seen as unstable, with frequent transitions between sleep and wakefulness. Panel C presents a model of insomnia informed by the “local sleep” conceptualization, and consistent with our proposed model. In this model, different brain regions may simultaneously show sleep-like and wake-like activity. Refer to Figure 4 and the text for further details.
Figure 4. Neurobiological Model of Insomnia, Part 2.
This figure depicts the Neurobiological Model of Insomnia in greater detail with regard to specific neural circuits and processes. The three panels present our conceptualization of normal sleep wake function (A), sleep-wake function in untreated insomnia (B), and the effects of treatment on sleep-wake function in insomnia (C). Within each panel, we depict specific brain systems (right column of boxes) which generate—and are in turn influenced by—a set of physiological processes (left column of boxes) to regulate overall sleep-wake function (far right box). (A) In the “normal” condition, neural circuits and structures that regulate sleep and wakefulness generate appropriate psychophysiological arousal, circadian rhythms, and homeostatic sleep drive. These structures include cortico-limbic circuits that regulate cognitive and emotional arousal, and the default mode network that may regulate self-awareness; hypothalamic centers (including the sleep-active median and ventrolateral preoptic areas and wake-promoting lateral hypothalamic hypocretin neurons) that control the “switching” between sleep and wakefulness; and brainstem-hypothalamic arousal centers (e.g., posterior hypothalamus, locus coeruleus, raphe nuclei, cholinergic brainstem nuclei) that in turn innervate cortico-limbic systems. The specific brain systems and physiological processes have extensive feedback interactions among themselves, and with each other. In insomnia (B), we hypothesize activation (pink boxes) of cortico-limbic and brainstem-hypothalamic centers and a relative increase in psychophysiolgical arousal. In addition, insomnia may also be characterized by impaired function (blue boxes) of circadian and homeostatic function. Increased arousal, coupled with reduced circadian and homeostatic sleep drive, may result in the sleep and waking features of insomnia. Deficient function of the sleep-wake “switch” (hatched green box) could also contribute to increased wakefulness in insomnia. Treatment effects in insomnia are shown in (C). Cognitive-behavioral treatments for insomnia may specifically target the dysregulated processes that characterize insomnia, whereas pharmacologic treatment may directly affect brain centers including cognitive-affective circuits and hypothalamic-brainstem arousal centers. Diagonally-filled boxes indicate changes anticipated with treatment. See text for details.
Animal studies
Cano and colleagues have presented the most face-valid animal model of insomnia [32] using a cage-exchange paradigm. In this paradigm, male rats are placed in a cage recently vacated by another male rate; the scent of another male is threatening and therefore stressful. Cano observed a period of “acute insomnia” beginning 5–6 hours after the stressor. EEG recordings documented decreased NREM sleep and increased wakefulness and high-frequency EEG power during NREM compared to control. Regional brain activation was examined using cfos expression. During NREM sleep in control animals, cfos expression was increased in sleep-active regions (MnPO and VLPO), with little activity present in the limbic system or hypothalamic and brainstem arousal centers. Conversely, wakefulness in control animals was characterized by absence of cfos expression in MnPO and VLPO, and increased expression in limbic and arousal structures. Cage-exchange “insomnia” animals showed a distinctive pattern of activation: Simultaneous activation of both sleep-promoting regions and wake-active limbic and arousal centers. This pattern also differed from that of control animals which had been awake for 50% of the time immediately prior to sacrifice. These animals showed minimal activation of MnPO and VLPO, and a smaller degree of activation in cortex and TMN. In summary, the cage-exchange model of insomnia was characterized by coactivation of sleep and arousal centers, and a sleep EEG pattern consistent with increased arousal.
Human functional neuroimaging studies
Human studies have used 15O-H2O and 18FFDG positron emission tomography (PET) and functional magnetic resonance imaging to study regional patterns of brain activity during sleep. In good sleepers, NREM sleep is characterized by widespread reduction in blood flow and relative glucose metabolism in frontal, parietal and temporal cortices, anterior and posterior cingulate gyri, thalamus, and hypothalamic-brainstem arousal centers (reviewed in [7]). Specific NREM waveforms, such as slow waves and spindles, are associated with distinct regional patterns [33].
Studies of patients with insomnia have used the 18F-FDG method to compare relative regional glucose metabolic rates in the brain during wakefulness and NREM sleep. (Relative regional measures correct for whole brain glucose metabolic rate, thereby emphasizing regional differences. However, they do not provide quantitative values of glucose metabolism in specific regions.) These studies show smaller differences between wakefulness and early NREM sleep in insomnia patients compared to controls in medial temporal cortex, thalamus, anterior cingulate, and brainstem arousal centers [34]. Wakefulness after sleep onset (measured by self-report or polysomnography) positively correlates with NREM relative regional metabolism in the dorsolateral prefrontal cortex, thalamus, and brainstem arousal structures [35]. Recent unpublished results in a larger group of insomnia and good sleeper subjects, matched for the amount of PSG-monitored NREM sleep during the glucose uptake period, confirm a smaller extent of deactivation in prefrontal cortex and thalamus among insomnia subjects. Moreover, a direct comparison of relative metabolism during NREM sleep in the two groups shows relatively greater metabolism in the frontoparietal cortex among insomnia subjects, most strikingly in the precuneus (Figure 5). Whole-brain cerebral metabolic rate of glucose during NREM sleep correlates with worse subjective sleep quality (r = .51, p = .003, n = 31), as does relative metabolism in the precuneus. The precuneus is one component of a “default mode network,” which also includes lateral parietal, ventromedial prefrontal, mid-dorsolateral prefrontal, and anterior temporal regions. This network has a high metabolic rate during resting wakefulness, which decreases with specific tasks. It is thought to play a central role in modulation of conscious processes, awareness, mental representations of the self, and inferential processing of context-related information [36,37].
Figure 5. Relative regional glucose metabolic activity during NREM sleep in primary insomnia and good sleeper subjects.
18F-fluoro-deoxyglucose was injected in 18 primary insomnia and 18 good sleeper subjects after they had been in NREM sleep for 10 minutes. Following 20 minutes of glucose uptake, subjects were awakened for 3-D PET scans. The amount of NREM sleep during the uptake period did not differ between the two groups. Using SPM-2, regions with greater relative glucose during the uptake period in insomnia patients were identified. The pseudo-color map shows a map of voxel-by-voxel t-values. Insomnia patients had greater relative glucose metabolism in broad regions of the dorsolateral and parietal cortex and the precuneus.
Drawing on these animal and human studies, our model suggests that insomnia results from persistent activity in wake-active brain regions and circuits during NREM sleep, including cortical regions (prefrontal, parietal, and precuneus), paralimbic cortex (anterior cingulate, mesial temporal), thalamus, and hypothalamic/brainstem arousal centers. Persistent activity in the precuneus during EEG-defined NREM sleep may be particularly relevant to the experience of insomnia: Such activity may contribute to the subjective experience of self-awareness. Increased activation of components of the default-mode network during slow wave activity has been suggested to facilitate neuronal interactions and information processing [33]; this interpretation may be consistent with the simultaneous increase in low-frequency and high-frequency EEG activity we have observed in at least some individuals with insomnia [18]. The proposed model is also consistent with the “local sleep” conceptualization that sleep is not a global all-or-none phenomenon, and that “partial” sleep states may occur. As Kreuger [23] notes, “global coordination of NREM sleep is not due to a single generator: It might largely reflect an emergent property of loosely-coupled local processes. This may help to explain the common observation that EEG indices of NREM sleep do not always correspond to the subjective experience of sleep quality.”
Unpublished observations from our center regarding the effects of pharmacologic and behavioral interventions in individuals with insomnia further support the framework of our model. Treatment with eszopiclone, a benzodiazepine receptor agonist, is associated with reduced NREM relative glucose metabolism in brainstem arousal centers, thalamus, and parietal cortex, including precuneus [38]. One night of sleep restriction to 2 hours, a more extreme version of the sleep restriction clinically recommended as part of behavioral insomnia treatments [10], results in reduced NREM relative glucose metabolism in medial and dorsolateral prefrontal cortex, amygdala, and precuneus. Thus, common pharmacologic and behavioral interventions may reverse some of the regionally-specific abnormalities observed in untreated patients.
Model Comparison
The proposed neurobiological model of insomnia differs from the most widely-cited models of insomnia primarily on the basis of level of inquiry. Most previous models have been developed from psychological and behavioral constructs which are measured by self-report or behavioral response patterns, or global EEG characteristics. The neurobiological model has been developed from clinical and basic neuroscience observations which are measured in the activity patterns of more specific brain regions or circuits. Ultimately, psychological and behavioral constructs must have neurobiological determinants. Conversely, the activity of brain regions and circuits is ultimately expressed in the behavior of the organism. Thus, the important future challenge will lie in joining these two levels of inquiry.
Conclusions
Insomnia is a common clinical problem with important consequences for health and functioning. Previous models of insomnia, built primarily on psychological and behavioral constructs, conform well to reported symptoms and observed behaviors. However, these models have generally not proposed an underlying neural basis. The neurobiological model of insomnia builds on basic and clinical neuroscience findings, and combines aspects of traditional top-down and more recent local sleep models. We propose that insomnia is characterized by simultaneous sleep and wake-like activation patterns in specific brain regions regulating sleep-wake state and self-awareness, and that these patterns can be modified by common pharmacologic and behavioral treatments. This model lends itself more readily to empirical testing and drug development for insomnia than other models.
Acknowledgments
The authors gratefully acknowledge the staff at the Neuroscience Clinical and Translational Research Center of the University of Pittsburgh Clinical and Translational Research Center, who conducted polysomnographic studies, as well as the staff of the University of Pittsburgh PET Center
Supported by National Institutes of Health grants MH024652, AG020677, MH061566, RR024153
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Dr. Buysse has served as a paid consultant, and/or has received compensation for CME activities indirectly sponsored by the following companies: Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, sanofi-aventis, Sepracor, Servier, Somnus Therapeutics, Stress Eraser, Takeda and Transcept Pharmaceuticals, Inc.
References
- 1.Lichstein KL, et al. Insomnia: Epidemiology and risk factors. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. Elsevier; 2011. pp. 827–837. [Google Scholar]
- 2.Roth T, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol.Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Morin CM, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Archives of Internal Medicine. 2009;169:447–453. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 4.Edinger JD, Means MK. Overview of insomnia: Definitions, epidemiology, differential diagnosis, and assessment. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. fourth edition Saunders; 2005. pp. 702–713. [Google Scholar]
- 5.Perlis M, et al. Models of insomnia. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. Elsevier; 2011. pp. 850–865. [Google Scholar]
- 6.Saper CB, et al. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 7.Nofzinger EA, Maquet P. What brain imaging reveals about sleep generation and maintenance. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. Elsevier; 2011. pp. 201–214. [Google Scholar]
- 8.Spielman AJ, et al. A behavioral perspective on insomnia treatment. Psychiatric Clinics of North America. 1987;10:541–553. [PubMed] [Google Scholar]
- 9.Bootzin RR. Stimulus control treatment for insomnia. Proceedings of the American Psychological Association. 1972;7:395–396. [Google Scholar]
- 10.Morin CM, et al. Psychological and behavioral treatment of insomnia: An update of recent evidence (1998–2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 11.Harvey AG. A cognitive model of insomnia. Behaviour Research and Therapy. 2002;40:869–893. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 12.Espie CA, et al. The attention-intention-effort pathway in the development of psychophysiologic insomnia: A theoretical review. Sleep Medicine Reviews. 2006:215–245. doi: 10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Jones BT, et al. Sleep-related attentional bias in good, moderate, and poor (primary insomnia) sleepers. Journal of Abnormal Psychology. 2005;114:249–258. doi: 10.1037/0021-843X.114.2.249. [DOI] [PubMed] [Google Scholar]
- 14.Spiegelhalder K, et al. Sleep-related attentional bias in patients with primary insomnia compared with sleep experts and healthy controls. Journal of Sleep Research. 2008;17:191–196. doi: 10.1111/j.1365-2869.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 15.Perlis ML, et al. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. Journal of Sleep Research. 1997;6:179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 16.Krystal AD, et al. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–640. [PubMed] [Google Scholar]
- 17.Perlis ML, et al. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–1682. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones BE. Neurobiology of waking and sleeping. Handb.Clin.Neurol. 2011;98:131–149. doi: 10.1016/B978-0-444-52006-7.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saper CB, et al. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steriade M, McCarley RW. Brain control of wakefulness and sleep. Kluwer Academic; 2005. [Google Scholar]
- 22.Basheer R, et al. Adenosine and sleep-wake regulation. Progress in Neurobiology. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Krueger JM, et al. Sleep as a fundamental property of neuronal assemblies. Nat.Rev.Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rector DM, et al. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto H, et al. Experience-dependent slow-wave sleep development. Nat.Neurosci. 2003;6:553–554. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 26.Kattler H, et al. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. Journal of Sleep Research. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 27.Massimini M, et al. Triggering sleep slow waves by transcranial magnetic stimulation. Proc.Natl.Acad.Sci.U.S.A. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De GL, et al. Cortical plasticity induced by transcranial magnetic stimulation during wakefulness affects electroencephalogram activity during sleep. PLoS.One. 2008;3:e2483. doi: 10.1371/journal.pone.0002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De GL, et al. Antero-posterior EEG changes during the wakefulness-sleep transition. Clin.Neurophysiol. 2001;112:1901–1911. doi: 10.1016/s1388-2457(01)00649-6. [DOI] [PubMed] [Google Scholar]
- 30.De GL, et al. An electroencephalographic fingerprint of human sleep. Neuroimage. 2005;26:114–122. doi: 10.1016/j.neuroimage.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, et al. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. Journal of Neuroscience. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cano G, et al. Neural circuitry of stress-induced insomnia in rats. Journal of Neuroscience. 2008;28:10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maquet P. Understanding non rapid eye movement sleep through neuroimaging. World J.Biol.Psychiatry. 2010;11(Suppl 1):9–15. doi: 10.3109/15622971003637736. [DOI] [PubMed] [Google Scholar]
- 34.Nofzinger EA, et al. Functional neuroimaging evidence for hyperarousal in insomnia. American Journal of Psychiatry. 2004;161:2126–2131. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 35.Nofzinger EA, et al. Regional cerebral metabolic correlates of WASO during sleep in insomnia. Journal of Clinical Sleep Medicine. 2006;2:316–322. [PubMed] [Google Scholar]
- 36.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 37.Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review. 2009;116:252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- 38.Nofzinger EA, et al. Eszopiclone reverses brain hyperarousal in insomnia: Evidence from [18]-FDG PET. Sleep. 2008;31:A232–A232. [Google Scholar]