Abstract
With using the carbon nano-tube (CNT) of high chemical activity, nano-TiCx particles with different growth shapes were synthesized through the self-propagating high temperature in the 80 wt.% metal (Cu, Al, and Fe)-Ti-CNT systems. The growth shapes of the TiCx particles are mainly octahedron in the Cu- and Al-Ti-CNT systems, while mainly cube- and sphere-like in the Fe-Ti-CNT system.
Keywords: self-propagating high-temperature synthesis (SHS), carbon nanotubes, nano-TiCx particles
Introduction
As known, some ceramic particles, such as titanium carbide (TiCx), are usually used as the reinforcing phases in the composites due to their unique properties such as high melting point, extreme hardness, and high resistance to corrosion and oxidation. Recently, many experimental and theoretical studies have indicated that decreasing the sizes of the reinforcing ceramic particulates can lead to substantial improvements in mechanical performance of the composites [1-11]. For example, Ma et al. [11] showed that the tensile strength of 1 vol.% Si3N4 (10 nm)/Al composite is comparable to that of the 15 vol.% SiCp (3.5 μm)/Al composite, and the yield strength of the former is much higher than that of the latter. Then, with significantly increasing intention to develop nanoparticle-reinforced composites with superior mechanical properties, the demand for nano-sized ceramic powders, including TiCx, has become more urgent.
Among the variety of the preparation methods for TiCx, self-propagating high-temperature synthesis (SHS) is noted by us because it is a convenient and efficient way to synthesize TiCx. However, the SHS is quite challenging to produce the nano-sized ceramic particles because the combustion temperature will lead to considerable coarsening of the ceramic particles. At present, the usual method for synthesizing the nano-ceramic particles through the SHS is the addition of volatile diluents such as NaCl into the reactants. Some nano-ceramic particles such as TiB2 and ZrB2 have been prepared by adding NaCl to the SHS reactants [12-14], and the nano-TiCx particles (20 to 100 nm) were also obtained by Nersisyan et al. [15] in the 30 wt.% NaCl-Ti-carbon black system.
On the other hand, the addition of a second metal (Me) such as Al, Cu, and Fe can also decrease the combustion temperature and thus prevent the ceramic particles from further growth. For example, with the increase in the Al incorporation from 10 to 40 wt.%, the sizes of the TiCx particles decrease from about 3 μm to 400 nm [16]. However, when more Me (≥50 wt.%) is incorporated, the SHS reaction tends to be incomplete or even cannot be ignited. Generally, this situation can be improved through using finer C-source particles because they can enlarge the area of the contact surface between the liquid and the carbon source and decrease the activation energy of the SHS reaction. At present, the source of C that are mostly used during the SHS are graphite (typically 1 to 150 μm) and C black (< 100 nm). In contrast to them, carbon nano-tube (CNT) has much finer size, usually 5 to 20 nm in diameter. In fact, CNT has been used to synthesize the nanostructured TiC-TiB2 [17] and carbide nanofibers [18] during the SHS.
In this paper, taking advantage of high chemical activity of the CNT, we tried to prepare the nano-sized TiCx particles during the SHS in the Me (Cu, Al, and Fe)-Ti-CNT systems with the high contents of the Me incorporation. The morphologies of the TiCx particles formed in these systems were investigated, and the mechanism for the difference in their morphology was discussed.
Experimental methods
The raw materials utilized were multi-walled carbon nanotubes (20 to 30 nm in diameter and approximately 30 μm in length, purity > 95 wt.%, Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences, Chengdu, China), Ti powders (> 99.5% purity, approximately 48 μm, Institute of Nonferrous Metals, Beijing, China), Al powders (> 99.0% purity, approximately 48 μm, Northeast Light Alloy Ltd. Co., Harbin, China), Cu powders (> 99.5% purity, approximately 48 μm, Institute of Nonferrous Metals, Beijing, China) and Fe powders (> 99.5% purity, approximately 48 μm, Institute of Nonferrous Metals, Beijing, China). The Ti and CNT powders with a molar ratio of 1:1 were mixed with the Me (Cu, Al, and Fe) powders in relative quantities of 50, 60, 70, and 80 wt.%, respectively. The reactants were mixed sufficiently by ball milling at a low speed (approximately 35 rpm) for 6 h and then pressed into the cylindrical compacts of approximately 22 mm in diameter and approximately 15 mm in height with green densities of approximately 60 ± 2% of theoretical. The SHS experiments were conducted in a self-made vacuum vessel in an Ar atmosphere using an arc as ignition source. During the SHS process, the temperature in the position about 3 mm beneath the center of the compact top surface was measured by W5-Re26 thermocouples, and the signals were recorded and processed by a data acquisition system using an acquisition speed of 50 ms per point.
The phase compositions in the reacted samples were identified by X-ray diffraction (XRD, Rigaku D/Max 2500PC, Rigaku Corporation, Tokyo, Japan) with CuKα radiation using a scanning speed of 4°/min. The reacted Cu-Ti-CNT samples were then dissolved in a saturated FeCl3 water solution, and the reacted Al- and Fe-Ti-CNT samples were dissolved in an 18 vol.% HCl-distilled water solution, to remove the Me coatings on the surfaces of the TiCx particles. The morphologies of the extracted TiCx particles were observed using a field emission scanning electron microscope (FESEM, JSM 6700F, JEOL, Tokyo, Japan) and a transmission electron microscope (TEM, JSM 200EX, JEOL).
Results and discussion
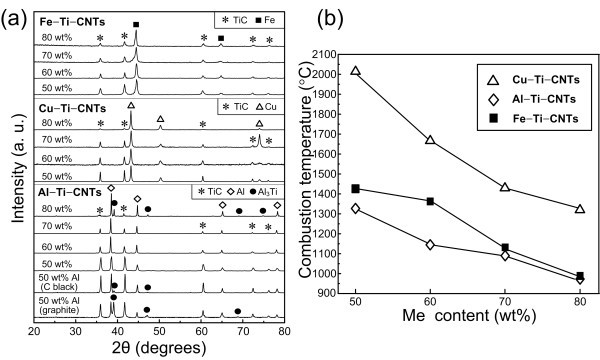
In the Me-Ti-C systems, the Me-Ti liquid forms firstly during the heating. The carbon then diffuses into the Me-Ti liquid, and when a critical concentration is achieved, the TiCx begins to form by reaction between [C] and [Ti]. Accordingly, the diffusion of carbon in the molten metals is a key step to form TiCx, and thus different carbon sources, i.e., graphite and C black, have great effects on the product morphology and the reaction rate of [Ti] and [C] to form TiCx. Generally speaking, the carbon source with finer sizes will make the combustion reaction proceed more thoroughly. For example, when C black was used as the carbon source in 50 wt.% Al-Ti-C system, the content of the intermediate phase Al3Ti decreases greatly than that of the graphite being used as the carbon source (Figure 1a). In contrast to the graphite and C black, carbon nano-tube (CNT) has much finer sizes. Furthermore, the defects such as pentagons, heptagons and vacancies in the structure of the CNT endow it with more chemical activity [19,20]. Therefore, the CNT will dissolve more rapidly in the liquid Me to provide dissociated [C], which promotes the SHS reaction. This speculation was proved as there is no Al3Ti formed in the 50 wt.% Al-Ti-CNT system. Actually, only when the Al content was increased to 80 wt.% in the Al-Ti-CNT system, a little amount of Al3Ti formed. In Cu- and Fe-Ti-CNT systems, within the range of 50 to 80 wt.% for the Me content, no Al3Ti is formed.
Figure 1.
XRD patterns of SHS products and the variation in the maximum combustion temperature. (a) XRD patterns of the SHS products and (b) the variation in the maximum combustion temperature with the Me content.
As known, according to Merzhanov's empirical criterion, for the reaction to be self-sustaining in the absence of preheat, the adiabatic temperature (Tad) should not be less than 1,800 K, corresponding to the maximum addition of 67.12 wt.% Cu, 46.65 wt.% Al [16], and 77.4 wt.% Fe [21] in the Me-Ti-C systems, respectively. However, in our experiments, because of the high activity of the CNT, the samples with 70 wt.% Al and 80 wt.% Cu and Fe can be ignited easily. Figure 1b shows the variation in the maximum combustion temperature with the Me content. Clearly, the maximum combustion temperature in all the systems decreases as the Me content increases, and the sequence is TCu-Ti-CNT >TFe-Ti-CNT >TAl-Ti-CNT. The difference in the combustion temperature in these systems, of course, will have an important influence on the shape and size of the synthesized TiCx particles.
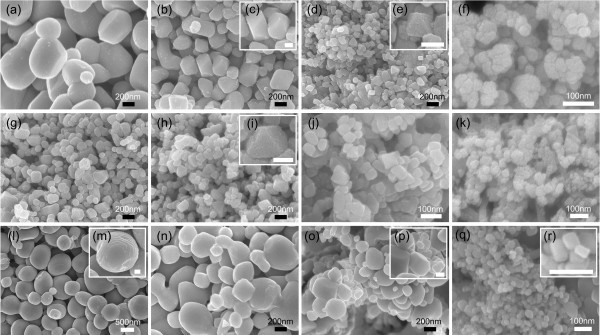
As indicated in Figure 2, with increasing the Me content, the TiCx particles formed in the Cu-, Al-, and Fe-Ti-CNT systems show a significant decrease in size. In the sample with 50 wt.% Cu, the sizes of the TiCx particles are about 600 nm (Figure 2a), while when the Cu content increases to 60, 70, and 80 wt.%, the sizes of the TiCx particles decrease to about 400, 100, and 60 nm, respectively (Figure 2b, d, f). Accompanying the decrease in the particle size, the TiCx particles change their shapes from sphere-like to regular octahedron (Figure 2c, e). The same growth shape as octahedron can be also observed in the TiCx particles formed in the samples with 50, 60, and 70 wt.% Al (Figure 2g, h, j), of which the particle sizes are about 200, 150, and 70 nm, respectively. When the Al content is increased to 80 wt.%, the shape of the TiCx particles cannot be observed clearly, and the particle size decreases to about 40 nm (Figure 2k). As we have suggested before, in the Al-Ti-C system, the TiCx particles grow through the deposition and lateral stacking of the growth units on the (111) surfaces [22,23]. In contrast to the growth mode of the TiCx particles in the Al-Ti-CNT system, the TiCx particles growing in the Fe-Ti-CNT system have a different growth mode, i.e., the lateral stack along the (100) surfaces (Figure 2m). Under this mode, the TiCx particles should grow into the cubic shapes. However, because of the round turning of the (100) surfaces, most of the TiCx particles in the Fe-Ti-CNT system show the sphere-like shapes (Figure 2l). When the Fe content increases, the sizes of the TiCx particles decrease and the cubic character of the TiCx particles becomes more and more distinct (Figure 2h). In the sample with 70 wt.% Fe, there are many TiCx particles with regular cubic shapes and sizes of about 200 nm (Figure 2o). Increasing the Fe content to 80 wt.% further decreases the sizes of the TiCx particles to approximately 70 nm, with primarily cubic shapes (Figure 2q).
Figure 2.
Morphologies of the TiCx particles formed in the Me-Ti-CNT systems. (a) 50 wt.% Cu, (b, c) 60 wt.% Cu, (d, e) 70 wt.% Cu, (f) 80 wt.% Cu, (g) 50 wt.% Al, (h, i) 60 wt.% Al, (j) 70 wt.% Al, (k) 80 wt.% Al, (l, m) 50 wt.% Fe, (n) 60 wt.% Fe, (o, p) 70 wt.% Fe, and (q, r) 80 wt.% Fe. The scale bars in the inset images represent 100 nm.
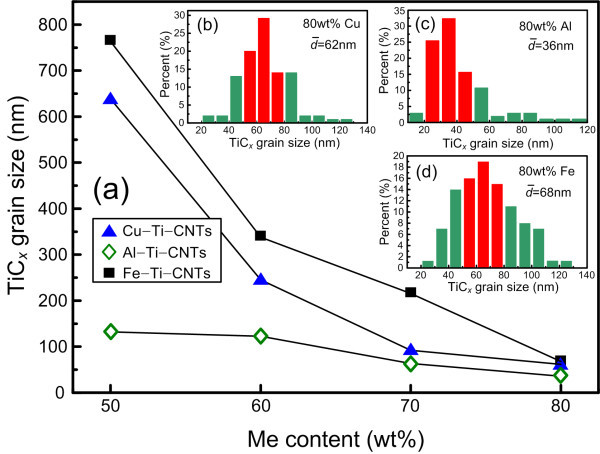
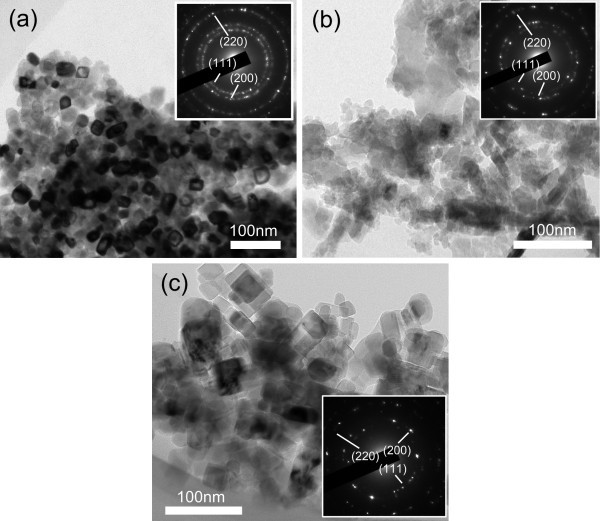
Figure 3a gives the mean sizes based on the statistic analysis of a hundred of TiCx particles in the FESEM images for the Me-Ti-CNT systems. The decrease in the TiCx particle sizes with the increase in the Me content is easy to understand because of the decreasing combustion temperature. When the Me content increases to 80 wt.% for Cu, Al, and Fe, the sizes of the TiCx particles decrease to about , , and nm, respectively. Furthermore, it can be noticed that in the above Me-Ti-CNT systems, the TiCx particles formed in the Al-Ti-CNT samples are the finest, which could be attributed to the lowest combustion temperatures. Nevertheless, the TiCx particles formed in the Fe-Ti-CNT samples have the largest sizes even though their combustion temperatures are quite lower than those formed in the Cu-Ti-CNT samples. This phenomenon is meaningful to the discussion in the following paragraphs on the mechanism of the TiCx shape variation with the different kinds of the Me addition. Figure 4 gives the TEM images of the TiCx particles formed in the samples with 80 wt.% Me. The diffraction rings from inner to outer in the inserted images in Figure 4a, b, c match the (111), (200), and (220) planes of the fcc TiC.
Figure 3.
Mean sizes and the size distribution of the TiCx particles. (a) Mean sizes calculated based on the statistic analysis of a hundred of TiCx particles in the FESEM images. (b, c, d) Size distribution of the TiCx particles formed in the samples with 80 wt.% Cu, Al, and Fe, respectively.
Figure 4.
TEM images of the TiCx particles formed in the Me-Ti-CNT samples. (a) 80 wt.% Cu, (b) 80 wt.% Al, and (c) 80 wt.% Fe. Inset images show the corresponding diffraction rings.
As we have mentioned, the shapes of the TiCx particles vary considerably in the different kinds of the Me incorporated Ti-CNT systems, i.e., the TiCx particles formed in the Cu- and Al-Ti-CNT systems are mainly with the octahedral shapes, while those formed in the Fe-Ti-CNT system are mainly with the cubic and sphere-like shapes. In our pervious paper [23], we have suggested that the growth shapes of the TiCx particles in the Al-Ti-C system should be directly related to their stoichiometry (x), i.e., when the stoichiometry is low, the TiCx (111) surfaces are the most stable and the growth shape is octahedron, while when the stoichiometry increases, the free energy of the (111) surfaces increases, which leads to the diminishing in the (111) surfaces on the TiCx crystals and the exposure of the (100) surfaces. According to this speculation, the stoichiometry of the TiCx crystals formed in the Cu- and Al-Ti-CNT systems should be low and that in the Fe-Ti-CNT system should be high. Here, we qualitatively estimate the stoichiometry of the TiCx formed in the combustion stage based on the phenomenon that the TiCx particles grown in the Fe-Ti-CNT samples are the largest while their combustion temperatures are relatively low. As known, carbon has good chemical affinity with Fe. Hence, the carbon atoms could dissolve rapidly in the Fe melt, which leads to the formation of the C-rich regions near the CNTs at the initial stage of the SHS. In these C-rich regions, the TiCx particles form and grow rapidly. That is why the sizes of the TiCx particles formed in the Fe-Ti-CNT system are generally large even though their combustion temperatures are quite low. As another consequence of the high C concentration, the stoichiometry of these primitively formed TiCx particles in the Fe melt is relatively high. Then, the (100) surfaces of TiCx are stable and the growth shape is cube. For the Cu- and Al-Ti-CNT systems, the CNT dissolves more slowly because of the poor chemical reactivity between carbon and the Cu (or Al) melt as well as very limited solubility of carbon in molten Cu and Al. In this case, the TiCx forms and grows under a condition of C scarcity. Hence, the TiCx particles grown in these two melts are with relatively small sizes, and the TiCx stoichiometry formed at the combustion stage is low. Accordingly, the TiCx growth shape is octahedron.
Frankly speaking, spending a great amount of metal (Al/Cu/Fe) to only synthesize the TiCx nanoparticles is really uneconomical. Nevertheless, considering that the TiCx particles reinforced metal matrix composites can be fabricated conveniently through following a pressing or forging treatment after the SHS [24], the real significance of this research is to provide a perspective to in situ synthesize the nano-TiCx particle reinforced composites more conveniently by using CNT. As known, the fabrication of ceramic nanoparticles reinforced metal matrix is an important development direction for the development of composites, and many papers have been published on this issue from 2000. In 99% of these works, the nanoparticles were introduced into the metal matrix through external addition. In these methods, the mixing of nano-sized particles in metal liquid is usually lengthy, expensive, and energy consuming. In fact, in contrast with the external addition methods, the method with nanoparticles in situ synthesis has the advantages of a more homogeneous distribution of the nanoparticles, clearer interface between nanoparticles and matrix, and lower chances to introduce impurity. However, when metal matrix is with high content (≥50 wt.%), the TiCx formation reaction tends to be incomplete or even cannot be ignited by using traditional C sources such as C black or graphite. To solve this key question in the SHS, we used CNT as the C source in this paper. The results indicate that the samples with more than 70 wt.% metals can still be ignited easily because of the high activity of the CNT. In fact, in our following study, by using CNT as C source, we have successfully in situ synthesized the TiCx nanoparticles in 97 wt.% Cu matrix, and the composite was fabricated conveniently by the SHS and a subsequent pressing or forging process. Moreover, our results suggest that other nano-sized transition metal carbides (such as SiC, ZrC, and NbC) and the corresponding reinforced composites could also be synthesized with using the high chemical activity of the CNT.
Conclusions
The using of CNT increases the reactivity in the Me (Cu, Al, and Fe)-Ti-CNT systems and makes SHS reaction more easily ignited. The sizes of the synthesized TiCx particles decrease with the increase in the Me content. When the Me content increases to 80 wt.% for Cu, Al, and Fe, the sizes of the TiCx particles decrease to about , , and nm, respectively. The shapes of the nano-TiCx particles formed in the Cu- and Al-Ti-CNT systems are mainly octahedral, while those formed in the Fe-Ti-CNT system are mainly cubic and sphere-like. This shape variation of the TiCx formed in different kinds of the Me liquid environment is believed to relate to the different stoichiometries of the TiCx formed during the combustion stage in these systems.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All the authors contributed to writing of the manuscript. SBJ carried out the experiments under the instruction of QCJ.
Contributor Information
Shenbao Jin, Email: jinshenbao@gmail.com.
Ping Shen, Email: shenping@jlu.edu.cn.
Dongshuai Zhou, Email: jsbking@126.com.
Qichuan Jiang, Email: jqc@jlu.edu.cn.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 51171071), National Basic Research Program of China (973 Program) (No. 2012CB619600), NNSFC (No. 50971065 and No. 50531030), the Project 985-High Performance Materials of Jilin University and Project 20092008 supported by Graduate Innovation Fund of Jilin University.
References
- Kang YC, Chan SL. Tensile properties of nanometric Al2O3 particulate-reinforced aluminum matrix composites. Mater Chem Phys. 2004;85:438–443. doi: 10.1016/j.matchemphys.2004.02.002. [DOI] [Google Scholar]
- Liu YQ, Cong HT, Wang W, Sun CH, Cheng HM. AlN nanoparticle-reinforced nanocrystalline Al matrix composites: fabrication and mechanical properties. Mater Sci Eng A. 2009;505:151–156. doi: 10.1016/j.msea.2008.12.045. [DOI] [Google Scholar]
- Hesabi ZR, Hafizpour HR, Simchi A. An investigation on the compressibility of aluminum/nano-alumina composite powder prepared by blending and mechanical milling. Mater Sci Eng A. 2007;454-455:89–98. [Google Scholar]
- Hemanth J. Development and property evaluation of aluminum alloy reinforced with nano-ZrO2 metal matrix composites (NMMCs) Mater Sci Eng A. 2009;507:110–113. doi: 10.1016/j.msea.2008.11.039. [DOI] [Google Scholar]
- Woo KD, Zhang DL. Fabrication of Al-7wt%Si-0.4wt%Mg/SiC nanocomposite powders and bulk nanocomposites by high energy ball milling and powder metallurgy. Curr Appl Phys. 2004;4:175–178. doi: 10.1016/j.cap.2003.11.002. [DOI] [Google Scholar]
- Ying DY, Zhang DL. Processing of Cu-Al2O3 metal matrix nanocomposite materials by using high energy ball milling. Mater Sci Eng A. 2000;286:152–156. doi: 10.1016/S0921-5093(00)00627-4. [DOI] [Google Scholar]
- Hassan SF, Gupta M. Development of high performance magnesium nano-composites using nano-Al2O3 as reinforcement. Mater Sci Eng A. 2005;392:163–168. doi: 10.1016/j.msea.2004.09.047. [DOI] [Google Scholar]
- Lee CJ, Huang JC, Hsieh PJ. Mg based nano-composites fabricated by friction stir processing. Scr Mater. 2006;54:1415–1420. doi: 10.1016/j.scriptamat.2005.11.056. [DOI] [Google Scholar]
- Wong WLE, Gupta M. Improving overall mechanical performance of magnesium using nano-alumina reinforcement and energy efficient microwave assisted processing route. Adv Eng Mater. 2007;9:902–909. doi: 10.1002/adem.200700169. [DOI] [Google Scholar]
- Artzt E. Size effects in materials due to microstructural and dimensional constraints: a comparative review. Acta Mater. 1998;46:5611–5626. doi: 10.1016/S1359-6454(98)00231-6. [DOI] [Google Scholar]
- Ma ZY, Tjong SC, Li YL, Liang Y. High temperature creep behavior of nanometric Si3N4 particulate reinforced aluminium composite. Mater Sci Eng A. 1997;225:125–134. doi: 10.1016/S0921-5093(96)10870-4. [DOI] [Google Scholar]
- Khanra AK, Pathak LC, Mishra SK, Godkhindi MM. Effect of NaCl on the synthesis of TiB2 powder by a self-propagating high-temperature synthesis technique. Mater Lett. 2004;58:733–738. doi: 10.1016/j.matlet.2003.06.003. [DOI] [Google Scholar]
- Khanra AK, Pathak LC, Mishra SK, Godkhindi MM. Self-propagating-high-temperature synthesis (SHS) of ultrafine ZrB2 powder. J Mater Sci Lett. 2003;22:1189–1191. doi: 10.1023/A:1025336230885. [DOI] [Google Scholar]
- Camurlu HE, Maglia F. Preparation of nano-size ZrB2 powder by self-propagating high-temperature synthesis. J Eur Ceram Soc. 2009;29:1501–1506. doi: 10.1016/j.jeurceramsoc.2008.09.006. [DOI] [Google Scholar]
- Nersisyan HH, Lee JH, Won CW. Self-propagating high-temperature synthesis of nano-sized titanium carbide powder. J Mater Res. 2002;17:2859–2864. doi: 10.1557/JMR.2002.0415. [DOI] [Google Scholar]
- Song MS, Huang B, Zhang MX, Li JG. Study of formation behavior of TiC ceramic obtained by self-propagating high-temperature synthesis from Al-Ti-C elemental powders. Int J Refractory Met Hard Mater. 2009;27:584–589. doi: 10.1016/j.ijrmhm.2008.09.009. [DOI] [Google Scholar]
- Deorsola FA, Atias Adrian IC, Ortigoza Villalba GA, DeBenedetti B. Nanostructured TiC-TiB2 composites obtained by adding carbon nanotubes into the self-propagating high-temperature synthesis process. Mater Res Bull. 2011;46:995–999. doi: 10.1016/j.materresbull.2011.03.017. [DOI] [Google Scholar]
- Lia XK, Westwood A, Brown A, Brydson R, Rand B. A convenient, general synthesis of carbide nanofibres via templated reactions on carbon nanotubes in molten salt media. Carbon. 2009;47:201–208. doi: 10.1016/j.carbon.2008.09.050. [DOI] [Google Scholar]
- Charlier JC. Defects in carbon nanotubes. Acc Chem Res. 2002;35:1063–1069. doi: 10.1021/ar010166k. [DOI] [PubMed] [Google Scholar]
- Mintmire JW, White CT. Electronic and structural properties of carbon nanotubes. Carbon. 1995;33:893–902. doi: 10.1016/0008-6223(95)00018-9. [DOI] [Google Scholar]
- Saidi A, Chrysanthou A, Wood JV, Kellie JLF. Characteristics of the combustion synthesis of TiC and Fe-TiC composites. J Mater Sci. 1994;29:4993–4998. doi: 10.1007/BF01151089. [DOI] [Google Scholar]
- Jin SB, Shen P, Zou BL, Jiang QC. Morphology evolution of TiCx grains during SHS in an Al-Ti-C system. Cryst Growth Des. 2009;9:646–649. doi: 10.1021/cg800527q. [DOI] [Google Scholar]
- Jin SB, Shen P, Lin QL, Zhan L, Jiang QC. Growth mechanism of TiCx during self-propagating high-temperature synthesis in an Al-Ti-C system. Cryst Growth Des. 2010;10:1590–1597. doi: 10.1021/cg9010983. [DOI] [Google Scholar]
- Shu SL, Lu JB, Qiu F, Xuan QQ, Jiang QC. Effects of alloy elements (Mg, Zn, Sn) on the microstructures and compression properties of high-volume-fraction TiCx/Al composites. Scr Mater. 2010;63:1209–1211. doi: 10.1016/j.scriptamat.2010.08.040. [DOI] [Google Scholar]