Abstract
In the title compound, C30H35B2NO4, the carbazole skeleton is essentially planar (r.m.s. deviation for all non-H atoms = 0.035 Å), and is oriented at a dihedral angle of 65.0 (3)° with respect to the adjacent phenyl ring.
Related literature
The title compound is an intermediate in the synthesis of 9-phenylcarbazole-based optical materials, see: Oliveira et al. (2005 ▶). For the synthesis of the title compound, see: Wong et al. (2005 ▶, 2006 ▶); Rashidnadimi et al. (2008 ▶). For related structures, see: Xu et al. (2010 ▶); Cui et al. (2009 ▶); Saeed et al. (2010 ▶). For standard bond lengths, see: Allen et al. (1987 ▶).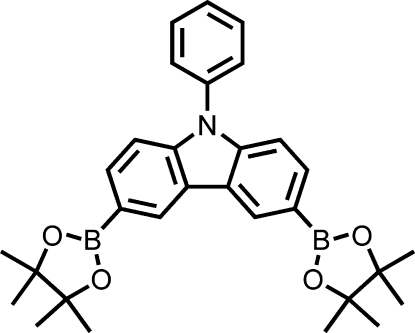
Experimental
Crystal data
C30H35B2NO4
M r = 495.21
Orthorhombic,

a = 13.974 (6) Å
b = 11.935 (5) Å
c = 34.494 (14) Å
V = 5753 (4) Å3
Z = 8
Mo Kα radiation
μ = 0.07 mm−1
T = 298 K
0.3 × 0.2 × 0.1 mm
Data collection
Rigaku Mercury2 diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku, 2005 ▶) T min = 0.9, T max = 1
49645 measured reflections
6553 independent reflections
5170 reflections with I > 2σ(I)
R int = 0.062
Refinement
R[F 2 > 2σ(F 2)] = 0.093
wR(F 2) = 0.242
S = 1.16
6553 reflections
342 parameters
15 restraints
H-atom parameters constrained
Δρmax = 0.72 e Å−3
Δρmin = −0.52 e Å−3
Data collection: CrystalClear (Rigaku, 2005 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811025645/jh2305sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811025645/jh2305Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811025645/jh2305Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors are grateful for financial support from the National Natural Science Foundation of China (No. 30972321) and the Basic Research Project of the Natural Science Foundation of Jiangsu Provincial Universities (10KJB53007).
supplementary crystallographic information
Comment
Carbazole - based materials have been investigated for their electrical and optical properties. Especially, introduction of substituents on the 3, 6-positions of carbazole represents a possible approach for designing carbazole-based materials with electrogenerated chemiluminescence. The title compound is a key intermediates, which can be used to synthesize 9-phenylcarbazole derivatives with substituents at 3, 6-positions (Wong et al., 2005, 2006; Rashidnadimi et al., 2008).
The central structural element of the title compound is a carbazole moiety substituted with two pinacolbronic ester at 3, 6-positions and a phenyl attached to atom N9. The carbazole moiety is essentially planar (maximum deviation=0.057 Å). The carbazole plane is inclined to the phenyl ring planes at dihedral angle of 115.0 (3)°. The C—B distances fall in the range to 1.550 (4) Å, consistent with the literature (Allen et al., 1987).The crystal packing is stabilized by van der Waals forces.
Experimental
To a solution of 5,8-dibromo-1-phenylcarbazole (400 mg, 1 mmol) in THF (15 ml) at -78°C was added 1.87 ml (3 mmol) of n-butyllithium (1.6 M in hexane). The mixture was stirred at -78°C for 2 h. 0.4 ml (2 mmol) of 2-isopropoxy-4,4,5,5-tetramethyl-[1,3,2]-dioxaborolane was added rapidly to the solution, and the resulting mixture was warmed to room temperature and stirred for 8 h. The mixture was poured into water and extracted with dichloromethane. The organic extracts were washed with brine and dried over magnesium sulfate. The solvent was removed by rotary evaporation, and recrystallization was made in a mixture of n-pentane/hexane to afford 356 mg (72%) of product as a whitesolid. The structure was confirmed by FTIR, 1H NMR and MS. Single crystals suitable for crystallographic analysis were obtained by slow evaporation of a ethanol/dichloromethane (1:1v/v) solution.
Refinement
All H atoms attached to C atoms were fixed geometrically and treated as riding with C—H = 0.96 Å(methyl) and C—H = 0.93 Å (aromatic) with Uĩso~(H) = 1.2U~eq~(aromatic) and Uĩso~(H) = 1.5U~eq~(methyl).
Figures
Fig. 1.
The molecular structure of the title molecule with the atom-numbering scheme. The displacement ellipsoids are drawn at the 30% probability level.
Crystal data
| C30H35B2NO4 | Dx = 1.143 Mg m−3 |
| Mr = 495.21 | Melting point: 476 K |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 10558 reflections |
| a = 13.974 (6) Å | θ = 2.1–27.5° |
| b = 11.935 (5) Å | µ = 0.07 mm−1 |
| c = 34.494 (14) Å | T = 298 K |
| V = 5753 (4) Å3 | Block, colourless |
| Z = 8 | 0.3 × 0.2 × 0.1 mm |
| F(000) = 2112 |
Data collection
| Rigaku Mercury2 diffractometer | 6553 independent reflections |
| Radiation source: fine-focus sealed tube | 5170 reflections with I > 2σ(I) |
| graphite | Rint = 0.062 |
| Detector resolution: 13.6612 pixels mm-1 | θmax = 27.5°, θmin = 1.9° |
| ω scans | h = −18→18 |
| Absorption correction: multi-scan (CrystalClear; Rigaku, 2005) | k = −15→15 |
| Tmin = 0.9, Tmax = 1 | l = −44→44 |
| 49645 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.093 | H-atom parameters constrained |
| wR(F2) = 0.242 | w = 1/[σ2(Fo2) + (0.0908P)2 + 3.0388P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.16 | (Δ/σ)max < 0.001 |
| 6553 reflections | Δρmax = 0.72 e Å−3 |
| 342 parameters | Δρmin = −0.52 e Å−3 |
| 15 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0056 (14) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.14754 (13) | 0.80983 (19) | 0.06118 (6) | 0.0611 (6) | |
| O2 | 0.16305 (13) | 0.7395 (2) | 0.12237 (6) | 0.0644 (6) | |
| C3 | 0.47750 (18) | 0.7413 (2) | 0.11098 (7) | 0.0454 (6) | |
| N1 | 0.61593 (15) | 0.8121 (2) | 0.08578 (6) | 0.0517 (6) | |
| C5 | 0.64049 (18) | 0.7510 (2) | 0.11878 (7) | 0.0475 (6) | |
| C6 | 0.51688 (17) | 0.8061 (2) | 0.08084 (7) | 0.0461 (6) | |
| C7 | 0.68001 (18) | 0.8756 (2) | 0.06208 (7) | 0.0465 (6) | |
| C8 | 0.31920 (18) | 0.7794 (2) | 0.08579 (8) | 0.0506 (6) | |
| C9 | 0.6547 (2) | 0.6093 (3) | 0.18283 (8) | 0.0533 (7) | |
| C10 | 0.56499 (19) | 0.6321 (2) | 0.16660 (7) | 0.0499 (6) | |
| H10A | 0.5105 | 0.5994 | 0.1771 | 0.060* | |
| C11 | 0.36216 (19) | 0.8401 (3) | 0.05558 (8) | 0.0562 (7) | |
| H11A | 0.3230 | 0.8716 | 0.0367 | 0.067* | |
| C12 | 0.55668 (17) | 0.7033 (2) | 0.13485 (7) | 0.0448 (6) | |
| C13 | 0.37813 (18) | 0.7292 (2) | 0.11331 (8) | 0.0491 (6) | |
| H13A | 0.3513 | 0.6874 | 0.1333 | 0.059* | |
| C14 | 0.7359 (2) | 0.6605 (3) | 0.16612 (8) | 0.0607 (7) | |
| H14A | 0.7956 | 0.6463 | 0.1769 | 0.073* | |
| C15 | 0.7300 (2) | 0.9648 (3) | 0.07770 (9) | 0.0603 (7) | |
| H15A | 0.7232 | 0.9829 | 0.1038 | 0.072* | |
| C16 | 0.73053 (19) | 0.7308 (3) | 0.13444 (8) | 0.0587 (7) | |
| H16A | 0.7851 | 0.7634 | 0.1239 | 0.070* | |
| C17 | 0.45997 (19) | 0.8554 (2) | 0.05254 (8) | 0.0544 (7) | |
| H17A | 0.4865 | 0.8968 | 0.0324 | 0.065* | |
| C18 | 0.6916 (2) | 0.8491 (2) | 0.02336 (8) | 0.0557 (7) | |
| H18A | 0.6590 | 0.7885 | 0.0127 | 0.067* | |
| C19 | 0.05223 (19) | 0.8181 (3) | 0.07865 (9) | 0.0611 (8) | |
| O3 | 0.59377 (17) | 0.4625 (2) | 0.23038 (7) | 0.0892 (7) | |
| C21 | 0.0605 (2) | 0.7369 (3) | 0.11389 (9) | 0.0630 (8) | |
| C22 | 0.7514 (2) | 0.9128 (3) | 0.00057 (8) | 0.0645 (8) | |
| H22A | 0.7584 | 0.8952 | −0.0255 | 0.077* | |
| B1 | 0.2088 (2) | 0.7751 (3) | 0.08979 (9) | 0.0516 (7) | |
| O4 | 0.74200 (18) | 0.5255 (2) | 0.24089 (7) | 0.0907 (7) | |
| C25 | 0.7291 (3) | 0.4402 (3) | 0.27042 (9) | 0.0707 (9) | |
| B2 | 0.6640 (3) | 0.5314 (4) | 0.21859 (12) | 0.0801 (8) | |
| C27 | 0.8004 (2) | 1.0010 (3) | 0.01569 (9) | 0.0668 (8) | |
| H27A | 0.8405 | 1.0436 | 0.0000 | 0.080* | |
| C28 | −0.0211 (2) | 0.7866 (4) | 0.04848 (10) | 0.0865 (12) | |
| H28A | −0.0135 | 0.7091 | 0.0417 | 0.130* | |
| H28B | −0.0123 | 0.8322 | 0.0258 | 0.130* | |
| H28C | −0.0842 | 0.7984 | 0.0587 | 0.130* | |
| C29 | 0.6219 (3) | 0.4071 (3) | 0.26585 (8) | 0.0664 (8) | |
| C30 | 0.7904 (2) | 1.0269 (3) | 0.05424 (10) | 0.0719 (9) | |
| H30A | 0.8244 | 1.0867 | 0.0647 | 0.086* | |
| C31 | 0.0404 (3) | 0.9398 (3) | 0.09067 (14) | 0.0974 (13) | |
| H31A | 0.0437 | 0.9869 | 0.0681 | 0.146* | |
| H31B | 0.0905 | 0.9599 | 0.1084 | 0.146* | |
| H31C | −0.0205 | 0.9495 | 0.1031 | 0.146* | |
| C32 | 0.0087 (3) | 0.7720 (5) | 0.15013 (10) | 0.1018 (15) | |
| H32A | 0.0196 | 0.7176 | 0.1702 | 0.153* | |
| H32B | −0.0587 | 0.7770 | 0.1449 | 0.153* | |
| H32C | 0.0320 | 0.8437 | 0.1585 | 0.153* | |
| C33 | 0.7992 (4) | 0.3483 (5) | 0.26240 (19) | 0.142 (2) | |
| H33A | 0.8631 | 0.3775 | 0.2638 | 0.212* | |
| H33B | 0.7880 | 0.3184 | 0.2370 | 0.212* | |
| H33C | 0.7916 | 0.2899 | 0.2813 | 0.212* | |
| C34 | 0.5556 (4) | 0.4521 (6) | 0.29700 (15) | 0.155 (3) | |
| H34A | 0.5692 | 0.5298 | 0.3014 | 0.232* | |
| H34B | 0.5651 | 0.4108 | 0.3206 | 0.232* | |
| H34C | 0.4904 | 0.4440 | 0.2887 | 0.232* | |
| C35 | 0.0369 (3) | 0.6160 (4) | 0.10317 (14) | 0.1071 (15) | |
| H35A | 0.0581 | 0.5669 | 0.1235 | 0.161* | |
| H35B | 0.0688 | 0.5967 | 0.0794 | 0.161* | |
| H35C | −0.0309 | 0.6082 | 0.0998 | 0.161* | |
| C36 | 0.6025 (4) | 0.2839 (4) | 0.26109 (18) | 0.1287 (19) | |
| H36A | 0.5353 | 0.2724 | 0.2569 | 0.193* | |
| H36B | 0.6219 | 0.2448 | 0.2841 | 0.193* | |
| H36C | 0.6377 | 0.2558 | 0.2393 | 0.193* | |
| C37 | 0.7538 (4) | 0.4952 (5) | 0.30874 (14) | 0.135 (2) | |
| H37A | 0.7156 | 0.5613 | 0.3121 | 0.202* | |
| H37B | 0.8203 | 0.5152 | 0.3089 | 0.202* | |
| H37C | 0.7411 | 0.4439 | 0.3295 | 0.202* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0418 (10) | 0.0836 (14) | 0.0579 (11) | 0.0092 (9) | −0.0044 (8) | 0.0047 (10) |
| O2 | 0.0427 (10) | 0.0916 (16) | 0.0588 (12) | 0.0036 (10) | −0.0048 (8) | 0.0047 (11) |
| C3 | 0.0420 (13) | 0.0488 (14) | 0.0454 (12) | 0.0058 (11) | −0.0023 (10) | −0.0002 (10) |
| N1 | 0.0401 (11) | 0.0684 (15) | 0.0465 (11) | −0.0006 (10) | −0.0049 (9) | 0.0088 (10) |
| C5 | 0.0423 (13) | 0.0583 (15) | 0.0418 (12) | 0.0012 (11) | −0.0031 (10) | 0.0024 (11) |
| C6 | 0.0404 (13) | 0.0523 (14) | 0.0455 (12) | 0.0026 (11) | −0.0039 (10) | 0.0000 (11) |
| C7 | 0.0415 (13) | 0.0520 (14) | 0.0462 (13) | 0.0027 (11) | −0.0034 (10) | 0.0052 (11) |
| C8 | 0.0408 (13) | 0.0582 (16) | 0.0528 (14) | 0.0048 (11) | −0.0060 (11) | −0.0011 (12) |
| C9 | 0.0506 (15) | 0.0639 (17) | 0.0455 (13) | 0.0036 (13) | −0.0062 (11) | 0.0035 (12) |
| C10 | 0.0455 (14) | 0.0585 (16) | 0.0456 (13) | 0.0022 (12) | −0.0009 (11) | 0.0037 (11) |
| C11 | 0.0454 (14) | 0.0678 (17) | 0.0554 (15) | 0.0095 (13) | −0.0103 (12) | 0.0044 (13) |
| C12 | 0.0391 (12) | 0.0517 (14) | 0.0435 (12) | 0.0031 (10) | −0.0028 (10) | −0.0026 (10) |
| C13 | 0.0406 (13) | 0.0564 (15) | 0.0502 (13) | 0.0029 (11) | 0.0003 (10) | 0.0002 (12) |
| C14 | 0.0456 (15) | 0.083 (2) | 0.0539 (15) | 0.0019 (14) | −0.0129 (12) | 0.0097 (15) |
| C15 | 0.0638 (18) | 0.0629 (18) | 0.0543 (15) | −0.0040 (14) | −0.0014 (13) | −0.0069 (13) |
| C16 | 0.0425 (15) | 0.082 (2) | 0.0520 (14) | −0.0036 (13) | −0.0061 (11) | 0.0098 (14) |
| C17 | 0.0473 (15) | 0.0645 (17) | 0.0514 (14) | 0.0036 (12) | −0.0037 (11) | 0.0093 (13) |
| C18 | 0.0602 (17) | 0.0594 (16) | 0.0476 (14) | −0.0033 (13) | −0.0051 (12) | −0.0003 (12) |
| C19 | 0.0394 (14) | 0.080 (2) | 0.0637 (17) | 0.0072 (14) | −0.0045 (12) | −0.0096 (15) |
| O3 | 0.0719 (13) | 0.1128 (17) | 0.0830 (14) | −0.0171 (11) | −0.0228 (11) | 0.0454 (13) |
| C21 | 0.0415 (14) | 0.087 (2) | 0.0602 (16) | −0.0044 (14) | −0.0053 (12) | −0.0049 (15) |
| C22 | 0.0708 (19) | 0.075 (2) | 0.0473 (14) | −0.0034 (16) | 0.0023 (14) | 0.0065 (14) |
| B1 | 0.0449 (16) | 0.0575 (18) | 0.0523 (16) | 0.0068 (13) | −0.0057 (13) | −0.0075 (14) |
| O4 | 0.0729 (13) | 0.1150 (17) | 0.0841 (14) | −0.0185 (12) | −0.0307 (11) | 0.0455 (13) |
| C25 | 0.085 (2) | 0.0637 (19) | 0.0635 (18) | −0.0032 (16) | −0.0241 (16) | 0.0160 (15) |
| B2 | 0.0655 (14) | 0.1032 (17) | 0.0718 (15) | −0.0136 (13) | −0.0238 (12) | 0.0382 (14) |
| C27 | 0.0590 (17) | 0.073 (2) | 0.0681 (19) | −0.0026 (16) | 0.0018 (14) | 0.0218 (16) |
| C28 | 0.0530 (19) | 0.132 (3) | 0.074 (2) | 0.008 (2) | −0.0207 (16) | −0.007 (2) |
| C29 | 0.081 (2) | 0.0681 (19) | 0.0498 (15) | 0.0003 (16) | −0.0061 (14) | 0.0114 (14) |
| C30 | 0.072 (2) | 0.0597 (18) | 0.084 (2) | −0.0156 (16) | −0.0073 (17) | 0.0009 (16) |
| C31 | 0.078 (2) | 0.083 (3) | 0.131 (4) | 0.026 (2) | −0.009 (2) | −0.018 (2) |
| C32 | 0.0523 (19) | 0.184 (5) | 0.069 (2) | 0.003 (2) | 0.0066 (16) | −0.007 (3) |
| C33 | 0.094 (3) | 0.110 (4) | 0.221 (7) | 0.029 (3) | −0.006 (4) | −0.002 (4) |
| C34 | 0.122 (4) | 0.234 (8) | 0.107 (4) | −0.007 (5) | 0.032 (3) | −0.037 (4) |
| C35 | 0.102 (3) | 0.087 (3) | 0.132 (4) | −0.027 (2) | −0.024 (3) | 0.007 (3) |
| C36 | 0.115 (4) | 0.081 (3) | 0.190 (6) | −0.020 (3) | −0.015 (4) | 0.004 (3) |
| C37 | 0.161 (5) | 0.153 (5) | 0.090 (3) | −0.037 (4) | −0.045 (3) | −0.014 (3) |
Geometric parameters (Å, °)
| O1—B1 | 1.371 (4) | C21—C32 | 1.504 (4) |
| O1—C19 | 1.465 (3) | C21—C35 | 1.526 (5) |
| O2—B1 | 1.361 (4) | C22—C27 | 1.361 (5) |
| O2—C21 | 1.463 (3) | C22—H22A | 0.9300 |
| C3—C13 | 1.398 (4) | O4—B2 | 1.336 (4) |
| C3—C6 | 1.408 (4) | O4—C25 | 1.452 (4) |
| C3—C12 | 1.452 (3) | C25—C33 | 1.497 (6) |
| N1—C5 | 1.395 (3) | C25—C37 | 1.516 (5) |
| N1—C6 | 1.396 (3) | C25—C29 | 1.556 (5) |
| N1—C7 | 1.429 (3) | C27—C30 | 1.373 (5) |
| C5—C16 | 1.390 (4) | C27—H27A | 0.9300 |
| C5—C12 | 1.415 (4) | C28—H28A | 0.9600 |
| C6—C17 | 1.389 (3) | C28—H28B | 0.9600 |
| C7—C18 | 1.382 (4) | C28—H28C | 0.9600 |
| C7—C15 | 1.383 (4) | C29—C36 | 1.505 (6) |
| C8—C13 | 1.392 (4) | C29—C34 | 1.517 (6) |
| C8—C11 | 1.404 (4) | C30—H30A | 0.9300 |
| C8—B1 | 1.550 (4) | C31—H31A | 0.9600 |
| C9—C10 | 1.399 (4) | C31—H31B | 0.9600 |
| C9—C14 | 1.412 (4) | C31—H31C | 0.9600 |
| C9—B2 | 1.550 (4) | C32—H32A | 0.9600 |
| C10—C12 | 1.391 (4) | C32—H32B | 0.9600 |
| C10—H10A | 0.9300 | C32—H32C | 0.9600 |
| C11—C17 | 1.383 (4) | C33—H33A | 0.9600 |
| C11—H11A | 0.9300 | C33—H33B | 0.9600 |
| C13—H13A | 0.9300 | C33—H33C | 0.9600 |
| C14—C16 | 1.379 (4) | C34—H34A | 0.9600 |
| C14—H14A | 0.9300 | C34—H34B | 0.9600 |
| C15—C30 | 1.384 (4) | C34—H34C | 0.9600 |
| C15—H15A | 0.9300 | C35—H35A | 0.9600 |
| C16—H16A | 0.9300 | C35—H35B | 0.9600 |
| C17—H17A | 0.9300 | C35—H35C | 0.9600 |
| C18—C22 | 1.376 (4) | C36—H36A | 0.9600 |
| C18—H18A | 0.9300 | C36—H36B | 0.9600 |
| C19—C28 | 1.508 (4) | C36—H36C | 0.9600 |
| C19—C31 | 1.519 (5) | C37—H37A | 0.9600 |
| C19—C21 | 1.559 (5) | C37—H37B | 0.9600 |
| O3—B2 | 1.343 (5) | C37—H37C | 0.9600 |
| O3—C29 | 1.445 (4) | ||
| B1—O1—C19 | 107.0 (2) | O4—C25—C33 | 107.6 (4) |
| B1—O2—C21 | 107.6 (2) | O4—C25—C37 | 106.2 (3) |
| C13—C3—C6 | 119.2 (2) | C33—C25—C37 | 109.2 (4) |
| C13—C3—C12 | 133.8 (2) | O4—C25—C29 | 103.1 (2) |
| C6—C3—C12 | 107.0 (2) | C33—C25—C29 | 115.2 (3) |
| C5—N1—C6 | 108.4 (2) | C37—C25—C29 | 114.7 (4) |
| C5—N1—C7 | 126.1 (2) | O4—B2—O3 | 113.0 (3) |
| C6—N1—C7 | 125.3 (2) | O4—B2—C9 | 123.9 (3) |
| C16—C5—N1 | 129.1 (2) | O3—B2—C9 | 123.1 (3) |
| C16—C5—C12 | 121.8 (2) | C22—C27—C30 | 119.6 (3) |
| N1—C5—C12 | 109.0 (2) | C22—C27—H27A | 120.2 |
| C17—C6—N1 | 129.1 (2) | C30—C27—H27A | 120.2 |
| C17—C6—C3 | 121.8 (2) | C19—C28—H28A | 109.5 |
| N1—C6—C3 | 109.0 (2) | C19—C28—H28B | 109.5 |
| C18—C7—C15 | 119.6 (3) | H28A—C28—H28B | 109.5 |
| C18—C7—N1 | 120.3 (2) | C19—C28—H28C | 109.5 |
| C15—C7—N1 | 120.1 (2) | H28A—C28—H28C | 109.5 |
| C13—C8—C11 | 118.4 (2) | H28B—C28—H28C | 109.5 |
| C13—C8—B1 | 120.9 (3) | O3—C29—C36 | 107.8 (3) |
| C11—C8—B1 | 120.6 (2) | O3—C29—C34 | 105.7 (4) |
| C10—C9—C14 | 118.2 (2) | C36—C29—C34 | 108.2 (4) |
| C10—C9—B2 | 120.7 (3) | O3—C29—C25 | 103.4 (2) |
| C14—C9—B2 | 121.1 (3) | C36—C29—C25 | 115.6 (3) |
| C12—C10—C9 | 120.6 (3) | C34—C29—C25 | 115.3 (3) |
| C12—C10—H10A | 119.7 | C27—C30—C15 | 120.5 (3) |
| C9—C10—H10A | 119.7 | C27—C30—H30A | 119.7 |
| C17—C11—C8 | 123.1 (2) | C15—C30—H30A | 119.7 |
| C17—C11—H11A | 118.4 | C19—C31—H31A | 109.5 |
| C8—C11—H11A | 118.4 | C19—C31—H31B | 109.5 |
| C10—C12—C5 | 119.0 (2) | H31A—C31—H31B | 109.5 |
| C10—C12—C3 | 134.5 (2) | C19—C31—H31C | 109.5 |
| C5—C12—C3 | 106.5 (2) | H31A—C31—H31C | 109.5 |
| C8—C13—C3 | 120.3 (2) | H31B—C31—H31C | 109.5 |
| C8—C13—H13A | 119.9 | C21—C32—H32A | 109.5 |
| C3—C13—H13A | 119.9 | C21—C32—H32B | 109.5 |
| C16—C14—C9 | 122.9 (3) | H32A—C32—H32B | 109.5 |
| C16—C14—H14A | 118.6 | C21—C32—H32C | 109.5 |
| C9—C14—H14A | 118.6 | H32A—C32—H32C | 109.5 |
| C7—C15—C30 | 119.5 (3) | H32B—C32—H32C | 109.5 |
| C7—C15—H15A | 120.2 | C25—C33—H33A | 109.5 |
| C30—C15—H15A | 120.2 | C25—C33—H33B | 109.5 |
| C14—C16—C5 | 117.5 (3) | H33A—C33—H33B | 109.5 |
| C14—C16—H16A | 121.2 | C25—C33—H33C | 109.5 |
| C5—C16—H16A | 121.2 | H33A—C33—H33C | 109.5 |
| C11—C17—C6 | 117.2 (3) | H33B—C33—H33C | 109.5 |
| C11—C17—H17A | 121.4 | C29—C34—H34A | 109.5 |
| C6—C17—H17A | 121.4 | C29—C34—H34B | 109.5 |
| C22—C18—C7 | 119.8 (3) | H34A—C34—H34B | 109.5 |
| C22—C18—H18A | 120.1 | C29—C34—H34C | 109.5 |
| C7—C18—H18A | 120.1 | H34A—C34—H34C | 109.5 |
| O1—C19—C28 | 108.5 (3) | H34B—C34—H34C | 109.5 |
| O1—C19—C31 | 106.0 (3) | C21—C35—H35A | 109.5 |
| C28—C19—C31 | 110.7 (3) | C21—C35—H35B | 109.5 |
| O1—C19—C21 | 102.2 (2) | H35A—C35—H35B | 109.5 |
| C28—C19—C21 | 115.7 (3) | C21—C35—H35C | 109.5 |
| C31—C19—C21 | 112.9 (3) | H35A—C35—H35C | 109.5 |
| B2—O3—C29 | 109.7 (3) | H35B—C35—H35C | 109.5 |
| O2—C21—C32 | 107.4 (2) | C29—C36—H36A | 109.5 |
| O2—C21—C35 | 106.2 (3) | C29—C36—H36B | 109.5 |
| C32—C21—C35 | 111.1 (3) | H36A—C36—H36B | 109.5 |
| O2—C21—C19 | 102.5 (2) | C29—C36—H36C | 109.5 |
| C32—C21—C19 | 116.1 (3) | H36A—C36—H36C | 109.5 |
| C35—C21—C19 | 112.5 (3) | H36B—C36—H36C | 109.5 |
| C27—C22—C18 | 121.0 (3) | C25—C37—H37A | 109.5 |
| C27—C22—H22A | 119.5 | C25—C37—H37B | 109.5 |
| C18—C22—H22A | 119.5 | H37A—C37—H37B | 109.5 |
| O2—B1—O1 | 113.3 (3) | C25—C37—H37C | 109.5 |
| O2—B1—C8 | 123.5 (2) | H37A—C37—H37C | 109.5 |
| O1—B1—C8 | 123.2 (3) | H37B—C37—H37C | 109.5 |
| B2—O4—C25 | 109.8 (3) | ||
| C6—N1—C5—C16 | 178.1 (3) | B1—O1—C19—C21 | −23.4 (3) |
| C7—N1—C5—C16 | −5.1 (5) | B1—O2—C21—C32 | −143.9 (3) |
| C6—N1—C5—C12 | 1.1 (3) | B1—O2—C21—C35 | 97.0 (3) |
| C7—N1—C5—C12 | 177.9 (2) | B1—O2—C21—C19 | −21.2 (3) |
| C5—N1—C6—C17 | 178.7 (3) | O1—C19—C21—O2 | 26.7 (3) |
| C7—N1—C6—C17 | 1.9 (4) | C28—C19—C21—O2 | 144.4 (3) |
| C5—N1—C6—C3 | 0.3 (3) | C31—C19—C21—O2 | −86.7 (3) |
| C7—N1—C6—C3 | −176.5 (2) | O1—C19—C21—C32 | 143.5 (3) |
| C13—C3—C6—C17 | −2.2 (4) | C28—C19—C21—C32 | −98.9 (4) |
| C12—C3—C6—C17 | 179.9 (2) | C31—C19—C21—C32 | 30.1 (4) |
| C13—C3—C6—N1 | 176.3 (2) | O1—C19—C21—C35 | −87.0 (3) |
| C12—C3—C6—N1 | −1.6 (3) | C28—C19—C21—C35 | 30.7 (4) |
| C5—N1—C7—C18 | 119.4 (3) | C31—C19—C21—C35 | 159.6 (3) |
| C6—N1—C7—C18 | −64.3 (4) | C7—C18—C22—C27 | 0.7 (5) |
| C5—N1—C7—C15 | −61.3 (4) | C21—O2—B1—O1 | 7.2 (3) |
| C6—N1—C7—C15 | 115.0 (3) | C21—O2—B1—C8 | −174.5 (3) |
| C14—C9—C10—C12 | 0.1 (4) | C19—O1—B1—O2 | 11.4 (3) |
| B2—C9—C10—C12 | 179.1 (3) | C19—O1—B1—C8 | −166.9 (3) |
| C13—C8—C11—C17 | −2.1 (4) | C13—C8—B1—O2 | 10.8 (4) |
| B1—C8—C11—C17 | 174.0 (3) | C11—C8—B1—O2 | −165.2 (3) |
| C9—C10—C12—C5 | 1.0 (4) | C13—C8—B1—O1 | −171.1 (3) |
| C9—C10—C12—C3 | 177.9 (3) | C11—C8—B1—O1 | 12.9 (4) |
| C16—C5—C12—C10 | −1.6 (4) | B2—O4—C25—C33 | 113.9 (4) |
| N1—C5—C12—C10 | 175.6 (2) | B2—O4—C25—C37 | −129.2 (4) |
| C16—C5—C12—C3 | −179.3 (3) | B2—O4—C25—C29 | −8.3 (4) |
| N1—C5—C12—C3 | −2.1 (3) | C25—O4—B2—O3 | 3.3 (5) |
| C13—C3—C12—C10 | 7.6 (5) | C25—O4—B2—C9 | −176.8 (4) |
| C6—C3—C12—C10 | −174.9 (3) | C29—O3—B2—O4 | 3.8 (5) |
| C13—C3—C12—C5 | −175.2 (3) | C29—O3—B2—C9 | −176.1 (4) |
| C6—C3—C12—C5 | 2.2 (3) | C10—C9—B2—O4 | −164.1 (4) |
| C11—C8—C13—C3 | 1.1 (4) | C14—C9—B2—O4 | 14.9 (6) |
| B1—C8—C13—C3 | −174.9 (2) | C10—C9—B2—O3 | 15.8 (6) |
| C6—C3—C13—C8 | 0.9 (4) | C14—C9—B2—O3 | −165.3 (4) |
| C12—C3—C13—C8 | 178.1 (3) | C18—C22—C27—C30 | 0.2 (5) |
| C10—C9—C14—C16 | −0.7 (5) | B2—O3—C29—C36 | −131.5 (4) |
| B2—C9—C14—C16 | −179.7 (3) | B2—O3—C29—C34 | 113.0 (4) |
| C18—C7—C15—C30 | 0.6 (4) | B2—O3—C29—C25 | −8.6 (4) |
| N1—C7—C15—C30 | −178.7 (3) | O4—C25—C29—O3 | 9.9 (3) |
| C9—C14—C16—C5 | 0.1 (5) | C33—C25—C29—O3 | −107.1 (4) |
| N1—C5—C16—C14 | −175.6 (3) | C37—C25—C29—O3 | 124.8 (4) |
| C12—C5—C16—C14 | 1.0 (4) | O4—C25—C29—C36 | 127.5 (4) |
| C8—C11—C17—C6 | 0.8 (4) | C33—C25—C29—C36 | 10.5 (5) |
| N1—C6—C17—C11 | −176.8 (3) | C37—C25—C29—C36 | −117.6 (4) |
| C3—C6—C17—C11 | 1.4 (4) | O4—C25—C29—C34 | −105.0 (4) |
| C15—C7—C18—C22 | −1.1 (4) | C33—C25—C29—C34 | 138.0 (5) |
| N1—C7—C18—C22 | 178.1 (3) | C37—C25—C29—C34 | 9.9 (5) |
| B1—O1—C19—C28 | −146.1 (3) | C22—C27—C30—C15 | −0.7 (5) |
| B1—O1—C19—C31 | 95.0 (3) | C7—C15—C30—C27 | 0.4 (5) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JH2305).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Cui, J., Duan, M. & Cai, L. (2009). Acta Cryst. E65, o215. [DOI] [PMC free article] [PubMed]
- Oliveira, M. M., Salvador, M. A., Coelho, P. J. & Carvalho, L. M. (2005). Tetrahedron, 61, 1681–1691.
- Rashidnadimi, S., Hung, T. H., Wong, K.-T. & Bard, A. J. (2008). J Am Chem Soc 130, 634–639. [DOI] [PubMed]
- Rigaku (2005). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Saeed, A., Kazmi, M., Ameen Samra, S., Irfan, M. & Bolte, M. (2010). Acta Cryst. E66, o2118. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wong, K.-T., Chao, T.-C., Chi, L.-C., Chu, Y.-Y., Balaiah, A., Chiu, S.-F., Liu, Y.-H. & Wang, Y. (2006). Org. Lett. 8, 5033–5036. [DOI] [PubMed]
- Wong, K.-T., Hung, T.-H., Chao, T.-C. & Ho, T.-I. (2005). Tetrahedron Lett. 46, 855–858.
- Xu, G.-Y., Geng, W.-Q. & Zhou, H.-P. (2010). Acta Cryst. E66, o571. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811025645/jh2305sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811025645/jh2305Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811025645/jh2305Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



