Abstract
Little is known about arousal to socially stressful situations in children with Autism Spectrum Disorders. This preliminary study investigates physiologic arousal in children with high functioning autism (HFA, n=19) compared to a comparison group (n=11) before, during, and after the Trier Social Stress Test. The HFA group was more likely to have a decrease in salivary cortisol following the stressor, while the comparison group was more likely to have an increase (p=.02). However, there was no difference in electrodermal activity, a measure of sympathetic arousal, or vagal tone, a measure of parasympathetic activity, between groups. These findings implicate a differential neuroendocrine response to social stress in children with HFA despite similar sympathetic and parasympathetic responses during a stressor. Further studies are required to substantiate this finding.
Keywords: psychophysiology, Trier Social Stress Test, high functioning autism, stress
Children with Autism Spectrum Disorders (ASDs) are known to have unique behavioral responses to social stimuli. However, identifying subjective, internal states in children with ASDs is difficult due to deficits in language and communication and difficulties processing and identifying emotions (Hill, Berthoz, & Frith, 2004; Losh & Capps, 2006). Thus, behavioral treatments to improve social and emotional functioning in children with ASDs are often challenged by this lack of important information. Measurements of psychophysiology have been used in prior studies to better elucidate how social stimuli are perceived differently in children with ASDs. However, these investigations have utilized a variety of protocols and stimuli, making conclusions about physiologic arousal in ASDs difficult.
Neuroendocrine markers of stress using cortisol reactivity (CORT) have shown greater reactivity than controls to a non-social stressor in some studies (Corbett, Mendoza, Abdullah, Wegelin, & Levine, 2006), but not others (Corbett, Mendoza, Wegelin, Carmean, & Levine, 2008; Corbett, Schupp, Levine, & Mendoza, 2009). In those studies using more socially relevant protocols, there was no difference in CORT from controls in children with ASDs undergoing a public speaking task (Jansen, Gispen-de Wied, van der Gaag, & van Engeland, 2003). One study examining children with high functioning ASDs found there was a significantly elevated CORT response to a social interaction with an unfamiliar peer when compared with a previous interaction with a familiar peer, but no difference if the order of the interactions was reversed (Lopata, Volker, Putnam, Thomeer, & Nida, 2008). Another study found children with autism engaged in a social playground paradigm had elevated CORT levels with consideration for age and behavioral patterns observed in these children when compared to controls (Corbett, Schupp, Simon, Ryan, & Mendoza, 2010).
Studies of sympathetic nervous system responses in ASDs have reported increased electrodermal activity (EDA) to eye gaze (Kylliainen & Hietanen, 2006), no difference from controls in reaction to pleasant, unpleasant, and neutral photographs (Ben Shalom, et al., 2006), and initial unresponsiveness to auditory stimuli (van Engeland, 1984). Children with ASDs have also demonstrated increased EDA in naturalistic settings (Hirstein, Iversen, & Ramachandran, 2001) and at baseline (Palkovitz & Wiesenfeld, 1980). Studies investigating the parasympathetic nervous system activity have found lower heart rate variability or vagal tone (VT) in children with ASDs at rest (Ming, Julu, Brimacombe, Connor, & Daniels, 2005), but greater VT during a mentally rigorous task (Toichi & Kamio, 2003) when compared to controls.
Our goal in this study was to explore potential differences in the level of physiological measures of arousal (CORT, EDA, and VT) at baseline and in reaction to a standardized social stressor in children with high functioning autism (HFA) compared to age matched controls (COMP). This study is one of the first to investigate physiological data using a standardized social stressor (The Trier Social Stress Test) to allow a deeper understanding into potential mechanisms underlying behavioral events in children with HFA. Our hypothesis was that children with HFA would demonstrate more physiologic arousal, as measured by elevated CORT and EDA, and less regulation of this stress, as measured by lower levels of VT, during a social stressor than COMP.
1. Methods
1.1. Participants
Participants included 19 children with high functioning autism (HFA), and 11 children in a non-autistic, healthy comparison group. Participants were 8 to 12 years old and provided assent along with parental consent in compliance with our institutional review board. Children in HFA group had a Diagnostics and Statistical Manual of Mental Disorders IV-TR diagnoses of Asperger’s Disorder, Autistic Disorder, or Pervasive Developmental Disorder NOS based on community-based diagnoses from the Providence, RI region as well as medical record review. This technique has been utilized in other studies examining HFA (Abell & Hare, 2005; Gillott, Furniss, & Walter, 2001; Lopata, Thomeer, Volker, Nida, & Lee, 2008). Diagnosticians were child and adolescent psychiatrists, psychologists, developmental pediatricians, and a pediatric neurologist. Exclusion criteria were diagnoses of mania, depression, psychosis, substance abuse, posttraumatic stress disorder, obsessive-compulsive disorder (all done via telephone screening), total Intelligence Quotients (IQs) less than 70, or current psychotropic medication use. The comparison group met the same criteria, but without the HFA diagnosis.
1.2. Stressor
All participants underwent the Trier Social Stress Test (TSST) which has been shown to induce psychosocial stress in children under laboratory conditions with changes in cortisol and cardiovascular parameters (Buske-Kirschbaum, et al., 1997). The TSST consists of the following activities which are performed in the presence of an observer and an examiner: 1) Preparation and completion of an oral story (10 minutes), 2) serial subtractions (5 minutes), 3) tracing a star through a mirror with an apparatus that beeps when errors are made (5 minutes, added by our lab to the protocol), and 4) a debriefing (approximately 5 minutes) where the child receives explanations about the test and praise for good work done.
1.3. Measures
1.3.1. Measures of Autistic Traits
Social Responsiveness Scale-Parent Version (SRS) The SRS is a 65-item parent report that provides quantitative measure of autistic traits for children 4 to 18 years old, completed in a separate room while the child underwent the TSST. The SRS generates both scale scores for specific symptom domains (social awareness, social cognition, social communication, social motivation, and autistic mannerisms) and a singular total score for autistic social impairment (Constantino, et al., 2003). This measure was used to validate the community HFA diagnoses based on total scores.
1.3.2. Measures of Intelligence and Socioeconomic Status
Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) is a short and reliable measure of intellectual function, designed for use with individuals aged 6–89 years. It consists of four sections (Vocabulary, Similarities, Block Design, and Matrix Reasoning) and is highly consistent with the Full Scale IQ.
Socioeconomic status (SES) was measured using the 4 factor Hollingshead Index of Social Position (Hollingshead, 1975). Lower scores reflect higher SES.
1.3.3. Physiologic measurement
Cortisol (CORT) was extracted from saliva samples as a measure of stress response to the TSST (measured in micrograms/dL). Five samples were taken; immediately following entry into the study (1), following placement of the ECG and EDA leads (2), and then at the start of (3), immediately following (4), and 30 minutes after the end of the TSST (5) (See Table 1). Each sample was frozen at −20°C and sent on dry ice for immunoassay at Salimetrics LLC (State College, PA). Duplicate assays were done on each sample and averaged for each time point in Table 1. Since we are most interested in the CORT reactivity resulting from the TSST, change scores were calculated between samples 3 and 4 and this change score was then dichotomized into Increasers (change score >0) and Decreasers (change scores ≤0).
Table 1.
Protocol Timeline
| Baseline | Stressor (TSST) | Post TSST | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Adjust1 | Start | Story | Math | Tracing | Debrief | Adjust2 | Post | |
| Time (min) | -- | 30 | -- | 10 | 5 | 5 | ~5 | 30 | -- |
| SRS | X | ||||||||
| Cortisol | X(1) | X(2) | X(3) | X(4) | X(5) | ||||
| WASI | X | ||||||||
| HR/EDA | Continuous | ||||||||
Vagal Tone (VT) is calculated as beat-to-beat heart rate (HR) variability that occurs as a result of vagal nerve regulation of the heart and reflects parasympathetic nervous system activity. Besides its implication in direct cardiovascular regulation, VT has been associated with emotional regulation and stimulus orientation at multiple developmental levels. The “polyvagal theory” endorses a role for the parasympathetic nervous system in regulation of social communication, self- soothing, and calming (Porges, 2001). To measure VT, electrocardiogram (ECG) signals were collected via electrodes placed on the child’s chest and abdomen and recorded continuously using the BIOPAC MP150 Data Acquisition System (BIOPAC Systems Inc., Goleta, CA) and the Acknowledge 3.8.2 software. HR was calculated from the interval between consecutive R-waves. VT was derived from time-series analysis of R–R intervals from digitized the ECG recordings. The resulting measure is one of HR variability in the frequency range of respiratory rate. The natural logarithm of heart period variance in this frequency range is a measure of respiratory sinus arrhythmia known as VT. VT was then averaged over 2 minute intervals during the following times (See Table 1): at the beginning and end of the 30 minute pre-TSST period (Adjust1); during all phases of the TSST (story preparation, story telling, math, tracing, and debrief); and then at the beginning and end of the 30 minute post-TSST period (Adjust 2).
Electrodermal Activity (EDA) is measured in microOhms and is a measure of sympathetic response obtained by measuring skin conductance in the ventral finger tips which have a high density of eccrine sweat glands. These glands have only sympathetic nervous innervation and their reactivity is a result of arousal from the sympathetic nervous system. EDA was recorded using a Biopac GSR100C Electro-dermal Activity Amplifier employing a constant voltage technique and sampling the absolute, direct skin conductance at the rate of 125 samples per second from the first two digits of the non-dominant hand. EDA was averaged over 4 minute intervals corresponding with the same 2 minute VT periods listed above. Since VT is calculated for each beat over a two minute interval, the four minute EDA average represents the same time period as the VT interval.
1.4. Procedure
All participants were seen at 3 o’clock in the afternoon to capture the approximate nadir of the CORT diurnal cycle. HR and EDA were continuously monitored and the session was video recorded to synchronize the physiology recording with events in the protocol. IQ scores were extracted from medical and assessment records if available. If unavailable, the WASI was administered following the protocol (see Table 1).
2. Statistical Analysis
All physiologic measures (CORT, EDA, VT) were compared at corresponding time points between HFA and COMP groups using analysis of variance (ANOVA). CORT reactivity from the TSST was compared between groups using ANOVA, a method used in other studies (Lester, et al., 2010). Fisher’s Exact Test was used to compare CORT Increasers and Decreasers between groups and correlations between physiologic measures (CORT, EDA, VT) within groups were performed as post hoc analyses.
3. Results
3.1. Participants
Groups did not differ in age, race/ethnicity, gender composition, or IQ (Table 2).
Table 2.
Participant Descriptives
| N | Age (Mean) | Gender | IQ (Mean) | Ethnicity | |
|---|---|---|---|---|---|
| HFA | 19 | 9.7 (SD=1.4) | 3 female 16 male |
99 (SD=12.9) | White 17 Latino 1 Asian 1 Black 0 |
| COMP | 11 | 9.6 (SD=1.4) | 4 female 7 male |
111 (SD=14.8) | White 8 Latino 1 Asian 0 Black 1 |
Of the 19 HFA children, 9 (47%) had a diagnosis of Asperger’s Disorder, 8 (42%) Pervasive Developmental Disorder NOS and 2 (11%) Autistic Disorder. The families of all participants were in Hollingshead SES groups 1 to 3, except for one family in the HFA group who was group 4. In the COMP group, 5 children were siblings of children with ASDs, 2 of whom were in the HFA group. Two children in the COMP group were siblings themselves, but did not have another sibling with an ASD. All participants from the HFA group had total score T-scores on the SRS >65 (M=82.4, SD 8.8), the clinical cutoff for mild ASD. All participants in the COMP group had total T-scores of <57 (M=42.7, SD 6.4). One participant from the COMP group was dropped secondary to having an SRS total T score >65 without a diagnosis of an ASD. One participant from the HFA group had an IQ of 69 but was included since he was able to perform the TSST tasks.
One participant from the control group did not have demographic information available or an IQ score recorded.
3.2. Cortisol Reactivity
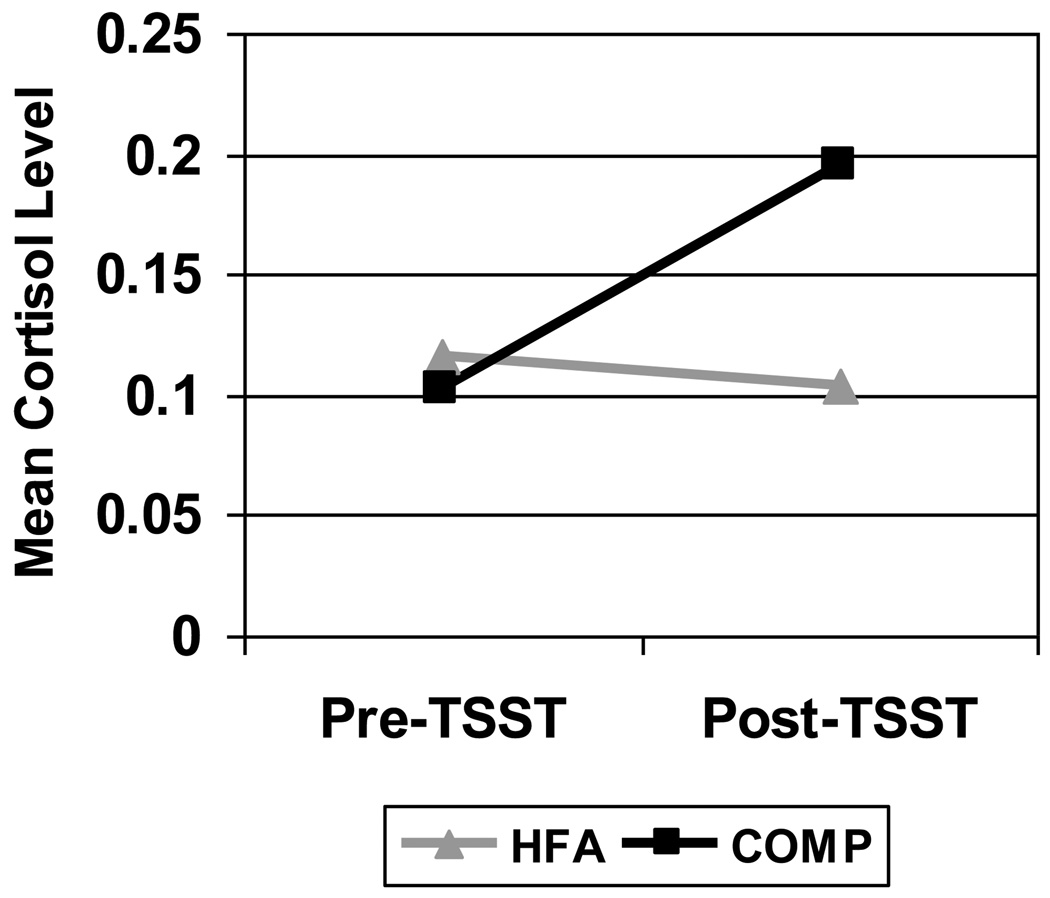
There were no significant differences in any of the 5 CORT samples (see Table 1) between groups. CORT change scores (micrograms/dL) before and after the TSST were significantly different between the HFA group, which decreased, and the COMP group, which increased [M=−.012 (SD .101) vs. M=.095 (SD .129), respectively, p=.02] (See Figure 1).
Figure 1.
Cortisol change comparison
There were more CORT Decreasers than Increasers in the HFA compared to COMP group (see Table 3), which approached statistical significance (p=.06).
Table 3.
Cortisol Groups
| HFA | COMP | |
|---|---|---|
| Increasers (N) | 6 | 8 |
| Decreasers (N) | 13 | 3 |
3.3. Autonomic Responses
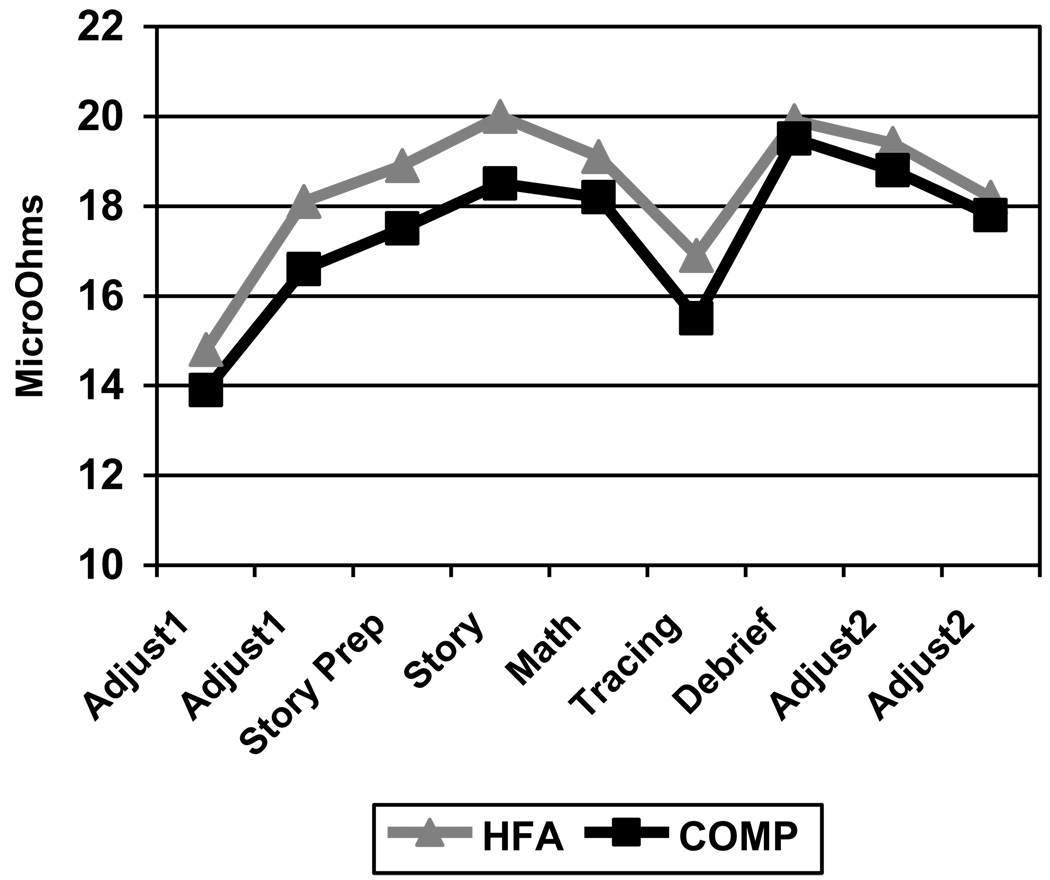
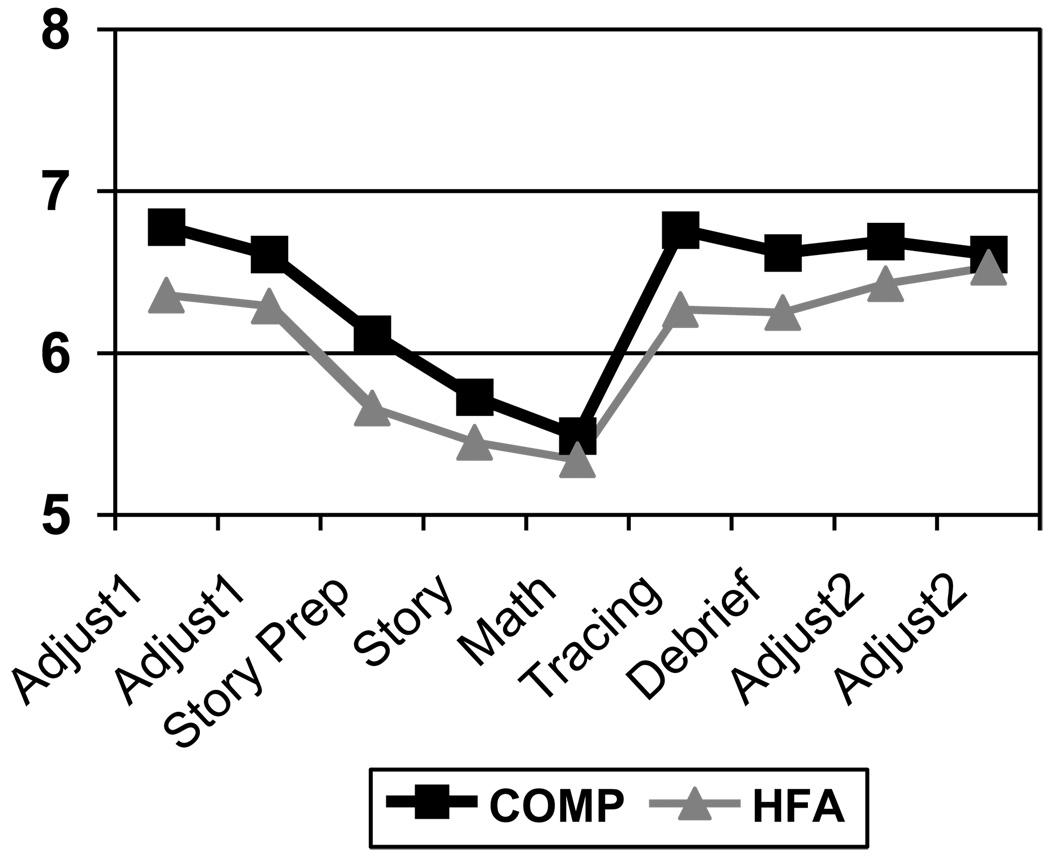
There were no statistically significant differences in EDA or VT at baseline, during the TSST, or post-TSST between the HFA and COMP groups (see Figures 2 & 3).
Figure 2.
Electrodermal activity (EDA) comparison
Figure 3.
Vagal tone (VT) comparison
However, both groups did demonstrate an increase in EDA in the events leading up to the TSST (Adjust 1) and during the TSST (Story Prep through Math). There was a decrease in EDA during the Tracing, followed by an increase during the Debrief, with a subsequent decrease through the rest of the protocol. VT showed a similar, yet inverse pattern in both groups with gradual decrease to the Math activity, a recovery at the Tracing, a decrease at the Debrief, and stabilization through the rest of the protocol. While not statistically significant, HFA children had consistently higher EDA and lower VT across the procedure as predicted.
No correlations between CORT, VT, or EDA levels were found within the HFA or COMP groups.
4. Discussion
We found that children with HFA did not demonstrate the increase in CORT in response to the social stressor that we found in the non-HFA children. In fact, the HFA children showed a decreased or blunted CORT response, indicating an attenuated hypothalamic-pituitary-adrenal axis (HPA axis) reaction to a social stressor. However, CORT reactivity in children tested in similar paradigms can vary by age, gender, pubertal level, availability of coping resources, and the quality of the parent-child relationship (Gunnar, Talge, & Herrera, 2009; Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Jessop & Turner-Cobb, 2008), making conclusions from this sample difficult. With this in mind, there are multiple possible explanations for this finding. First, children with ASDs may have hypoactivation of the HPA axis secondary to differential functioning in the amygdala (Schultz, 2005) and prefrontal cortex (Kennedy & Courchesne, 2008) which have connectivity to the HPA axis and may contribute to its activation. Further neuroimaging studies as well as testing of other hormones in the HPA axis (corticotropin releasing factor and adrenocorticotropin hormone) would be necessary to confirm this. Secondly, children with HFA may have a blunted CORT response secondary to chronic stress experienced through a lifetime of difficulty with social interactions and function. This is modeled from similar findings in adults that experienced adverse events in childhood (Carpenter, et al., 2007; Elzinga, et al., 2008; Klaassens, et al., 2009). Lastly, while the TSST is an effective social stressor in non-autistic children, children with HFA may not have found the TSST stressful or had better coping strategies with which to handle the stress. However, EDA and VT measurements indicate that the HFA group experienced physiologic arousal, indicating that the paradigm induced stress. Further investigations into these areas are important to elucidate this significant difference in neuroendocrine reactivity in children with HFA in this paradigm.
These results do not support our hypothesis that children with HFA would demonstrate more sympathetic arousal, as measured by elevated EDA, and less regulation of this stress, as measured by lower levels of VT, during a social stressor than COMP. However, these findings are intriguing since they show that children with HFA experienced the same moment-to-moment sympathetic and parasympathetic reactivity compared to COMP as demonstrated by having similar EDA and VT patterns throughout the protocol. While our sample might not have been large enough to detect significant difference between groups, the deeper meaning to this could be that despite difficulties with social relatedness, children with HFA may experience components of social stress as do children without HFA.
5. Limitations
This study had a small sample size and the diagnoses of ASDs in the HFA group were not performed using “gold standard” measures including the Autism Diagnostic Observation Schedule (Lord, et al., 2000) and the Autism Diagnostic Interview-Revised (Rutter, Lecouteur, & Lord, 2003). However, we did use the SRS to confirm that the HFA group met diagnostic criteria on this measure and the COMP group did not. Another major limitation is the fact that 5 of the children in the COMP group were siblings of children with ASDs. However, based on research supporting that family members of children with ASDs are likely to demonstrate autistic phenotypic characteristics (Bailey, Palferman, Heavey, & Le Couteur, 1998; Szatmari, et al., 2000), our differences in CORT reactivity may be more robust with a COMP group not including biological relatives. Also, it is possible with greater sample size, the differences between groups in VT and EDA across the protocol may be statistically significant.
Research Highlights.
Study of arousal to social stress in children with high functioning autism (HFA)
HFA group decreased salivary cortisol level after the stress, controls had increase
No difference in sympathetic or parasympathetic activity between HFA and controls
Findings implicate different neuroendocrine response to social stress in HFA
Acknowledgements
This study was funded by National Institutes of Health grant T32MH19927: Research Training in Child Mental Health, principal investigator: Gregory Fritz, MD. Funding was also provided by The Brown Center for the Study of Children at Risk and Women and Infants’ Hospital, Providence, RI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell F, Hare DJ. An experimental investigation of the phenomenology of delusional beliefs in people with Asperger syndrome. Autism. 2005;9(5):515–531. doi: 10.1177/1362361305057857. [DOI] [PubMed] [Google Scholar]
- Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: the phenotype in relatives. J Autism Dev Disord. 1998;28(5):369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- Ben Shalom D, Mostofsky SH, Hazlett RL, Goldberg MC, Landa RJ, Faran Y, Hoehn-Saric R. Normal physiological emotions but differences in expression of conscious feelings in children with high-functioning autism. J Autism Dev Disord. 2006;36(3):395–400. doi: 10.1007/s10803-006-0077-2. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31(1):59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, Carmean V, Levine S. Variable cortisol circadian rhythms in children with autism and anticipatory stress. J Psychiatry Neurosci. 2008;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Levine S, Mendoza S. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Res. 2009;2(1):39–49. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Simon D, Ryan N, Mendoza S. Elevated cortisol during play is associated with age and social engagement in children with autism. Mol Autism. 2010;1(1):13. doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33(2):227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Gillott A, Furniss F, Walter A. Anxiety in high-functioning children with autism. Autism. 2001;5(3):277–286. doi: 10.1177/1362361301005003005. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U. Brief report: cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. J Autism Dev Disord. 2004;34(2):229–235. doi: 10.1023/b:jadd.0000022613.41399.14. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proc Biol Sci. 2001;268(1479):1883–1888. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Jansen LM, Gispen-de Wied CC, van der Gaag RJ, van Engeland H. Differentiation between autism and multiple complex developmental disorder in response to psychosocial stress. Neuropsychopharmacology. 2003;28(3):582–590. doi: 10.1038/sj.npp.1300046. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress. 2008;11(1):1–14. doi: 10.1080/10253890701365527. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(5):889–894. doi: 10.1016/j.pnpbp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen JK. Skin conductance responses to another person's gaze in children with autism. J Autism Dev Disord. 2006;36(4):517–525. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Lester BM, Lagasse LL, Shankaran S, Bada HS, Bauer CR, Lin R, Higgins R. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. J Pediatr. 2010;157(2):288–295. doi: 10.1016/j.jpeds.2010.02.039. e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata C, Thomeer ML, Volker MA, Nida RE, Lee GK. Effectiveness of a manualized summer social treatment program for high-functioning children with autism spectrum disorders. J Autism Dev Disord. 2008;38(5):890–904. doi: 10.1007/s10803-007-0460-7. [DOI] [PubMed] [Google Scholar]
- Lopata C, Volker MA, Putnam SK, Thomeer ML, Nida RE. Effect of Social Familiarity on Salivary Cortisol and Self-Reports of Social Anxiety and Stress in Children with High Functioning Autism Spectrum Disorders. J Autism Dev Disord. 2008 doi: 10.1007/s10803-008-0575-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Losh M, Capps L. Understanding of emotional experience in autism: insights from the personal accounts of high-functioning children with autism. Dev Psychol. 2006;42(5):809–818. doi: 10.1037/0012-1649.42.5.809. [DOI] [PubMed] [Google Scholar]
- Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain Dev. 2005;27(7):509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Palkovitz RJ, Wiesenfeld AR. Differential autonomic responses of autistic and normal children. J Autism Dev Disord. 1980;10(3):347–360. doi: 10.1007/BF02408294. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Rutter M, Lecouteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Szatmari P, MacLean JE, Jones MB, Bryson SE, Zwaigenbaum L, Bartolucci G, Tuff L. The familial aggregation of the lesser variant in biological and nonbiological relatives of PDD probands: a family history study. J Child Psychol Psychiatry. 2000;41(5):579–586. doi: 10.1111/1469-7610.00644. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kamio Y. Paradoxical autonomic response to mental tasks in autism. J Autism Dev Disord. 2003;33(4):417–426. doi: 10.1023/a:1025062812374. [DOI] [PubMed] [Google Scholar]
- van Engeland H. The electrodermal orienting response to auditive stimuli in autistic children, normal children, mentally retarded children, and child psychiatric patients. J Autism Dev Disord. 1984;14(3):261–279. doi: 10.1007/BF02409578. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, Tx: The Psychological Corporation; 1999. [Google Scholar]