Abstract
The non-H atoms of the title compound, C9H8ClN3O3, lie approximately on a plane (r.m.s. deviation = 0.111 Å), and the C=N double bond has a Z configuration. In the crystal, adjacent molecules are linked by an N—H⋯Ocarbonyl hydrogen bond, forming a chain running along [101].
Related literature
For the synthesis, see: Benincori et al. (1990 ▶); Sayed et al. (2002 ▶). For background to the title compound, see: Asiri et al. (2010 ▶).
Experimental
Crystal data
C9H8ClN3O3
M r = 241.63
Monoclinic,

a = 7.0628 (3) Å
b = 13.4182 (5) Å
c = 11.2884 (5) Å
β = 95.589 (4)°
V = 1064.72 (8) Å3
Z = 4
Cu Kα radiation
μ = 3.19 mm−1
T = 100 K
0.20 × 0.10 × 0.05 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2010 ▶) T min = 0.568, T max = 0.857
4113 measured reflections
2105 independent reflections
1839 reflections with I > 2σ(I)
R int = 0.020
Refinement
R[F 2 > 2σ(F 2)] = 0.054
wR(F 2) = 0.152
S = 1.09
2105 reflections
150 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 1.21 e Å−3
Δρmin = −0.48 e Å−3
Data collection: CrysAlis PRO (Agilent, 2010 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811026407/xu5261sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811026407/xu5261Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811026407/xu5261Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯O1i | 0.85 (4) | 2.26 (4) | 3.000 (3) | 145 (3) |
Symmetry code: (i) 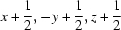 .
.
Acknowledgments
We thank King Abdulaziz University and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
We have previously reported the synthesis of ethyl (Z)-2-chloro-2-(2-phenylhydrazin-1-ylidene) acetate by the reaction of benzenediazonium chloride with ethyl 2-chloro-3-oxobutanoate (Asiri et al., 2010). The compound is an ester. In the present study, the use of a substituted benzenediazonium chloride and the methyl ester (instead of the ethyl ester) afforded a 1-chloro-1-(arylhydrazono)-2-propanone. Such ketones are intermediates in the synthesis of pyrazoles (Sayed et al., 2002) and other heterocycles (Benincori et al., 1990). In the 4-nitro substituted compound (Scheme I, Fig. 1), the non-hydrogen atoms lie on a plane [r.m.s. deviation 0.111 Å] (Scheme I, Fig. 1). The Caryl–N(H)–N═ C(S)═O portion adopts an extended zigzag conformation. Adjacent molecules are linked by an N–H···Ocarbonyl hydrogen bond to form a chain running [1 0 1].
Experimental
To a stirred solution of methyl 2-chloro-3-oxobutanoate (1.64 g, 10 mmol) in ethanol (100 ml) was added sodium acetate trihydrate (1.30 g, 10 mmol). The mixture was chilled to 273 K and then treated with a cold solution of p-nitrobenzenediazonium chloride, prepared by diazotizing p-nitroaniline (1.38 g, 10 mmol) dissolved in 6M hydrochloric acid (6 ml) with a solution of sodium nitrite (0.70 g, 10 mmol) in water (10 ml). The addition of the diazonium salt solution was carried out with rapid stirring over a period of 20 min. The reaction mixture was stirred for further 15 min. and left for 3 h in refrigerator. The resulting solid was collected by filtration and washed thoroughly with water. The crude product was crystallized from ethanol to give the corresponding hydrazonoyl chloride.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C—H 0.95 to 0.98 Å, Uiso(H) 1.2 to 1.5Ueq(C)] and were included in the refinement in the riding model approximation.
The amino H-atom was located in a difference Fourier map, and was freely refined.
The final difference Fourier map had a peak in the vicinity of H6.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of C9H8ClN3O3 at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C9H8ClN3O3 | F(000) = 496 |
| Mr = 241.63 | Dx = 1.507 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: -P 2yn | Cell parameters from 1647 reflections |
| a = 7.0628 (3) Å | θ = 3.3–74.3° |
| b = 13.4182 (5) Å | µ = 3.19 mm−1 |
| c = 11.2884 (5) Å | T = 100 K |
| β = 95.589 (4)° | Prism, yellow |
| V = 1064.72 (8) Å3 | 0.20 × 0.10 × 0.05 mm |
| Z = 4 |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 2105 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 1839 reflections with I > 2σ(I) |
| Mirror | Rint = 0.020 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 74.4°, θmin = 5.1° |
| ω scans | h = −8→5 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2010) | k = −16→16 |
| Tmin = 0.568, Tmax = 0.857 | l = −14→14 |
| 4113 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.054 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.152 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.09 | w = 1/[σ2(Fo2) + (0.0849P)2 + 0.8946P] where P = (Fo2 + 2Fc2)/3 |
| 2105 reflections | (Δ/σ)max = 0.001 |
| 150 parameters | Δρmax = 1.21 e Å−3 |
| 0 restraints | Δρmin = −0.48 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.63780 (10) | 0.29860 (4) | 0.39614 (6) | 0.0299 (2) | |
| O1 | 0.4446 (3) | 0.25504 (13) | 0.15943 (16) | 0.0275 (4) | |
| O2 | 0.8827 (3) | −0.34157 (14) | 0.6658 (2) | 0.0369 (5) | |
| O3 | 1.0132 (3) | −0.25969 (16) | 0.81885 (18) | 0.0383 (5) | |
| N1 | 0.6623 (3) | 0.10048 (16) | 0.37953 (18) | 0.0213 (5) | |
| N2 | 0.7385 (3) | 0.09908 (17) | 0.49160 (19) | 0.0226 (5) | |
| H2 | 0.753 (5) | 0.154 (3) | 0.528 (3) | 0.042 (10)* | |
| N3 | 0.9267 (3) | −0.26356 (18) | 0.7182 (2) | 0.0300 (5) | |
| C1 | 0.5294 (4) | 0.0846 (2) | 0.1360 (2) | 0.0304 (6) | |
| H1A | 0.4572 | 0.0912 | 0.0578 | 0.046* | |
| H1B | 0.4743 | 0.0311 | 0.1808 | 0.046* | |
| H1C | 0.6622 | 0.0686 | 0.1258 | 0.046* | |
| C2 | 0.5211 (4) | 0.1804 (2) | 0.2026 (2) | 0.0251 (6) | |
| C3 | 0.6129 (4) | 0.18280 (19) | 0.3275 (2) | 0.0224 (5) | |
| C4 | 0.7818 (3) | 0.00828 (19) | 0.5472 (2) | 0.0209 (5) | |
| C5 | 0.8644 (4) | 0.0084 (2) | 0.6644 (2) | 0.0246 (5) | |
| H5 | 0.8885 | 0.0696 | 0.7054 | 0.030* | |
| C6 | 0.9115 (4) | −0.0816 (2) | 0.7210 (2) | 0.0250 (5) | |
| H6 | 0.9677 | −0.0825 | 0.8009 | 0.030* | |
| C7 | 0.8754 (4) | −0.16926 (19) | 0.6594 (2) | 0.0237 (5) | |
| C8 | 0.7916 (4) | −0.17086 (19) | 0.5427 (2) | 0.0241 (5) | |
| H8 | 0.7668 | −0.2324 | 0.5026 | 0.029* | |
| C9 | 0.7449 (4) | −0.08185 (19) | 0.4860 (2) | 0.0229 (5) | |
| H9 | 0.6882 | −0.0815 | 0.4061 | 0.027* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0424 (4) | 0.0166 (4) | 0.0299 (4) | 0.0005 (2) | 0.0002 (3) | 0.0006 (2) |
| O1 | 0.0353 (10) | 0.0225 (9) | 0.0246 (9) | 0.0050 (8) | 0.0026 (8) | 0.0069 (7) |
| O2 | 0.0469 (12) | 0.0195 (10) | 0.0448 (12) | 0.0028 (9) | 0.0070 (10) | 0.0060 (9) |
| O3 | 0.0513 (13) | 0.0354 (11) | 0.0272 (10) | 0.0140 (10) | −0.0015 (9) | 0.0142 (9) |
| N1 | 0.0244 (10) | 0.0193 (10) | 0.0202 (10) | 0.0008 (8) | 0.0019 (8) | 0.0023 (8) |
| N2 | 0.0300 (11) | 0.0183 (10) | 0.0195 (10) | 0.0001 (9) | 0.0014 (8) | −0.0007 (9) |
| N3 | 0.0329 (12) | 0.0262 (12) | 0.0322 (12) | 0.0067 (10) | 0.0099 (10) | 0.0094 (10) |
| C1 | 0.0410 (15) | 0.0266 (14) | 0.0233 (12) | 0.0013 (12) | 0.0021 (11) | −0.0004 (11) |
| C2 | 0.0279 (12) | 0.0229 (12) | 0.0253 (13) | 0.0009 (10) | 0.0066 (10) | 0.0058 (10) |
| C3 | 0.0265 (12) | 0.0169 (11) | 0.0244 (12) | 0.0022 (10) | 0.0062 (10) | 0.0026 (10) |
| C4 | 0.0214 (11) | 0.0179 (12) | 0.0242 (12) | 0.0006 (9) | 0.0060 (9) | 0.0034 (10) |
| C5 | 0.0291 (12) | 0.0206 (13) | 0.0245 (12) | −0.0018 (10) | 0.0046 (10) | −0.0028 (10) |
| C6 | 0.0259 (12) | 0.0297 (14) | 0.0193 (11) | 0.0032 (10) | 0.0016 (9) | 0.0044 (10) |
| C7 | 0.0266 (12) | 0.0197 (13) | 0.0254 (13) | 0.0052 (10) | 0.0059 (10) | 0.0097 (10) |
| C8 | 0.0287 (12) | 0.0186 (12) | 0.0259 (13) | −0.0007 (10) | 0.0071 (10) | −0.0007 (10) |
| C9 | 0.0275 (12) | 0.0198 (12) | 0.0214 (12) | −0.0009 (10) | 0.0027 (9) | 0.0002 (10) |
Geometric parameters (Å, °)
| Cl1—C3 | 1.737 (3) | C1—H1C | 0.9800 |
| O1—C2 | 1.217 (3) | C2—C3 | 1.494 (4) |
| O2—N3 | 1.227 (3) | C4—C5 | 1.393 (4) |
| O3—N3 | 1.238 (3) | C4—C9 | 1.405 (4) |
| N1—C3 | 1.283 (3) | C5—C6 | 1.391 (4) |
| N1—N2 | 1.326 (3) | C5—H5 | 0.9500 |
| N2—C4 | 1.391 (3) | C6—C7 | 1.377 (4) |
| N2—H2 | 0.85 (4) | C6—H6 | 0.9500 |
| N3—C7 | 1.458 (3) | C7—C8 | 1.391 (4) |
| C1—C2 | 1.493 (4) | C8—C9 | 1.380 (4) |
| C1—H1A | 0.9800 | C8—H8 | 0.9500 |
| C1—H1B | 0.9800 | C9—H9 | 0.9500 |
| C3—N1—N2 | 121.0 (2) | N2—C4—C5 | 118.7 (2) |
| N1—N2—C4 | 119.6 (2) | N2—C4—C9 | 120.7 (2) |
| N1—N2—H2 | 118 (3) | C5—C4—C9 | 120.6 (2) |
| C4—N2—H2 | 122 (3) | C6—C5—C4 | 119.6 (2) |
| O2—N3—O3 | 123.9 (2) | C6—C5—H5 | 120.2 |
| O2—N3—C7 | 118.7 (2) | C4—C5—H5 | 120.2 |
| O3—N3—C7 | 117.4 (2) | C7—C6—C5 | 119.1 (2) |
| C2—C1—H1A | 109.5 | C7—C6—H6 | 120.5 |
| C2—C1—H1B | 109.5 | C5—C6—H6 | 120.5 |
| H1A—C1—H1B | 109.5 | C6—C7—C8 | 122.1 (2) |
| C2—C1—H1C | 109.5 | C6—C7—N3 | 119.1 (2) |
| H1A—C1—H1C | 109.5 | C8—C7—N3 | 118.8 (2) |
| H1B—C1—H1C | 109.5 | C9—C8—C7 | 119.1 (2) |
| O1—C2—C1 | 123.0 (2) | C9—C8—H8 | 120.4 |
| O1—C2—C3 | 119.7 (2) | C7—C8—H8 | 120.4 |
| C1—C2—C3 | 117.3 (2) | C8—C9—C4 | 119.5 (2) |
| N1—C3—C2 | 119.1 (2) | C8—C9—H9 | 120.2 |
| N1—C3—Cl1 | 123.7 (2) | C4—C9—H9 | 120.2 |
| C2—C3—Cl1 | 117.12 (18) | ||
| C3—N1—N2—C4 | 176.8 (2) | C5—C6—C7—C8 | −0.6 (4) |
| N2—N1—C3—C2 | −177.8 (2) | C5—C6—C7—N3 | 179.3 (2) |
| N2—N1—C3—Cl1 | −0.4 (3) | O2—N3—C7—C6 | 175.2 (2) |
| O1—C2—C3—N1 | 167.5 (2) | O3—N3—C7—C6 | −4.9 (4) |
| C1—C2—C3—N1 | −12.9 (4) | O2—N3—C7—C8 | −4.9 (4) |
| O1—C2—C3—Cl1 | −10.0 (3) | O3—N3—C7—C8 | 175.0 (2) |
| C1—C2—C3—Cl1 | 169.63 (19) | C6—C7—C8—C9 | 0.8 (4) |
| N1—N2—C4—C5 | 179.0 (2) | N3—C7—C8—C9 | −179.1 (2) |
| N1—N2—C4—C9 | −0.3 (3) | C7—C8—C9—C4 | −0.3 (4) |
| N2—C4—C5—C6 | −179.0 (2) | N2—C4—C9—C8 | 179.2 (2) |
| C9—C4—C5—C6 | 0.3 (4) | C5—C4—C9—C8 | −0.2 (4) |
| C4—C5—C6—C7 | 0.1 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···O1i | 0.85 (4) | 2.26 (4) | 3.000 (3) | 145 (3) |
Symmetry codes: (i) x+1/2, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU5261).
References
- Agilent (2010). CrysAlis PRO Agilent Technologies, Yarnton, Oxfordshire, England.
- Asiri, A. M., Zayed, M. E. M. & Ng, S. W. (2010). Acta Cryst. E66, o2374. [DOI] [PMC free article] [PubMed]
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Benincori, T., Fusco, R. & Sannicolo, F. (1990). Gazz. Chim. Ital. 120, 635–659.
- Sayed, S. M., Khalil, M. A., Ahmed, M. A. & Raslan, M. A. (2002). Synth. Commun. 32, 481–495.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811026407/xu5261sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811026407/xu5261Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811026407/xu5261Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



