Abstract
The development of metastatic disease is often correlated with poor patient outcome in a variety of different cancers. The metastatic cascade is a complex, multistep process that involves the growth of the primary tumor and angiogenesis, invasion into the local environment, intravasation into the vasculature, tumor cell survival in the circulation, extravasation from the vasculature and sustained growth at secondary organ sites to form metastases. Although in vitro assays of single cell types can provide information regarding cell autonomous mechanisms contributing to metastasis, the in vivo microenvironment entails a network of interactions between cells which is also important. Insight into the mechanisms underlying tumor cell migration, invasion and metastasis in vivo has been aided by development of multiphoton microscopy and in vivo assays, which we will review here.
Introduction
Cancer is the leading cause of death among individuals under the age of 75 in the United States, and the high mortality rate associated with this disease often stems from the development of metastases. In order for tumors to grow and develop into life threatening entities, they must acquire the ability to invade, degrade extracellular matrix (ECM), survive in circulation and undergo sustained growth in new organ sites; and the tumor microenvironment plays a crucial role in this process [1]. The tumor microenvironment consists of the ECM, blood and lymphatic vessels, nerves and a variety of different cell types, such as fibroblasts and inflammatory and immune cells. Cross-talk between invasive carcinoma cells and the tumor microenvironment can be an important determinant of the malignancy of the tumor [2, 3]. For example, cancer-associated fibroblasts can secrete growth factors and chemokines, which can subsequently alter the ECM and enhance carcinoma cell proliferation and invasion via activation of oncogenic signals [2]. Similarly, tumor-associated macrophages can suppress local immune responses to the tumor as well as stimulate tumor invasion [4]. Since the microenvironment of a tumor can affect the growth and progression of the tumor as well as the development of metastatic disease, it is essential to better understand the interplay between the tumor and its surroundings. However, in vitro assays used to evaluate invasion and metastasis are limited in their ability to recapitulate this complex environment. In addition, most in vivo assays used in metastasis research are endpoint assays; although they can determine the overall efficiency of the metastatic process, very little information is provided about the intermediate steps [5]. Until recently, the steps of the metastatic cascade preceding intravasation have not been well studied in vivo, mostly due to difficulties in observing this process directly [6]. However, the advent of in vivo assays and intravital multiphoton microscopy has led to powerful insights into the mechanisms underlying tumor cell migration and metastasis during the processes of invasion and intravasation [5–13].
Animal Models of Metastasis
The field of cancer biology has greatly benefited from the use of animal models, which are unique in their ability to recapitulate the in vivo systemic and local environment of the developing tumor [14]. To study breast cancer metastasis, for example, transgenic mouse models are the most accurate mimics of the process of metastasis – the tumors arise from the appropriate tissue type (i.e. mammary gland epithelium for breast cancer) and usually mirror their respective human histotypes [15]. However, limitations of using transgenic mouse models include: (1) the amount of time it takes for tumors to develop, (2) the high cost and difficulty in maintaining and manipulating protein expression, (3) the low incidence of developing metastatic disease, and (4) the fact that human tumor cells are not being utilized [15]. Thus, to overcome the limitation of not being able to use and study human carcinoma cells in transgenic mouse models, immunodeficient mouse models have been used to reduce reaction against allogenic antigens [14]. Strains with varying levels of immunodeficiency from athymic to NOG (NOD/Shi-scid/IL-2Rγnull) have been used [16]. The advantage of using human tumor samples is offset by the loss of the ability to examine how the adaptive immune system may contribute to tumor malignancy and metastasis.
In both immunocompetent and immunodeficient models, comparing the metastatic behavior of carcinoma cells using the spontaneous and experimental metastasis assays allows for the evaluation of the efficiency of specific steps within the metastatic cascade [11]. The spontaneous metastasis assay involves orthotopic injection of the tumor, i.e. injecting cells into their tissue of origin, such as breast cancers in mammary fat pads, to create a primary tumor that is subsequently allowed to grow and spread in the host. This assay incorporates most steps in the metastatic cascade and thus more closely resembles clinical disease in terms of its spread and manifestation [11]. To complement the study of tumor cell dissemination using the spontaneous metastasis assay in murine models, the chick embryo chorioallantoic membrane (CAM) model has been introduced as an alternative in vivo model of study since the CAM is capable of efficiently supporting the growth of inoculated xenogenic tumor cells [12]. Briefly, the CAM is lowered by generating an air pocket between the CAM and separated shell membrane to create a “dropped” CAM. Tumor cells are then inoculated in ovo via a small window that is created in the shell above the “dropped” CAM. Approximately one week post-inoculation, large primary tumors develop – from which aggressive carcinoma cells are capable of escaping and ultimately developing micrometastases at other sites, such as the distal CAM and internal organs. These assays are not only cheaper and require much less complex incubation requirements, they circumvent the disadvantage present in murine models of spontaneous metastasis in which a longer period of time is required for evident metastatic disease [11, 12]. However, the later steps of the metastatic cascade, such as extravasation of the tumor cells, remain poorly understood [17]. Using end point assays in murine or chick CAM models, tumor cell extravasation is usually inferred by quantifying secondary tumor formation upon the direct inoculation of tumor cells into the circulation. Intravital imaging of transplanted human carcinoma cells in optically transparent, transgenic zebrafish has demonstrated that extravasation requires adhesion of the tumor cells to the endothelium as well as migration of the tumor cells along the luminal surface of the blood vessels [17].
Unlike the spontaneous metastasis assay, the experimental metastasis assay allows for the study of the target organ seeding step of the metastatic cascade in vivo by injecting the carcinoma cells directly into the circulation. As a result, the early steps of spontaneous dissemination are surpassed and the events following the entry of carcinoma cells into the vasculature, i.e. survival of tumor cells in the circulation, extravasation and the ability to seed and form micrometastases in secondary organ sites, can be studied directly. Using the experimental metastasis model, the tissue specificity of metastases has been studied extensively [reviewed in 11]. Also, this assay has allowed for more rapid screening of targeted cancer therapeutics. As with spontaneous metastasis, the chick embryo CAM model has been proposed to complement and provide an alternative to examining experimental metastasis in murine models.
Intravital Imaging using Multiphoton Microscopy
Conventional single-photon confocal microscopy, a technique that utilizes a pinhole aperture to remove out-of-focus contributions, can be used to obtain dynamic measurements of physiological processes occurring in real-time in three dimensions. Multiphoton microscopy utilizes long wavelength light from pulsed lasers to excite molecules in a limited focal plane through simultaneous absorption of two or even three photons. This confers several technical advantages over conventional single-photon methods [6, 7]. First, although significant light scattering results from the presence of multiple refractive index interfaces present in live tissue samples, (a) the longer wavelengths used for excitation experience less scattering, thus allowing for deeper penetration into tissues, and (b) the excitation light beam is not weakened by fluorophore absorption above the focal plane. Second, because excitation is limited only to the optical section being examined due to the nonlinear dependence on photon density, a pinhole aperture is not required for confocality; hence, 100% of the collected fluorescent light is used by the external detector, which is more efficient. Third, there is reduced bleaching of out of focus elements. Fourth, multiphoton microscopy is capable of achieving true registration in the Z-axis, i.e. the registration of several probes in the Z-axis. Finally, generation of second-harmonic scattering in multiphoton microscopy permits visualization of periodic noncentrosymmetric structures in the extracellular matrix (ECM), such as collagen fibers.
Alternatives to Multiphoton Microscopy for Intravital Imaging
Although multiphoton microscopy for intravital imaging offers several advantages (as described above), currently it is a rather expensive technique and its penetration is typically limited to less than a millimeter. As a result, alternative low resolution imaging methods have been developed to study metastases over the entire animal (Table 1) [18–20]. For example, luminescence has been utilized to examine metastases to the lungs and bone in murine models of osteosarcoma and breast cancer, respectively [18, 19]. Also, metastases can be visualized using real time whole body fluorescent imaging [20].
Table 1.
Comparison of various intravital imaging modalities.
| Imaging Modality | Imaging depth | Image resolution | Cost | Labeling reagents | Invasive technique | Advantages | Limitations |
|---|---|---|---|---|---|---|---|
| Multiphoton microscopy[13] | < 1000 mm | < 0.5μm | High | Fluorophores | Yes | Generation of second harmonic. Minimal photobleaching and toxicity. | High cost. Technology still in development – not used clinically. |
| OFDI[13] | > 1 mm | < 15 μm | High | N/A | No | Exogenous contrast reagents not required. Lack of photobleaching or toxicity. | Very high cost. Technology still in development – not used clinically. |
| Bioluminescence[55] | > 2 cm | > 1 mm | Medium | Luciferins | No | Very sensitive, rapid and noninvasive detection of tumor growth and micrometastases. | Low anatomic resolution. |
| Whole body fluorescent imaging[55,56] | > 2 mm | 100 – 1000 μm | Low | Fluorophores | No | Non-invasive. | Not used clinically. |
| MRI[55,57] | No limit | 25 – 100 μm | High | Magnetic compounds | No | Used clinically | High contrast only generated in soft tissues. Long imaging times required for high resolution. |
| CT[55,57] | No limit | 50 μm | Medium | Iodinated contrast agents | No | Used clinically. | High contrast generated in lungs and bones. High radiation doses needed for high resolution. |
| PET[55,57] | No limit | 1 – 2 mm | High | Radioactive compounds | No | Used clinically. | High cost. Detection capability limited by availability of appropriate compounds. |
OFDI, optical frequency domain imaging; MRI, magnetic resonance imaging; CR, X-ray computed tomography; PET, positron emission tomography.
Recently, a second-generation optical coherence tomography (OCT) technology called intravital optical frequency domain imaging (OFDI) has been used to quantify processes such as tumor angiogenesis and lymphangiogenesis [8]. Despite the advantages offered by multiphoton microscopy for intravital imaging, only superficial visualization of the tumor microenvironment and vasculature are permitted. OFDI, however, avoids the technical limitations of multiphoton microscopy; because of its enhanced depth penetration, repetitive imaging at depths of several millimeters is possible. Clinically, magnetic resonance imaging (MRI), computed tomography (CT) and positron emission tomography (PET) are used to assess for the presence of metastatic disease. These imaging modalities as well as technology that synergizes two pre-existing imaging methodologies, such as PET-MRI, can also be used in animals to bridge the gap between the laboratory and the clinic [21,22].
In Vitro and In Vivo Cancer Cell Migration
Tumor cell motility can be subdivided roughly into amoeboid, mesenchymal and collective migration. Rounded or ovoid cells lacking mature focal adhesions and stress fibers undergo amoeboid migration (of which there are two subtypes – blebby and pseudopodal/filopodal) [23]. Cells exhibiting blebby, amoeboid migration have a low speed of migration and move about by using a propulsive, pushing mechanism. Cells undergoing pseudopodal/filopodal, amoeboid migration create actin-rich filopodia at the leading edge that are poorly adhesive. In a specific case of this movement, dynamic actin-rich dendrites, rather than blebs, are found at the leading edge and subsequently adhere to the ECM during migration, thus resulting in relatively rapid migration. Individual cells demonstrating elevated levels of attachment and cytoskeletal contractility utilize mesenchymal migration, which involves localized cell-ECM interactions and movement in a fibroblast-like fashion. During collective invasion, cell-cell adhesions are maintained; as a result, cell-cell communication can take place and the resulting collective migration of cells occurs as multicellular tubes, strands, amorphous masses of sheets. Although collective invasion is common among a variety of cancer types, it is challenging to follow by direct microscopic observation due to prolonged observation times required.
The difference in velocity between in vitro and in vivo adenocarcinoma cell migration is quite remarkable. In the primary tumor, invasive carcinoma cells can move at velocities much greater than the velocity of similar cells grown in culture in vitro, and up to 30 times the velocity of similar cells in 3D networks of ECM [6]. Another striking difference between in vitro and in vivo migration of adenocarcinoma cells is the extent of persistent linear motion. Unlike cells in 2D cultures, cells in tumors can display a high degree of polarized linear walking [7], which can play a role in permitting carcinoma cells to respond to chemotactic signals and to travel along fibers of the extracellular matrix (ECM) [24].
Intravital Imaging of Tumor Cell Metastasis In Vivo
Motile cells are capable of metastasizing by intravasating into the circulatory or lymphatic system. An important subject to examine is whether cell migration behavior is different in metastatic and non-metastatic tumors. The motility of metastatic MTLn3 and non-metastatic MTC cells was studied in primary mammary carcinoma tumors using intravital microscopy [7,10]. While both types of carcinoma cells moved at similar rates in tumors in vivo, only the rapidly migrating, metastatic MTLn3 cells remained closely associated with and migrated along the collagen fibers in a linear manner [25]. In addition, metastatic cells were polarized and demonstrated increased motility near the tumor vasculature, which indicating their ability to respond to a gradient, a behavior that is absent in non-metastatic MTC cells. Because metastatic tumors have high levels of EGFR expression and could respond to EGF gradients originating from the tumor stroma and vasculature [26], it was postulated that the EGFR could play a role in cell guidance. In fact, overexpression of the EGFR was later shown to increase intravital motility and intravasation [27]. Later experiments [24] strengthened the model that the coordinated deregulation of processes such as chemotaxis and cellular polarity, all of which are pathways involved in directional cell motility, can increase the metastatic potential of carcinoma cells.
The use of confocal microscopy in intravital imaging was instrumental in discovering fundamental differences between in vivo and in vitro tumor cell movement. The disparity between the two may suggests that there are certain interactions between the tumor cells and their microenvironment that cannot be recreated easily in vitro [6]. The migration of cells is a multifaceted and diverse process, and structural and molecular elements present in the tumor microenvironment can influence whether cells will migrate individually through amoeboid or mesenchymal modes or collectively as a cohesive unit [23]. To further investigate the mechanisms underlying tumor cell invasiveness, a breast cancer window model was combined with genetically encoded photoconvertible proteins [28]. Tumor cells expressing Dendra2, a photoswitchable protein whose fluorescence can be altered by intense UV illumination, were orthotopically injected into the mammary fat pads of mice. Using confocal imaging, it was determined that photomarked carcinoma cells remained close together in regions devoid of blood vessels whereas in highly vascular regions photomarked tumor cells spread out. Indeed, a reduction in the number of photomarked cancer cells in the vascular regions suggested that intravasation was occurring. Thus it is highly probable that tumor angiogenesis and vascularization can increase the metastatic potential of cancer cells by affecting their migratory behavior.
Studying the Tumor Microvasculature and Microenvironment using Intravital Imaging
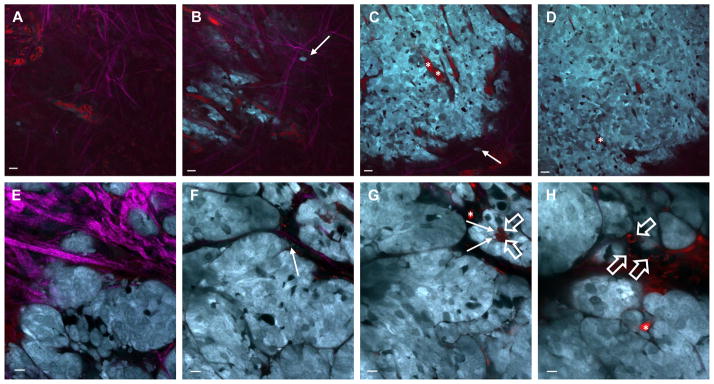
The microvasculature found in solid tumors plays a fundamental role in response to therapeutics [8]. Intravital imaging has been used extensively to gain a better understanding of the size and structure of tumor vasculature (Figure 1) [7, 29–31]. Measuring parameters such as blood vessel diameter, surface area, and branching patterns in growing or regressing tumors can be done with intravital imaging upon the injection of FITC-dextran, which temporarily enhances contrast in blood vessels [29,30]. Unlike normal vascular networks, the tumor vasculature is highly disorganized – intravital imaging has shown that not only are tumor vessels dilated and tortuous, they trifurcate and branch unevenly [31]. In addition, these tumor-associated vessels often lack normal basement membranes and have an increased number of fenestrations and the pericytes found lining the tumor vessels have an abnormal morphology [32,33]. Consistent with these structural changes, time-course intravital imaging of injected fluorescent macromolecules revealed a higher degree of permeability in tumor vessels when compared with normal vessels [30,34].
Figure 1. Visualizing the tumor architecture and microenvironment using multiphoton microscopy.
FVB mice transgenic for the Neu gene having a small activating deletion (neu-NDL) and the add-back mutant constructions Neu-YB or Neu-YD, [58,59], driven by the mouse mammary tumor virus long terminal repeat (MMTV LTR) were crossed with a strain that expressed the cyan fluorescent protein, CFP, using the mammary gland specific MMTV promoter (MMTV-icre-CAG-CAC-ECFP) [60,61]. Mice were anesthetized, skin flap surgeries were done to expose the tumor, and the tumors were imaged using an Olympus Fluoview FV1000-MPE microscope. The imaging was performed using an excitation wavelength of 880 nm using a 25X 1.05NA water objective with varying zoom. The tumor vasculature (asterisks, red) and macrophages (open arrows, red) were labeled using Texas Red 70kD dextran. Neu-YD (A-D) and Neu-YB (E-H) tumors are shown. The extracellular matrix as detected by second harmonic imaging (fuschia) is most dense at the surface of the tumor (A, B, E). Tumor cells (closed arrows, cyan) can be seen interacting with and extending protrusions towards individual fibers of the ECM (B, C, F) or with macrophages (G). Some tumors contain vasculature that is only elaborated closer to the surface (A, B) and begins to disappear upon imaging deeper into the tumor (C, D). However, the vasculature can be seen to wrap around and envelope the tumor, (H). Relative distances from the surface of the tumor are as follows: A, 0μm; B, 5μm; C, 20μm; D, 30μm; E, 15μm; F, 35μm; G, 75μm; H, 90μm. A – D, scale bar, 50 μm. F – I, scale bar, 25μm.
Valuable information regarding the pathophysiology of tumor-associated lymphatics has been obtained by fluorescence micro-lymphangiography; hyperplastic lymphatic vessels were seen to promote local metastasis as determined by the presence of GFP-labeled metastatic cancer cells in nearby lymph nodes by intravital imaging [35]. However, major disadvantages of this technique include visualization of lymphatic vessels draining only the area of the injection site and obstruction of structures near the injection site [8]. These shortcomings are circumvented with the use of OFDI, which eliminates the need for exogenous contrast agents. OFDI lymphangiography of human sarcoma tumors grown in the murine dorsal skin fold showed the presence of hyperplastic lympatics and cellular masses within the lymphatic vessels [8]. Interestingly, additional lymphatic vessels were detected and individual lymphatic valves were resolved in normal dorsal skin folds of mice using OFDI lymphangiography when compared with traditional lymphangiography methods [8].
Moreover, intravital imaging has been modified to make use of specific molecular probes capable of modifying their optical properties as a function of pO2 and pH in order to study the tumor metabolic microenvironment [36]. Although hypoxia (low pO2) and acidic pH have been shown to induce expression of VEGF in vitro [37,38], the effect of these two parameters on in vivo VEGF expression was not known for many years due to the lack of appropriate techniques and animal models. Ultimately, the coordinated study of pH, pO2 and in vivo VEGF expression was made possible by the discovery of live fluorescent reporters that allowed for the transgenic visualization of VEGF promoter activity and intravital imaging techniques used to measure tumor microenvironmental factors, such as pO2 and pH [39].
The tumor microenvironment also contains a variety of other cell types, such as stromal cells and macrophages. Using transgenic models that express fluorescent proteins in specific cell types through tissue-specific promoters, the behavior of stromal cells relative to tumor cells can also be imaged [40,41]. Phagocytic cells such as macrophages can also be imaged through the uptake of fluorescent dextran to follow their effects on tumor biology [43]. While macrophages play a role in tumor rejection, they can also cause increased malignancy [42,43]. Evidence for how macrophages lead to increased invasion and metastasis has been provided using intravital imaging and in vivo invasion assays as discussed in the next section [9,42,44].
Chemotaxis and In Vivo Invasion Assay for Tumor Cell Invasion
Intravital imaging has revealed the importance of chemotaxis for tumor cell invasion and metastasis. Chemotaxis is a process involving the directed movement of cells in response to chemoattractant gradients [6]. A major limitation of in vitro chemotaxis assays is that cell motility is studied on flat, 2D surfaces, and these results still need to be compared to in vivo cellular behavior. An in vivo invasion assay was developed to study invasion in tumors by allowing for the direct collection and stimulation of cells from living animals [9, 10]. In brief, microneedles containing a mixture of components of the extracellular matrix such as Matrigel in the presence or absence of a chemoattractant are introduced into the primary tumor. Invasive cells are allowed to enter the needles over a period of 4 hours as the animal is kept under anesthesia. Upon the conclusion of the assay, cells are extruded from the microneedles and subsequently analyzed for both the quantity and type of cells which have invaded. Advantages of this assay include (1) evaluating invasion under a variety of conditions, (2) determining types of invasive cells, and (3) dissecting mechanisms of invasion in vivo through the use of pharmacological agents.
The in vivo invasion assay has provided valuable information regarding how in vitro chemotactic properties of breast cancer cells correlate with their in vivo invasive and metastatic characteristics. In particular, using both xenograft and transgenic breast cancer models, the results suggested the potential importance of the epidermal growth factor receptor (EGFR) in mediating metastasis in metastatic carcinoma cells [10, 27]. The invasive behavior of carcinoma cells to other chemoattractants, such as transforming growth factor-alpha and heregulin, was also evaluated using the in vivo invasion assay [44, 45].
Most importantly, upon identification of the invasive cells, it was found that both cancer cells and macrophages invaded into the collection needles of the in vivo invasion assay, which ultimately led to the discovery of a paracrine interaction between these two cell types during the process of invasion [44]. Briefly, secretion of colony stimulating factor-1 (CSF-1) by carcinoma cells stimulates macrophages to secrete EGF, which causes further cancer cell stimulation. Inclusion of EGFR or CSF-1 receptor inhibitors in the presence of these chemoattractants blocks this paracrine signaling interaction between tumor cells and macrophages. In addition, this assay has been used in a human mammary tumor model to demonstrate both a paracrine interaction between tumor cells and host macrophages as well as an autocrine signaling loop present in the tumor cells themselves [46].
The collected invasive carcinoma cells can be extruded and further characterized using other assays, such as cDNA microarray analysis, in order to generate gene expression signatures that are specific to invasive cells [7, 47, 48]. For example, gene expression characterization of invasive carcinoma cells showed that there is an upregulation of motility pathways, which are required for disseminating from the primary tumor. Because this method allows for the separation of invasive cells from other cell populations within the primary tumor, it allows for the collection and subsequent characterization of this specific subset of tumor cells.
Translational Value of In Vivo Assays and Multiphoton Microscopy to Humans & Conclusions
In summary, dynamic measurements and parameters obtained through the use of in vivo assays and intravital imaging have provided mechanistic and integrated insights into the biology underlying tumor cell migration, invasion and metastasis. The combination of intravital imaging along with the in vivo invasion assay has allowed for the correlation of an observed phenomenon, such as increased invasiveness, with an underlying mechanistic explanation for said behavior. Intravital imaging has demonstrated that the metastatic potential of tumor cells not only depends on their intrinsic properties but also on their interactions with the tumor microenvironment, as evident by the paracrine invasion loop.
For the future, the challenge remains to image secondary organ sites that are susceptible to the development of micrometastases, such as the lungs [49] and bone marrow [50]. A common disadvantage of current multiphoton experiments is that surgical dissection is required in order to expose the site to be imaged, which prevents imaging for long periods of time and the subsequent visualization of tumor cell colonization and dormancy. While the utilization of imaging windows in the mammary fat pads and brain [28, 51] has started to address this limitation, the expansion of this technique to other tissues prone to developing metastases, e.g. lymph nodes and liver, will aid in resolving some of the unanswered questions that remain – why are carcinoma cells more likely to colonize certain organs while failing to form colonies in others as well as how long carcinoma cells remain dormant and what causes them to reactivate. The use of biosensors in intravital imaging will allow investigators to follow intracellular signaling pathways during the various steps of metastasis. For example, with the use of reactive oxygen species biosensors [52], we anticipate a better understanding of how the tumor microenviroment influences carcinoma cell escape from the primary tumor and colonization in other organs. Similarly, intravital characterization of the underlying transcriptional and differentiation state of carcinoma cells will help in identifying intermediate stages in metastasis. Using genetically engineered melanoma cells, it was demonstrated that when melanoma cells leave the primary tumor in order to development distant metastatic sites, they switch from a less to a more differentiated state [53]. Conducting experiments like these will help in addressing questions that remain in the field, such as whether tumor cells undergo the epithelial-mesenchymal transition (EMT) only transiently or permanently switch to have a more stem cell phenotype.
With respect to therapeutic intervention, the role of tumor associated macrophages in tumor cell invasion has been identified as a target. Further elucidation of the relationship between the structure of the tumor microenvironment (including blood vessels, lymphatics and extracellular matrix as well as stromal cells) and tumor cell malignancy will continue to rely upon intravital imaging to identify new ways to attack cancer through interruption of tumor cell interactions with the microenvironment. The next frontiers for these studies will be the metastasis microenvironments [54]. Interactions between tumor cells and the local microenvironment are likely to be site specific and thus combinations of therapies attacking interactions that occur in different microenvironments need to be evaluated for their ability to improve patient survival.
Acknowledgments
Financial support: This work was supported by grants CA77522 and CA100324 from the NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chambers AF, et al. Dissemination and growth of cancer cells in metastatic sites. Nature Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 3.Mbeunkui F, Johann DJ., Jr Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571–582. doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migrations, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Giampieri S, et al. Intravital imaging illuminates transforming growth factor b signaling switches during metastasis. Cancer Res. 2010;70:3435–3439. doi: 10.1158/0008-5472.CAN-10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–6288. [PubMed] [Google Scholar]
- 8.Vakoc BJ, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nature Med. 2009;15:1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez L, et al. In vivo assay for tumor cell invasion. Methods Mol Biol. 2009;571:227–238. doi: 10.1007/978-1-60761-198-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyckoff JB, et al. The collection of the motile population of cells from a living tumor. Cancer Res. 2000;60:5401–5404. [PubMed] [Google Scholar]
- 11.Francia G, et al. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nature Rev Cancer. 2011;11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deryugina EI, Quigley JP. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem Cell Biol. 2008;130:1119–1130. doi: 10.1007/s00418-008-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohela M, Werb Z. Intravital imaging of stromal cell dynamics in tumors. Curr Opin Genet Dev. 2010;20:72–78. doi: 10.1016/j.gde.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cariati M, et al. Xenotransplantation of breast cancers. Methods Mol Biol. 2011;731:471–482. doi: 10.1007/978-1-61779-080-5_38. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson JN, Muller WJ. Transgenic mouse models of human breast cancer. Oncogene. 2000;53:6130–6137. doi: 10.1038/sj.onc.1203970. [DOI] [PubMed] [Google Scholar]
- 16.Brehm MA, et al. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17:120–125. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoletov K, et al. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci. 2010;123:2332–2341. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miretti S, et al. A mouse model of pulmonary metastasis from spontaneous osteosarcoma monitored in vivo by luciferase imaging. PLoS One. 2008;3:e1828. doi: 10.1371/journal.pone.0001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowey S, et al. Breast cancer metastasis to bone: evaluation of bioluminescent imaging and microSPECT/CT for detecting bone metastasis in immunodeficient mice. Clin Exp Metastasis. 2007;24:389–401. doi: 10.1007/s10585-007-9076-8. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman RM. Whole-body fluorescence imaging with green fluorescence protein. Methods Mol Biol. 2002;183:135–148. doi: 10.1385/1-59259-280-5:135. [DOI] [PubMed] [Google Scholar]
- 21.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 22.Judenhofer MS, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 23.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, et al. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 2007;67:3505–3511. doi: 10.1158/0008-5472.CAN-06-3714. [DOI] [PubMed] [Google Scholar]
- 25.Wyckoff JB, et al. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–2511. [PubMed] [Google Scholar]
- 26.Quaranta V. Motility cues in the tumor microenvironment. Differentiation. 2002;70:590–598. doi: 10.1046/j.1432-0436.2002.700912.x. [DOI] [PubMed] [Google Scholar]
- 27.Xue C, et al. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 2006;66:192–197. doi: 10.1158/0008-5472.CAN-05-1242. [DOI] [PubMed] [Google Scholar]
- 28.Kedrin D, et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Meth. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan F, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan F, et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial window. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 31.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 32.Dvorak HF, et al. Tumor architecture and targeted delivery. In: Abrams PG, Fritzberg AR, editors. Radioimmunotherapy of Cancer. Marcel Dekker, Inc; 2002. pp. 107–135. [Google Scholar]
- 33.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1 and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Yuan F, et al. Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc Res. 1993;45:269–289. doi: 10.1006/mvre.1993.1024. [DOI] [PubMed] [Google Scholar]
- 35.Hoshida T, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:236–243. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 36.Helmlinger G, et al. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 37.Harris AL. Hypoxia: a key regulatory factor in tumor growth. Nature Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 38.Xu L, et al. Acidic extracellular pH induces vascular endothelial growth factor (VEGF) in human glioblastoma cells via ERK1/2 MAPK signaling pathway – mechanism of low pH-induced BEGF. J Biol Chem. 2002;277:11368–11374. doi: 10.1074/jbc.M108347200. [DOI] [PubMed] [Google Scholar]
- 39.Fukumura D, et al. Hypoxia and acidosis independently up-regulate vascular endothelial factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- 40.Nolte C, et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- 41.Ahmed F, et al. GFP expression in the mammary gland for imaging of mammary tumor cells in transgenic mice. Cancer Res. 2002;62:7166–7169. [PubMed] [Google Scholar]
- 42.Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 43.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 44.Wyckoff JB, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez, et al. The EGF/CSF-1 paracrine invasion loop can be triggered by heregulin beta1 and CXCL12. Cancer Res. 2009;69:3221–3227. doi: 10.1158/0008-5472.CAN-08-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patsialou A, et al. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009;69:9498–506. doi: 10.1158/0008-5472.CAN-09-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 48.Condeelis J, et al. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Mol Cell Biol. 2005;12:954–961. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- 49.Kimura H, et al. Real-time imaging of single cancer-cell dynamics of lung metastasis. J Cell Biochem. 2010;109:58–64. doi: 10.1002/jcb.22379. [DOI] [PubMed] [Google Scholar]
- 50.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumor engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kienast Y, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 52.Guzy RD, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Pinner S, Sahai E. Imaging amoeboid cancer cell motility in vivo. J Microsc. 2008;231:441–445. doi: 10.1111/j.1365-2818.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 54.Qian BZ, Pollard JW. Macrophage density enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyons SK. Advances in imaging mouse tumour models in vivo. J Pathol. 2005;205:194–205. doi: 10.1002/path.1697. [DOI] [PubMed] [Google Scholar]
- 56.Leblond F, et al. Pre-clinical whole-body fluorescence imaging: review of instruments, methods and applications. J Photochem Photobiol B. 2010;98:77–94. doi: 10.1016/j.jphotobiol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez-Suarez M, Ting AY. Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol. 2008;9:929–943. doi: 10.1038/nrm2531. [DOI] [PubMed] [Google Scholar]
- 58.Siegel PM, et al. Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol Cell Biol. 1994;14:7068–7077. doi: 10.1128/mcb.14.11.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siegel PM, et al. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. Embo J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyckoff JW, et al. High-resolution multiphoton imaging of tumors in vivo. Cold Spring Harbor Chapter. 2011;24:441–461. doi: 10.1101/pdb.top065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Entenberg D, et al. Two laser multiphoton microscope and analysis software for multichannel intravital fluorescence imaging. Nature Protocols. 2011 doi: 10.1038/nprot.2011.376. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]