Abstract
Tuning the electronic band structures such as band-edge position and bandgap of organic semiconductors is crucial to maximize the performance of organic photovoltaic devices. We present a simple yet effective electron irradiation approach to tune the band structure of [6, 6]-phenyl-C61-butyric acid methyl ester (PCBM) that is the most widely used organic acceptor material. We have found that the lowest unoccupied molecular orbital (LUMO) level of PCBM up-shifts toward the vacuum energy level, while the highest occupied molecular orbital (HOMO) level down-shifts when PCBM is electron-irradiated. The shift of the HOMO and the LUMO levels increases as the irradiated electron fluence increases. Accordingly, the band-edge position and the bandgap of PCBM can be controlled by adjusting the electron fluence. Characterization of electron-irradiated PCBM reveals that the variation of the band structure is attributed to the molecular structural change of PCBM by electron irradiation.
Keywords: tunable band structure, HOMO, LUMO, organic semiconductor, PCBM, electron irradiation
Introduction
Organic semiconductors such as small molecules [1,2] and conjugated polymers [3,4] are widely used in organic photovoltaic cells [4-6], dye-sensitized solar cells [2,7], organic field-effect transistors [8-10], and organic light-emitting diodes [3,11]. In particular, [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) is a small molecule that is most widely used as an electron acceptor in organic photovoltaic (OPV) cells [1]. To improve the power conversion efficiency of OPV cells, open-circuit voltage (Voc) of the cells should be increased. The upper limit of the Voc is determined by the energy difference between the highest occupied molecular orbital (HOMO) level of the electron donor and the lowest unoccupied molecular orbital (LUMO) level of the electron acceptor [12]. Thus, several efforts have been made to increase the LUMO level of PCBM by chemical approach, for instance, placing electron-donating and electron-withdrawing substituents on the phenyl ring or synthesizing bisadduct analogue of PCBM [12-14]. However, these approaches generally require complicated synthetic procedures and result in a low yield of the products [13]. As an alternative, radiation chemistry can be a good strategy to modify the chemical structures of particularly organic materials [15-18]. As a result of the chemical structural modification, the optical properties of the organic materials can be changed [19-21]. Here, we present a simple and novel approach to tune the HOMO and LUMO levels of PCBM based on electron irradiation. Only by irradiating an electron beam onto PCBM, the bandgap as well as the HOMO and LUMO levels of PCBM can be changed, and furthermore the electronic band structures of PCBM can be controlled by adjusting the electron fluence.
Results and discussion
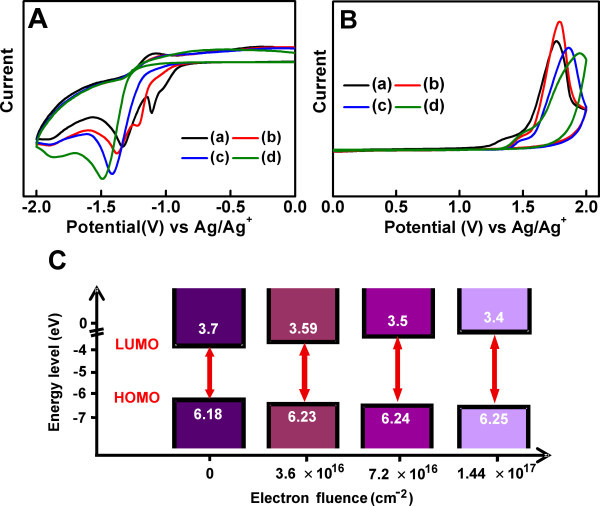
Figure 1 shows the reduction and oxidation properties of electron-irradiated PCBM thin films as well as pristine PCBM measured by cyclic voltammetry (CV). Pristine PCBM exhibits three reduction peaks (-1.11 V, -1.33 V, and -1.92 V vs. Ag/Ag+) and one oxidation peak (+1.76 V vs. Ag/Ag+). Electron irradiation led to a negative shift of the reduction peaks and a positive shift of the oxidation peak. Interestingly, only two reduction peaks appeared for the electron-irradiated PCBM at fluences higher than 7.2 × 1016 cm-2. The first reduction peak of pristine PCBM was located at -1.11 V vs. Ag/Ag+; however, the peak was negatively shifted to -1.22 V, -1.41 V, and -1.49 V as the electron fluence was increased to 3.6 × 1016, 7.2 × 1016, and 1.44 × 1017 cm-2, respectively. On the contrary, the oxidation peak was positively shifted from the pristine value of 1.76 to 1.79, 1.86, and 1.94 V with increasing the electron fluence to 3.6 × 1016, 7.2 ×, and 1.44 × 1017, respectively.
Figure 1.
Reduction and oxidation properties of electron-irradiated PCBM thin films, pristine PCBM measured by cyclic voltammetry. Cyclic voltammogram of cathodic scan (A) and anodic scan (B) of PCBM film after electron irradiation at different fluences of (a) 0, (b) 3.6 × 1016 cm-2, (c) 7.2 × 1016 cm-2, and (d) 1.44 × 1017 cm-2. (C) HOMO and LUMO levels of PCBM changed by electron irradiation as a function of electron fluence.
From the CV measurements, the LUMO and HOMO levels of PCBM were calculated using the following equation [22]:
| (1) |
where Eon is the onset potential of the first reduction peak for LUMO or the onset potential of the oxidation peak for HOMO, and E1/2 (ferrocene) is the half-wave potential of a ferrocene redox reaction. The LUMO level up-shifts toward the vacuum energy level, while the HOMO level down-shifts with increasing the electron fluence (Figure 1C, the detailed values are shown in Table 1). The LUMO level of the irradiated PCBM was increased by 0.3 eV and the HOMO level was decreased by 0.07 eV compared to the respective values of the pristine PCBM when the electron fluence was 1.44 × 1017 cm-2. Consequentially, the bandgap of electron-irradiated PCBM gradually increases with increasing the electron fluence: the bandgap increases from the pristine value of 2.48 to 2.85 eV at the electron fluence of 1.44 × 1017 cm-2.
Table 1.
HOMO and LUMO levels of PCBM changed by electron irradiation as a function of electron fluence
| Ep, red(1) | Eon, red (1) | LUMO | Ep, ox | Eon, ox | HOMO | |
|---|---|---|---|---|---|---|
| PCBM | -1.11 | -0.98 | -3.70 | 1.76 | 1.50 | -6.18 |
| PCBM (3.6 × 1016 cm-2) | -1.22 | -1.09 | -3.59 | 1.79 | 1.55 | -6.23 |
| PCBM (7.2 × 1016 cm-2) | -1.41 | -1.18 | -3.50 | 1.86 | 1.56 | -6.24 |
| PCBM (1.44 × 1017 cm-2) | -1.49 | -1.28 | -3.40 | 1.94 | 1.57 | -6.25 |
The first reduction and oxidation peak potentials (voltage vs. Ag/Ag+), onset potentials, and LUMO and HOMO level (electron volts) of electron-irradiated PCBM at different fluences.
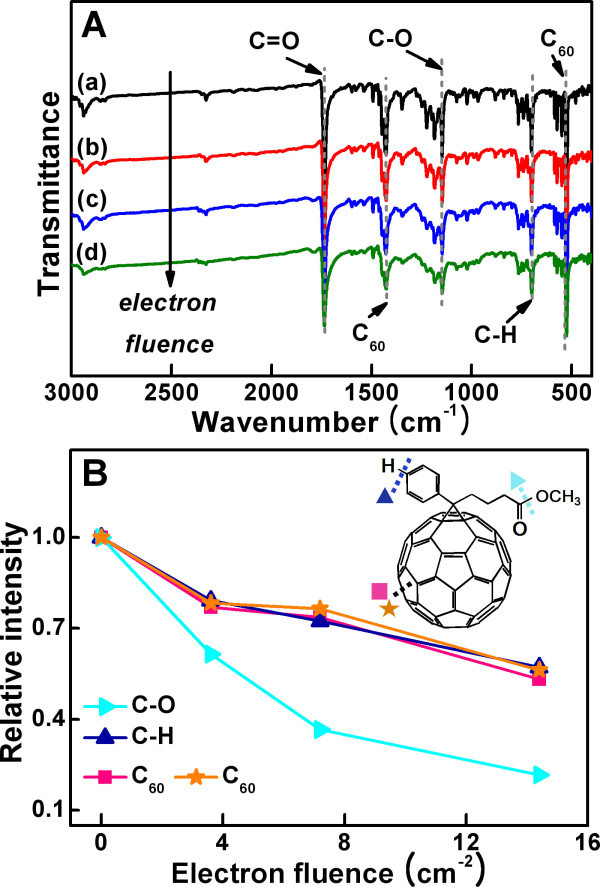
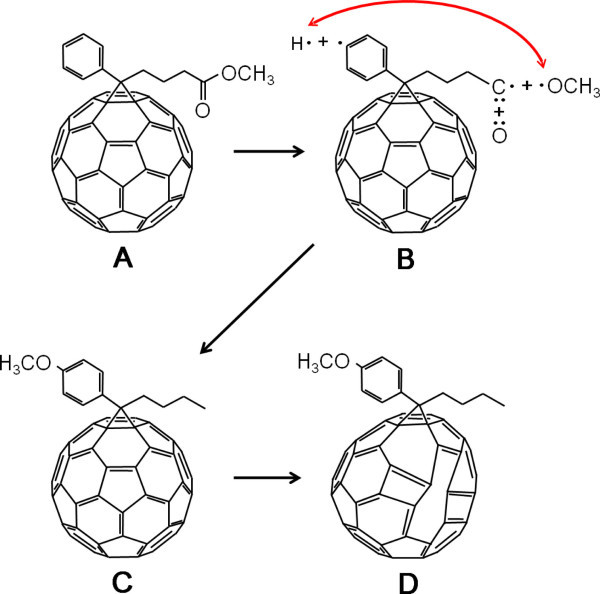
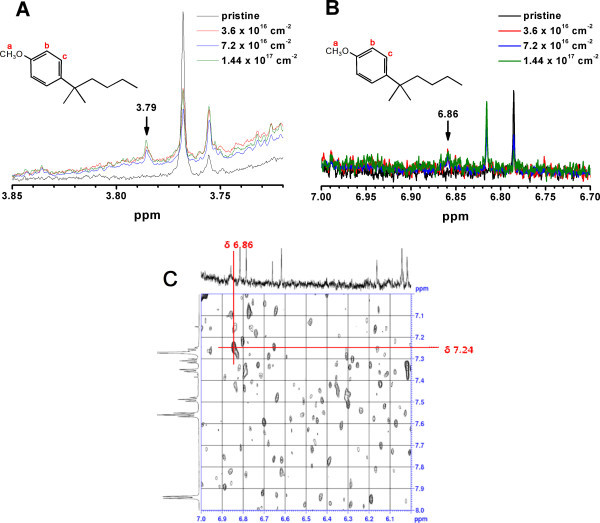
To investigate the origin of the change in the band structure of electron-irradiated PCBM, the molecular structure of electron-irradiated PCBM was characterized by 1H nuclear magnetic resonance (NMR), Fourier transform infrared (FTIR), and Raman spectroscopy. PCBM is a fullerene derivative of which molecular structure comprises a side chain of butyric acid methyl ester and a phenyl ring attached on a C60 cage [1], FTIR spectra show that the intensities of all the peaks decreased with increasing the electron fluence (Figure 2A), suggesting that the molecular bonds of PCBM are gradually decomposed by electron irradiation. Analysis on the peak intensity variation reveals the C-O bond of the butyric acid methyl ester is most rapidly decomposed (Figure 2B). This indicates that the C-O bond can be easily detached from the side chain, forming methoxy radicals (Figure 3B). In addition, one of the C-H bonds in the phenyl ring was also broken by electron irradiation, producing phenyl radicals (Figure 3B). These two radicals produced by radiolysis make bonds with each other to form methoxy-substituted phenyl ring on PCBM (Figure 3C). The presence of methoxy-substituted phenyl ring is verified by 1H NMR spectra. First, a new signal at 3.79 ppm appeared and this signal corresponds to the hydrogen a marked in Figure 4A, that is, the hydrogen of methoxy group attached on the para position of phenyl ring in electron-irradiated PCBM. In addition, another new signal at 6.86 ppm (Figure 4B) and 7.24 ppm (Figure 4C) emerged and these signals are attributed to the hydrogen b on the meta position and hydrogen c on the ortho position of methoxy-substituted phenyl ring, respectively. It has been reported that attaching methoxy group on phenyl ring up-shifts the LUMO level of PCBM [12,23]. Therefore, we propose that the up-shift in the LUMO level of electron-irradiated PCBM is partly attributed to the attachment of methoxy group on the phenyl ring of PCBM.
Figure 2.
FTIR spectra. (A) FTIR spectrum of electron-irradiated PCBM at electron fluence of (a) 0, (b) 3.6 × 1016 cm-2, (c) 7.2 × 1016 cm-2, and (d) 1.44 × 1017 cm-2 including assignment of several vibration modes with its peak position. (B) Relative intensity of vibration modes in the butyric acid methyl ester side chain and phenyl ring of electron-irradiated PCBM.
Figure 3.
Schematic illustration of the modification in the molecular structure of PCBM by electron irradiation. (A) Molecular structure of pristine PCBM. (B) Formation of methoxy radical and phenyl radical by electron irradiation. (C) Formation of new methoxy-substituted phenyl ring by the combination of the methoxy radical and the phenyl radical. (D) Destruction of the C60 cage by high-dose electron irradiation.
Figure 4.
1H NMR spectra of the electron-irradiated PCBM at different fluences. (A) a new signal at 3.79 ppm develops, which corresponds to the hydrogen a of the methoxy group attached on the para position of phenyl ring in the PCBM. (B) Another new signal at 6.86 ppm corresponds to the hydrogen b on the meta position of phenyl ring in the PCBM. (C) The hydrogen c on the ortho position of phenyl ring is displayed in the 2D H-H COSY, which is positioned at 7.24 ppm.
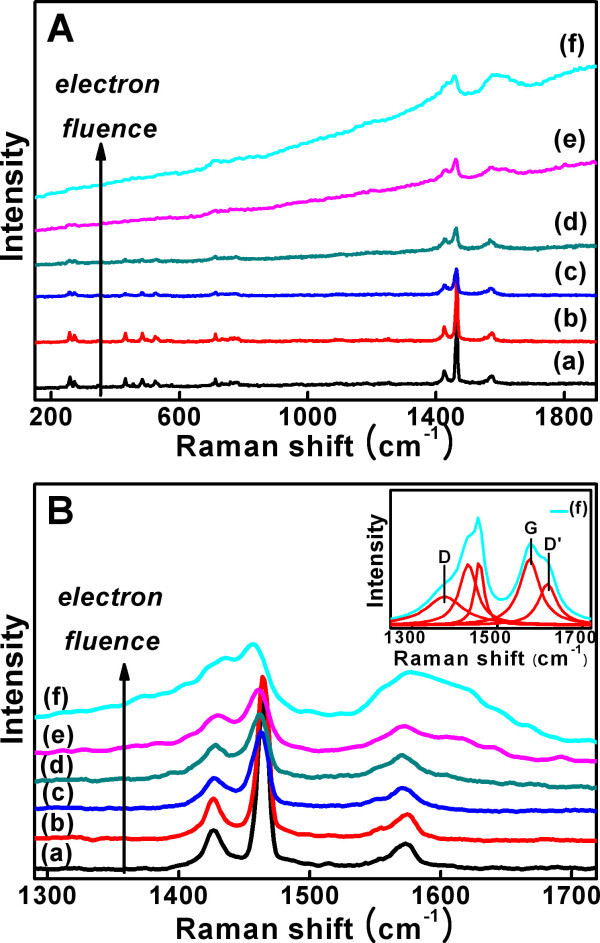
Along with the modification in the side chain of butyric acid methyl ester, C60 backbone in PCBM molecule was also deformed by electron irradiation. Figure 2B verifies this fact that the intensities of peaks associated with the C60 cage gradually decreases as the electron fluence increases. For more detailed interpretation of the modification in C60 cage, electron-irradiated PCBM was analyzed by Raman spectroscopy. PCBM shows ten Raman-active vibration modes, which originates from the icosahedral symmetry (Ih) of C60 [24]. These vibration modes are sorely affected by change in the Ih symmetry of C60, therefore, the structural change of C60 at different fluences of electron irradiation was analyzed by observing the variation of those vibration modes. At electron fluence of 3.6 × 1016 cm-2, no significant change was observed in the Raman spectrum, which indicates the C60 cage was intact at low fluence of electron irradiation. However, the ten Raman-active vibration modes show progressive decrease in the peak intensity as the electron fluence increases. Decrease in the peak intensity and the peak broadening of the strongest peak at 1,464 cm-1 (Ag(2), "pentagonal pinch" mode) clearly proves that the cage was destroyed by further electron irradiation [25-28]. These facts lead to a conclusion that the C60 cage was degraded from its icosahedral symmetry by high dose of electron irradiation. After the electron irradiation at a high fluence of 2.88 × 1017 cm-2, broad bands at 1,376, 1,618, and 1,578 cm-1, which respectively correspond to D-, D'-, and G-bands of graphite [29,30] appeared (inset of Figure 5B). In addition, the Raman spectra of the electron-irradiated PCBM exhibit photoluminescence background with a positive slope and the slope of the background increases with increasing the electron fluence (Figure 5A) [25,31]. These two results indicate that PCBM is transformed into hydrogenated amorphous carbon structure at high dose of electron irradiation (Figure 3D).
Figure 5.
Raman spectra. (A) Raman spectra of electron-irradiated PCBM at different electron fluences of (a) 0, (b) 3.6 × 1016 cm-2, (c) 7.2 × 1016 cm-2, and (d) 1.44 × 1017 cm-2, (e) 2.88 × 1017 cm-2, and (f) 3.96 × 1017 cm-2. (B) Raman spectra of 1,300- to approximately 1,700-cm-1 region with the subtraction of the linear Raman scattering background for peak analysis. The upper right inset shows the deconvoluting peaks in the 1,300- to approximately 1,700-cm-1 spectra region of (f) in (B).
Consequently, from these analyses, we can conclude that the change in the band structure of electron-irradiated PCBM is attributed to the modification of the molecular structure of PCBM by electron irradiation (Figure 3). Formation of methoxy-substituted phenyl ring on PCBM up-shifts the LUMO level at low electron fluencies; further electron irradiation deforms the C60 cage and gradually converts it to hydrogenated amorphous carbon, resulting in the increase of the HOMO-LUMO gap. This also indicates that the band structure of PCBM can be tuned by adjusting the electron dose.
Conclusions
We have found that the electronic band structure of PCBM is changed by electron irradiation. The LUMO level of PCBM gradually up-shifts toward the vacuum energy level, while the HOMO level slightly down-shifts against the vacuum energy level as the electron fluence increase. Consequently, the bandgap of PCBM can be controlled by adjusting the electron fluence. The variation of the band structure is attributed to the change in the molecular structure of PCBM by electron irradiation. The electron irradiation technique can also be used to control the electronic band structures of other organic semiconductors and thus this irradiation technique can provide a useful strategy to improve the performances of organic photovoltaic and organic optoelectronic devices.
Methods
PCBM solution was prepared by dissolving PCBM (99.5% purity, Nano-C, Inc., Westwood, MA, USA) powder into chlorobenzene (≥ 99.5% purity, Sigma-Aldrich, St. Luois, Mo, USA). PCBM films were fabricated on glassy carbon electrodes by spin-coating a chlorobenzene solution containing 24 mM PCBM at 2,000 rpm for 60 s. The irradiation of an electron beam on PCBM films were carried out at room temperature and in vacuum lower than 2 × 10-5 Torr. An electron beam was generated from a thermionic electron gun with electron energy of 50 keV and current density of the electron beam was 1.6 μA cm-2 [16,32]. The electron fluence was varied by adjusting the irradiation time. PCBM films were irradiated by 1, 2, and 4 h, which corresponds to electron fluence of 3.6 × 1016, 7.2 × 1016, and 1.44 × 1017 cm-2, respectively.
After electron irradiation of PCBM, the reduction and oxidation properties of PCBM were characterized by CV. The CV measurements were carried out using a three-electrode system consisting of the glassy carbon electrode as a working electrode, a platinum (Pt) wire as a counter electrode, and a Ag/Ag+ electrode as a reference electrode in an acetonitrile solution of 0.1 M Bu4NPF6. Potentials were quoted with reference to the internal ferrocene standard (E1/2 = 0.120 V vs. Ag/Ag+) that was measured in the same electrolyte. The scan rate was 100 mV s-1 for all measurements. The changes in molecular structure of PCBM due to electron irradiation were investigated by 1H NMR (Bruker Biospin AvanceII 900, Bruker, Billerica, MA, USA), FTIR and high-resolution dispersive Raman spectroscopy (Jasco FT/IR-4100 (JASCO, Easton, MD, USA) and LabRAM HR UV/Vis/NIR (HORIBA Jobin Yvon, Edison, NJ, USA), respectively).
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
The work was carried out by collaboration between all authors. SOC initiated the idea of electron irradiation on PCBM. SHY performed the electron irradiation experiments and JMK carried out the FTIR, Raman, and NMR measurements of electron-irradiated PCBM. SOC and SHY analyzed the data and suggested the mechanism of band-tuning of electron-irradiated PCBM. All authors read and approved the final manuscript.
Contributor Information
Seung Hwa Yoo, Email: yoo3841@kaist.ac.kr.
Jong Min Kum, Email: hhh@kaist.ac.kr.
Sung Oh Cho, Email: socho@kaist.ac.kr.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (no. 2011-0020764).
References
- Hummelen JC, Knight BW, Lepeq F, Wudl F, Yao J, Wilkins CL. Preparation and characterization of fulleroid and methanofullerene derivatives. J Org Chem. 1995;60:532. doi: 10.1021/jo00108a012. [DOI] [Google Scholar]
- Oregan B, Gratzel M. A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353:737. doi: 10.1038/353737a0. [DOI] [Google Scholar]
- Burroughes JH, Bradley DDC, Brown AR, Marks RN, Mackay K, Friend RH, Burns PL, Holmes AB. Light-emitting-diodes based on conjugated polymers. Nature. 1990;347:539. doi: 10.1038/347539a0. [DOI] [Google Scholar]
- Gunes S, Neugebauer H, Sariciftci NS. Conjugated polymer-based organic solar cells. Chem Rev. 2007;107:1324. doi: 10.1021/cr050149z. [DOI] [PubMed] [Google Scholar]
- Chamberlain GA. Organic Solar-Cells - a Review. Sol Cells. 1983;8:47. doi: 10.1016/0379-6787(83)90039-X. [DOI] [Google Scholar]
- Facchetti A. pi-conjugated polymers for organic electronics and photovoltaic cell applications. Chem Mater. 2011;23:733. doi: 10.1021/cm102419z. [DOI] [Google Scholar]
- Hagberg DP, Yum JH, Lee H, De Angelis F, Marinado T, Karlsson KM, Humphry-Baker R, Sun LC, Hagfeldt A, Gratzel M, Nazeeruddin MK. Molecular engineering of organic sensitizers for dye-sensitized solar cell applications. J Am Chem Soc. 2008;130:6259. doi: 10.1021/ja800066y. [DOI] [PubMed] [Google Scholar]
- Garnier F, Horowitz G, Peng XH, Fichou D. An all-organic soft thin-film transistor with very high carrier mobility. Adv Mater. 1990;2:592. doi: 10.1002/adma.19900021207. [DOI] [Google Scholar]
- Ortiz RP, Herrera H, Blanco R, Huang H, Facchetti A, Marks TJ, Zheng Y, Segura JL. Organic n-channel field-effect transistors based on arylenediimide-thiophene derivatives. J Am Chem Soc. 2010;132:8440. doi: 10.1021/ja1018783. [DOI] [PubMed] [Google Scholar]
- Di CA, Yu G, Liu YQ, Guo YL, Sun XN, Zheng J, Wen YG, Wu WP, Zhu DB. Selective crystallization of organic semiconductors for high performance organic field-effect transistors. Chem Mater. 2009;21:4873. doi: 10.1021/cm902594y. [DOI] [Google Scholar]
- Kulkarni AP, Tonzola CJ, Babel A, Jenekhe SA. Electron transport materials for organic light-emitting diodes. Chem Mater. 2004;16:4556. doi: 10.1021/cm049473l. [DOI] [Google Scholar]
- Kooistra FB, Knol J, Kastenberg F, Popescu LM, Verhees WJH, Kroon JM, Hummelen JC. Increasing the open circuit voltage of bulk-heterojunction solar cells by raising the LUMO level of the acceptor. Org Lett. 2007;9:551. doi: 10.1021/ol062666p. [DOI] [PubMed] [Google Scholar]
- Lenes M, Wetzelaer GJAH, Kooistra FB, Veenstra SC, Hummelen JC, Blom PWM. Fullerene bisadducts for enhanced open-circuit voltages and efficiencies in polymer solar cells. Adv Mater. 2008;20:2116. doi: 10.1002/adma.200702438. [DOI] [Google Scholar]
- Yang C, Kim JY, Cho S, Lee JK, Heeger AJ, Wudl F. Functionalized methanofullerenes used as n-type materials in bulk-heterojunction polymer solar cells and in field-effect transistors. J Am Chem Soc. 2008;130:6444. doi: 10.1021/ja710621j. [DOI] [PubMed] [Google Scholar]
- Miller AA. Radiation chemistry of polydimethylsiloxane. I. Crosslinking and gas yields. J Am Chem Soc. 1959;82:3519. [Google Scholar]
- Kang HY, Saito O, Dole M. The radiation chemistry of polyethylene. IX. temperature coefficient of cross-linking and other effects. J Am Chem Soc. 1967;89:1980. doi: 10.1021/ja00985a002. [DOI] [Google Scholar]
- Li Y, Lee EJ, Cai W, Kim KY, Cho SO. Unconventional method for morphology-controlled carbonaceous nanoarrays based on electron irradiation of a polystyrene colloidal monolayer. Acs Nano. 2008;2:1108. doi: 10.1021/nn8001483. [DOI] [PubMed] [Google Scholar]
- Novembre AE, Tai WW, Kometani JM, Hanson JE, Nalamasu O, Taylor GN, Reichmanis E, Thompson LF. Radiation-induced chemistry of poly(4-[(tert-butoxycarbonyl)oxy]styrene-co-sulfur dioxide) Chem Mater. 1992;4:278. doi: 10.1021/cm00020a012. [DOI] [Google Scholar]
- Lee HM, Kim YN, Kim BH, Kim SO, Cho SO. Fabrication of luminescent nanoarchitectures by electron irradiation of polystyrene. Adv Mater. 2008;20:2094. doi: 10.1002/adma.200702221. [DOI] [Google Scholar]
- Davenas J, Boiteux G, Xu XL, Adem E. Role of the modifications induced by ion-beam irradiation in the optical and conducting properties of polyimide. Nucl Instrum Meth B. 1988;32:136. doi: 10.1016/0168-583X(88)90196-6. [DOI] [Google Scholar]
- Hioki T, Noda S, Sugiura M, Kakeno M, Yamada K, Kawamoto J. Electrical and optical-properties of ion-irradiated organic polymer Kapton-H. Appl Phys Lett. 1983;43:30. doi: 10.1063/1.94157. [DOI] [Google Scholar]
- Al-Ibrahim M, Roth HK, Zhokhavets U, Gobsch G, Sensfuss S. Flexible large area polymer solar cells based on poly(3-hexylthiophene)/fullerene. Sol Energ Mat Sol C. 2005;85:13. [Google Scholar]
- Morvillo P, Bobeico E. Tuning the LUMO level of the acceptor to increase the open-circuit voltage of polymer-fullerene solar cells: a quantum chemical study. Sol Energ Mat Sol C. 2008;92:1192. doi: 10.1016/j.solmat.2008.04.010. [DOI] [Google Scholar]
- Bethune DS, Meijer G, Tang WC, Rosen HJ. The vibrational Raman-spectra of purified solid films of C-60 and C-70. Chem Phys Lett. 1990;174:219. doi: 10.1016/0009-2614(90)85335-A. [DOI] [Google Scholar]
- Dmytrenko OP, Kulish NP, Belyi NM, Prylutskyy YI, Poperenko LV, Stashchuk VS, Poroshin VG, Pavlenko EL, Shlapatskaya VV, Bernas H, Scharff P. Dose dependences of the optical properties of fullerene films subjected to the electron irradiation. Thin Solid Films. 2006;495:365. doi: 10.1016/j.tsf.2005.08.237. [DOI] [Google Scholar]
- Robinson APG, Palmer RE, Tada T, Kanayama T, Preece JA, Philp D, Jonas U, Deiderich F. Electron beam induced fragmentation of fullerene derivatives. Chem Phys Lett. 1998;289:586. doi: 10.1016/S0009-2614(98)00455-2. [DOI] [Google Scholar]
- Hunt MRC, Schmidt J, Palmer RE. Electron-beam-induced fragmentation in ultrathin C-60 films on Si(100)-2 × 1-H: mechanisms of cage destruction. Phys Rev B. 1999;60:5927. doi: 10.1103/PhysRevB.60.5927. [DOI] [Google Scholar]
- Liu Z, Suenaga K, Iijima S. Imaging the structure of an individual C-60 fullerene molecule and its deformation process using HRTEM with atomic sensitivity. J Am Chem Soc. 2007;129:6666. doi: 10.1021/ja068516r. [DOI] [PubMed] [Google Scholar]
- Cataldo F, Baratta GA, Strazzulla G. He+ ion bombardment of C-60 fullerene: an FT-IR and Raman study. Fuller Nanotub Car N. 2002;10:197. doi: 10.1081/FST-120014734. [DOI] [Google Scholar]
- Meinardi F, Paleari A, Manfredini M, Milani P. Raman characterization of amorphous-carbon produced by fullerite laser-induced transformation. Solid State Commun. 1995;93:335. doi: 10.1016/0038-1098(94)00789-6. [DOI] [Google Scholar]
- Marchon B, Gui J, Grannen K, Rauch GC, Ager JW, Silva SRP, Robertson J. Photoluminescence and Raman spectroscopy in hydrogenated carbon films. Ieee T Magn. 1997;33:3148. doi: 10.1109/20.617873. [DOI] [Google Scholar]
- Cho SO, Lee BC, Jeong YU. Effects of beam loss on the stability of a recirculating electrostatic accelerator for a long-pulse free-electron laser. Nucl Instrum Meth A. 2003;515:402. doi: 10.1016/j.nima.2003.07.028. [DOI] [Google Scholar]