Abstract
One of the key features of cardiovascular complications, such as hypertension or diabetes, is that they often appear at the same time in the same individual together with other forms of co-morbidities. While clinically a recognized phenomenon, no molecular mechanism for such co-morbidities has received universal acceptance. We propose a new hypothesis that provides a molecular basis for co-morbidities in hypertension due to unchecked proteolytic activity and receptor destruction. Testing of the hypothesis in the spontaneously hypertensive rat reveals an unchecked matrix metalloproteinase and serine protease activity in plasma and on several cardiovascular and parenchymal cells. The elevated proteolytic activity causes extracellular cleavage of multiple receptor types, such that cleavage of one receptor type leads to loss of the function carried out by this receptor. Proteolytic cleavage of the extracellular domain of the β2 adrenergic receptor in arteries and arterioles causes vasoconstriction and elevation of the central blood pressure while cleavage of the extracellular domain of the insulin receptor leads to insulin resistance and lack of transmembrane glucose transport. A diverse set of cell dysfunctions in the spontaneously hypertensive rat are accompanied by cleavage of the membrane receptors that are involved in these functions. Chronic inhibition of the unchecked protease activity in the spontaneously hypertensive rat serves to restore the extracellular receptor density and alleviates the corresponding cell dysfunctions. The mild unchecked proteolytic activity in the spontaneously hypertensive rat points towards a chronic autodigestion process as a contributor to the end organ injury encountered in this rat strain. The presence of various soluble receptors, which consist of extracellular fragments of membrane receptors, in the plasma of hypertensive and diabetic patients suggest that the autodigestion process may also be present in man.
Keywords: Metabolic Syndrome X, protease activity, hypertension, insulin resistance, capillary rarefaction
1. Introduction
One of the key characteristics of the metabolic syndrome X is the simultaneous presence of multiple co-morbidities. Besides obesity and an elevated level of triglycerides, there is an elevated arterial blood pressure, an increased blood glucose level with signs of insulin resistance (1,2) and a clustering of microvascular dysfunctions (3). These diverse dysfunctions may appear in the same individual to different degrees and involve different receptors and molecular pathways. In current management protocols, conditions like elevated blood pressure in hypertension or elevated glucose levels in diabetes are often subject to different types of drug treatments, each individually targeted towards one symptom at a time. The consequence is that patients may need to take a multitude of medications. This situation may be contrasted with interventions against the metabolic syndrome X, such as calorie restriction, which serves to interfere at the same time with both hypertension and diabetes (4).
To date no hypothesis has been advanced that places the multifaceted dysfunctions encountered in the metabolic syndrome X under one conceptual roof. Development of such an overarching concept is potentially of major importance for more effective treatments of patients and it is the subject of this review.
We propose here a common mechanism that leads at the same time to the symptoms associated with diabetes and with hypertension and their corresponding but different cell dysfunctions. Accordingly we will advance a new hypothesis for the origin of these dysfunctions that we have tested in a genetic model, the spontaneously hypertensive rat (SHR) (5).
2. The Spontaneously Hypertensive Rat – Is Elevated Blood Pressure Really its Problem?
While originally developed for the spontaneous elevation of its arterial blood pressure (6), a large body of evidence shows that the SHR has many other defects besides an elevated arterial blood pressure, including type II diabetes with insulin resistance (7–10), insomnia (11–14), loss of microvessels (capillary rarefaction) (15–18), signs of immune suppression (19–24) with impaired leukocyte-endothelial interaction (25–27) and a chronically elevated leukocyte count compared to their control strains (28). The SHR’s vascular cells exhibit a reduce response to fluid shear stress (29–31), to name just a few cell and tissue dysfunctions (32). It appears the elevated blood pressure is not its major problem; other issues may be more relevant for its cell dysfunctions. The SHR also exhibits greatly attenuated responses to circulatory challenges, e.g. ischemia-reperfusion (33) but in an apparent dichotomy exhibits an attenuated response to acute inflammatory stimuli (34).
3. Oxygen Free Radical Formation
One form of cell injury that has received in the past extraordinary attention is oxygen free radical production. Indeed, the SHR exhibits an elevated level of superoxide at all vascular levels (35–39). Various interventions against oxygen and nitrogen radicals have been tested (40,41). In spite of many innovative approaches, clinical trials investigating the cardiovascular benefits of antioxidants have yielded few robust results to support a primary role of oxygen free radicals in the cell dysfunction associated with hypertension or its co-morbidities (42). No molecular mechanism has been advanced to explain the diverse cellular dysfunctions, such as the combination of hypertension, insulin resistance, capillary rarefaction, etc., in the SHR.
We need another mechanism to explain these diverse cellular dysfunctions. This issue is the focus of the following discussion.
4. Unchecked Protease Activity in the SHR
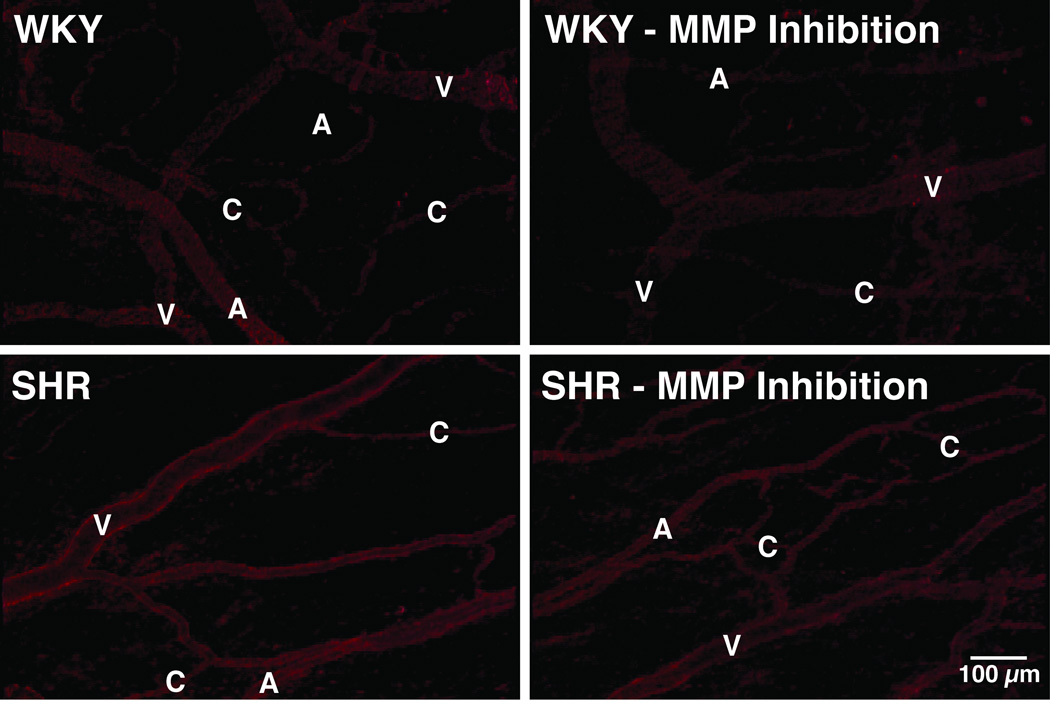
The SHR exhibits an enhanced protease activity that is detectable with fluorescently quenched substrates applied either in-vitro or in-vivo with microzymographic techniques (Figure 1) (43).
Figure 1.
Digital fluorescent micrographs of in-vivo protease activity as detected with microzymography using a quenched fluorogenic peptide substrate for MMP (MMP-1 and -9) activity in mesenteric microcirculation of WKY and SHR before and after chronic MMP inhibition (with doxycycline) (43). Note the enhanced fluorescent emission over the endothelial cells in the SHR arterioles (A), capillaries (C), and venules (V).
One family of proteases of special interest in this context is the metalloproteinases. While described originally for their ability to cleave extracellular proteins, e.g. collagen (by MMP-1, -8, -13) and elastin (by MMP-12), by a common catalytic Zn++ domain, in recent time an expanding body of evidence suggest that they may also have other substrates and participate in pathophysiological mechanisms for which hypertension and diabetes are a risk factor, e.g. atherosclerosis, stroke, heart and kidney failure (44,45).
The plasma protease activity is detectable in venous plasma derived from serine proteases as well as matrix metalloproteinases (43). The activity involves the gelatinases MMP-2 and MMP-9 as well as the matrilysin MMP-7 in plasma, while MM-1, MMP-3, MMP-8 and MMP-13 activities were not found to be significantly elevated in plasma. MMP-14 activity (a membrane-type MMP) was significantly reduced in the SHR (46). The elevations of plasma MMP-2, 7-and -9 activity are relatively modest and in the range between 10 and 25%. But the fact that they are present at all is notable in light of the ability of proteases to be blocked by endogenous protease inhibitors. In young adult SHR the protease activity is present mostly in venous plasma; arterial plasma exhibits in some experiments less or no elevation of protease activity in the SHR and is influenced by calorie consumption (47). It is interesting to note that the low-pressure control to the SHR, the Wistar-Kyoto (WKY) strain, also tends to exhibit elevated protease activities compared to the normotensive Wistar strain from which the SHR and WKY rats were bred (46).
In addition to the plasma protease activity, we also find a significantly elevated MMP activity on the endothelium along mesenteric microvessels of the SHR, which includes MMP-1, MMP-1/-9, MMP-7, and MMP-8 activities, but less MMP-2 and MMP-3 activities, compared to its control strains (43,48). In the mesentery the enzyme activity is present in arterioles and venules as well as in tissue mast cells. The protease activity is especially prominent in venules even though these vessels are not exposed to an elevated blood pressure. The elevated protease level in SHR endothelial cells involves an enhanced MMP-9 expression level (detected with immunohistochemistry). The MMP-9 protein levels and activity are also elevated in SHR venules as well as on its circulating leukocytes, which pass periodically through the circulation from high to low blood pressure regions in the vasculature (43). Thus, in the microcirculation there is no close association between elevated blood pressure and elevated MMP activity.
Furthermore direct microzymographic evidence suggests that MMPs are present in multiple organs of the SHR and can be further induced by ischemia. The renal cortex and medulla exhibit elevated levels of MMP-2 and -9 even at young age (2 and 6 weeks) together with an enhanced level of tissue inhibitor of MMPs (TIMP-4) (49). Salt supplementation enhances MMP-2 activity in the kidney of stroke-prone SHR (50).
MMP-2, -9 and -13 are elevated in the heart of the spontaneously hypertensive heart failure (SHHF) rat and is associated with increased TIMP-1, -2 but decreased TIMP-4 protein levels (51). The cardiac muscle of the SHR or the SHHF also has elevated MMP-2 activity (52,53). MMP-1 and TIMP-1 mRNA measurements indicate an age dependency (54). The elevated MMP activity in the heart is not seen at a statistically significant level in all studies (55) but is age-dependent (56). In the brain astrocytes of the SHR, MMP-2 is present and during ischemia MMP-9 and MMP-3 are produced as well (57–61).
Besides an elevated MMP activity, the SHR also has elevated levels of protease activity derived from the granules of its circulating leukocytes. In its chronically elevated pool of circulating leukocytes (28), a high proportion of granulocytes spontaneously degranulate (62) and have elevated elastase activity on their membrane (63).
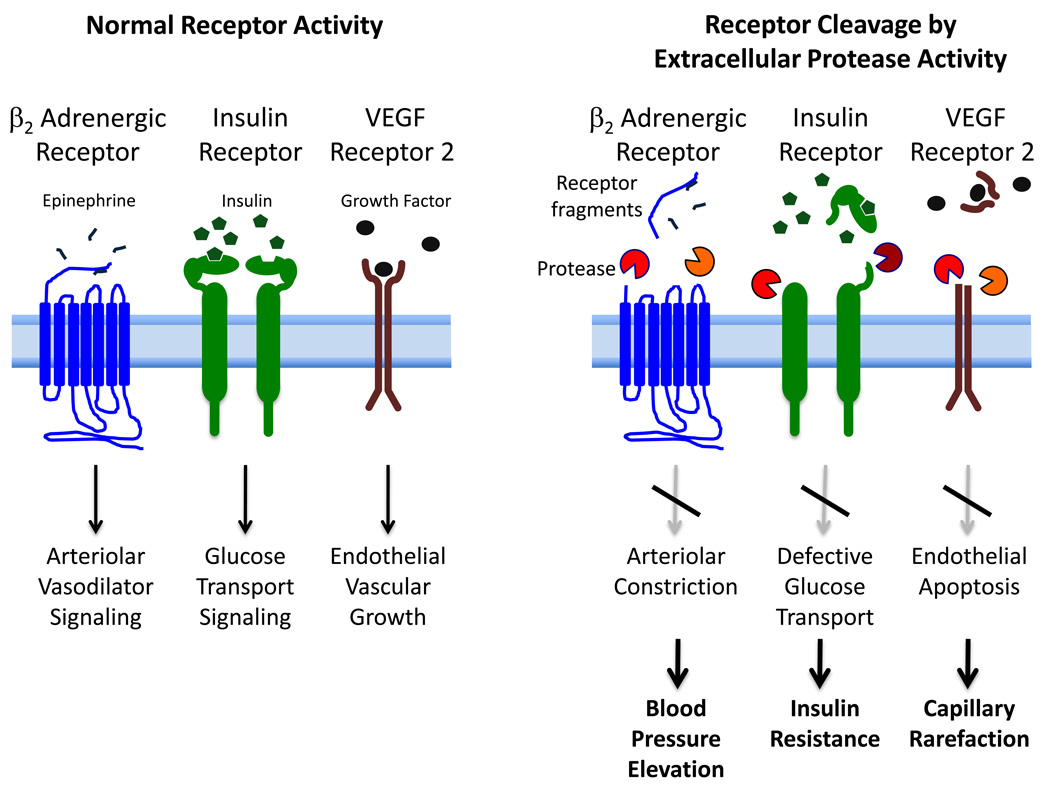
5. Receptor Cleavage in the SHR
In addition to enzymatic cleavage of fluorescently quenched substrates, the immediate question arises whether the unchecked protease activity in the SHR may cause destruction of important biomolecules, i.e. an autodigestion process. To examine this possibility we recently embarked on an analysis of several important types of receptors that carry out key cellular activities and whose function may be compromised in the SHR due to enzymatic destruction.
5.1. β2 Adrenergic Receptor
The SHR has an elevated constriction of its arterioles, which leads to elevation of its characteristic arterial blood pressure and for which this strain received its name (64,65). Among multiple possibilities that may cause the elevation of the tone, use of agonists and antagonists to the β2 adrenergic receptor point towards a defective β2 receptor signaling in the SHR, e.g. a reduced smooth arteriolar vasodilation in the presence of epinephrine (66). Direct infusions of MMP-7, MMP-9 or a combination of both into microvessels leads to constriction of venules or arterioles, which is blocked by a MMP inhibitor. Immunolabeling of the receptor with an antibody against the extracellular domain (N-terminus) and as control against the intracellular domain (C-terminus) shows that a part of the β2 adrenergic receptor population is cleaved on SHR endothelium. The density of the extracellular receptor label is significantly lower in the aorta and in cardiac microvessels of the SHR compared to the WKY rats. Treatment of the aorta and the heart muscle of control Wistar rats with plasma from SHRs, but not plasma from WKY rats, reduced the number of extracellular domains, but not intracellular domains, of the β2 adrenergic receptor. The ability of the SHR plasma to cleave the receptor can be blocked by MMP inhibitors (66). As the SHR is chronically treated with a broad acting MMP inhibitor (doxycycline), its plasma and endothelial MMP activity is reduced to levels of the normotensive WKY rats and its elevated blood pressure is reduced to the level of the WKY rat (43).
5.2. Insulin Receptor
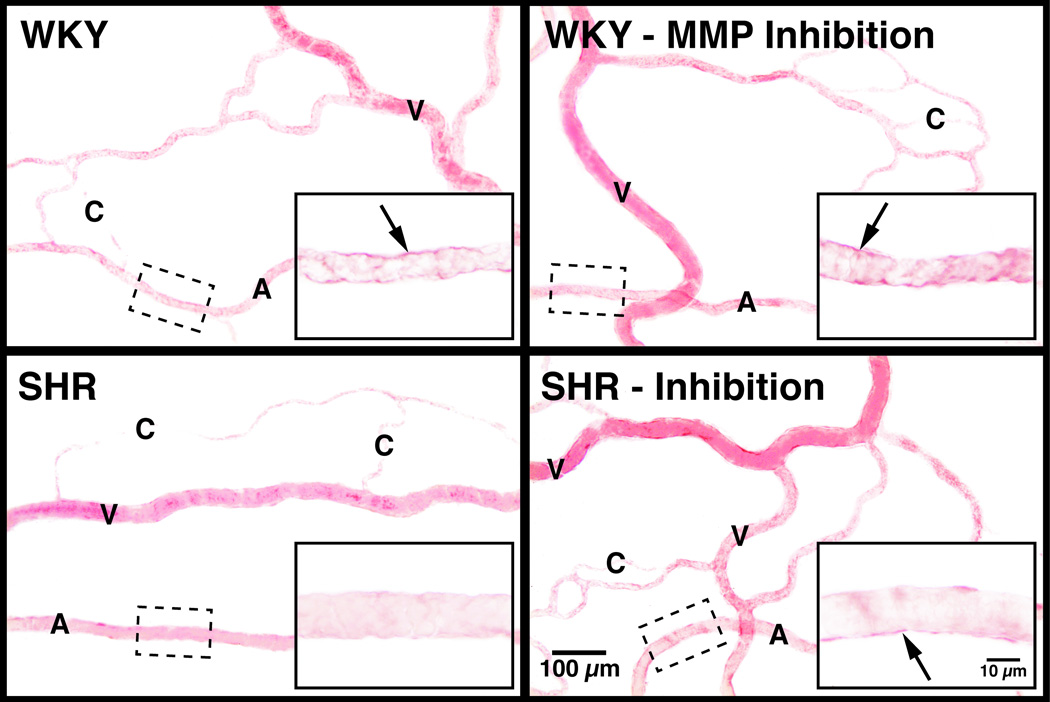
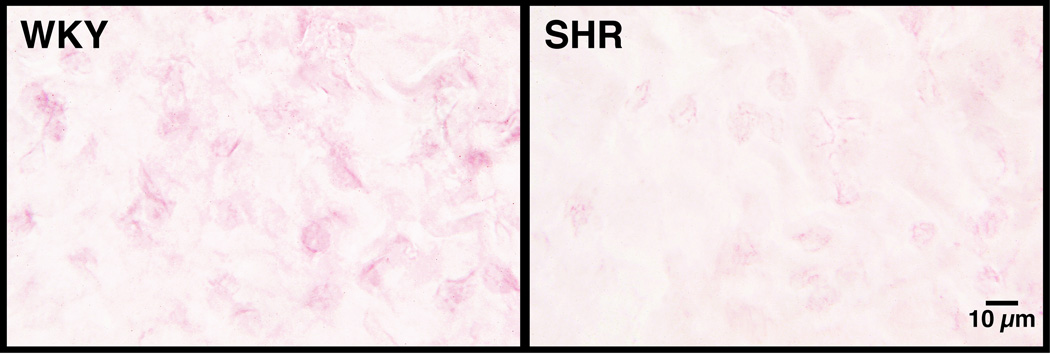
Insulin resistance in the SHR is accompanied by a reduced density of the extracellular domain of the insulin receptor on various cells, including leukocytes and mesentery interstitial cells (43) (Figure 2). If naïve cells of the Wistar rat, the strain from which the SHR is derived, is incubated with venous plasma of the SHR, the extracellular domain of the insulin receptor is cleaved and consequently the transport of glucose into the cell is impaired, a key marker for insulin resistance. Furthermore, if the SHR is treated chronically with an MMP inhibitor, the density of the extracellular insulin receptor domains is restored to control values and the plasma of such treated SHRs no longer has the ability to cleave the receptor on control cells. The elevated plasma glucose levels in the SHR are reduced after chronic MMP inhibition, which is in line with the improved glucose transport due to restoration of the membrane insulin receptor density (43).
Figure 2.
(A) Insulin receptor immunolabel density in mesentery microvessels of WKY and SHR before and after chronic MMP inhibition with doxycycline as detected by immunohistochemistry using an antibody against the extracellular domain of the receptor with VECTOR NovaRED substrates (for details see Reference (43)). Note the dark label for the insulin receptor (as compared to the lighter hemoglobin) on the endothelium, which is attenuated in the SHR and restored after chronic MMP inhibition. (B) Extracellular insulin receptor label density in the interstitial space of the WKY and SHR mesentery recorded at higher image resolution and lower image contrast. Note that the level of receptor labeling is lower in the mesentery interstitium and was not recorded in Panel A at higher contrast.
5.3. β2 Integrin Receptor
The chronically enhanced circulating leukocyte count of the SHR (28) is associated with reduced density of P-selectin on postcapillary venules and sialyl Lewis X-like carbohydrates on circulating neutrophils and a reduced tendency of leukocytes to roll on postcapillary venules (25). In addition, SHR neutrophils have a lower density of β2 integrins on their membrane (26). SHR venous plasma also cleaves the extracellular domain of the β2 integrins, an effect that is also observed to a lower degree with plasma of the WKY strain but is negligible with plasma of the Wistar strain. In contrast, the venous plasma of SHRs treated chronically with an MMP inhibitor has reduced ability to cleave the outer membrane domain of the β2 adhesion receptor (43).
5.4. ICAM
The counter receptor to β2 integrins on the endothelium, ICAM-1, is predominantly expressed on venules and is also affected by proteolytic enzyme cleavage on the endothelium of the SHR. But with this receptor, we observed a reversed pattern when compared with the control Wistar strain. The extracellular domain of ICAM-1 has a higher labeling density in SHR liver sinusoids, central veins, renal glomeruli, and the glomerular capsules. Comparison with the intracellular label density of ICAM-1 suggests that the higher label density of the extracellular domain in liver sinusoids and the renal glomeruli may be due to accumulation of extracellular fragments of ICAM-1 from the systemic circulation. In the SHR plasma, the density of soluble ICAM-1 was lower than in Wistar plasma, a feature that may be associated in part with continued proteolytic cleavage of soluble fragments when suspended in plasma. Thus the SHR may in fact filter extracellular receptor fragments in the renal glomeruli (67).
5.5. Vascular Endothelial Growth Factor Receptor 2 (VEGFR2)
The SHR exhibits enhanced apoptosis in its entire vasculature, including arterioles, venules and the capillary network, an effect that leads to actual loss of capillaries (rarefaction) (68,69). In addition the SHR has attenuated angiogenesis, which is associated with impaired VEGFR2 signaling (70). Labeling of the SHR extracellular, but not the intracellular, domain of the VEGFR2 shows a reduced density in cardiac microvessels (arterioles and capillaries) and on the aortic endothelium (46). SHR plasma has the ability to cleave the extracellular domain of VEGFR2 on naive control cells, a process that is also caused by purified forms of MMP-7 and MMP-9. Chronic inhibition of MMP activity with doxycycline in the SHR abolishes the ability of its plasma to cleave the receptor, an effect that is not observed to the same degree in the WKY plasma. The chronic abolishment of MMP activity in the SHR also serves to reduce significantly its endothelial apoptosis and at the same time increases the capillary density in cardiac and skeletal muscles as well as in the mesentery (46).
5.6. The Formyl-Peptide Receptor (FPR)
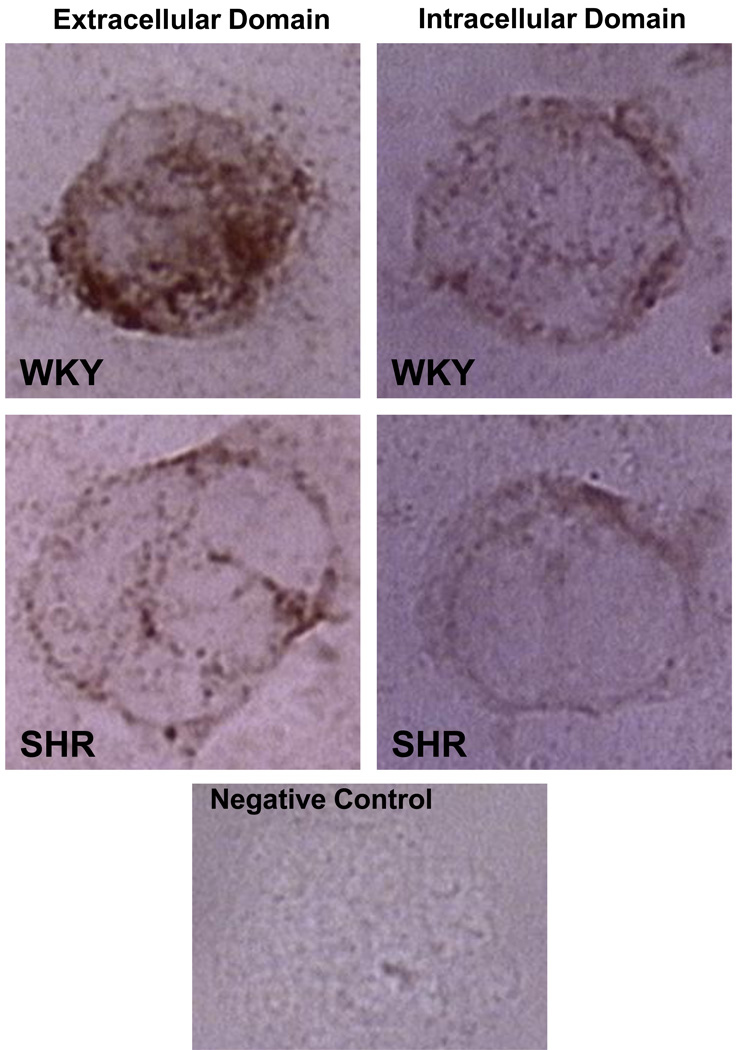
The FPR plays a central role in the fluid shear stress response of circulating leukocytes as a mechanosensor (71). Leukocytes retract their pseudopods when subject to fluid shear stress at magnitudes typical for the circulation. The pseudopod retraction is due to depolymerization of the actin inside pseudopods, a process controlled by the FPR. This form of shear stress response in leukocytes is impaired in cells derived from SHR and also in naïve control leukocytes exposed to SHR plasma for 20 minutes (72). The impaired fluid shear stress response in the SHR causes a situation that leads to increased tendency for pseudopod formation by actin-polymerization and reduced ability to reduce pseudopods when exposed to fluid shear stress (31). The impairment is accompanied by a reduced density of the extracellular domain of the FPR on SHR neutrophils (72). Exposure of control neutrophils to SHR plasma or just to MMP-9 leads to reduction of the extracellular but not intracellular domain of the FPR, i.e. cleavage of the receptor (Figure 3), and a reduced shear stress response as well as a reduced fMLP response. Chronic inhibition of MMPs in the SHR serves to restore the receptor density and the fluid shear stress response (72).
Figure 3.
Selected micrographs of the formyl peptide receptor (FPR) density on typical WKY and SHR leukocytes after immunolabeling with an antibody against its extracellular (left images) and intracellular domain (right images). The images are representative for the average receptor density as measured previously (74). Bottom image shows control label density without primary antibody. Note the reduced extracellular labeling density on SHR leukocytes as compared to the normotensive WKY, a trend that is not present when an antibody against the intracellular domain is used, suggesting FPR cleavage in the SHR.
Receptor cleavage may also be involved in the anomalous behavior of blood cells after traditional centrifugation used in a standard fashion to separate different blood cell components. Depending on the time and magnitude of centrifugation of whole blood, the process leads to a reduction and even complete loss of the fluid shear stress response in leukocytes (73). Measurement of the extra- and intracellular domain densities of the FPR before and after centrifugation shows cleavage of the extracellular domain, possibly due to neutrophil degranulation and release of proteolytic activity at the membrane (72). Unless the endocytosis of degrading protease from cytoplasmic granules and other cytoplasmic compartments is prevented or the degrading protease activity is blocked, it is possible that membrane receptors other than the FPR are cleaved as well after standard centrifugation protocols.
5.7. Endothelial Tight Junction Proteins
Degradation of the tight junction forming proteins (occludin and claudin-5) and opening of the blood-brain barrier in SHR cerebral vessels during a 90-minute ischemic period followed by reperfusion may be due to MMPs (74). The degradation of the tight junction proteins correlates with an increase in microvascular permeability and involves MMP-2, MMP-9, and the activator of MMP-2, MT-1 MMP, as well as its activator, the serine endopeptidases paired basic amino acid cleaving enzyme (furin). The activation under these ischemic conditions can be prevented with an MMP inhibitor. Both claudin-5 and occludin fragments can be detected in brain tissue with appearance of lower molecular weight fragments. Activation of MMPs in acute focal ischemia is observed in species, other than just the SHR, and involves degradation of proteins in the extracellular space with a direct effect on the function of the neuropil (75). The impact of the acute MMP activation after ischemia remains largely unexplored.
5.8. Hypothalamic Receptor Cleavage and Reduced Sleep Quality - Insomnia
The SHR has a reduced sleep quality with a reduced quiet sleep period (76). We obtained initial evidence that the antibody label density against the extracellular domain of the 5HT-1A receptor is lower in the SHR hypothalamic region compared to its normotensive control (77).
5.9. Regulation of MMP Expression in SHR
The SHR has an enhanced level of nuclear factor κB (NF-κB) expression levels. The overexpression is detectable with immunohistochemistry in arterioles and in parenchymal cells of kidney and brain hypothalamus, but not myocardium and cerebral cortex (78). The overexpression is also present in circulating leukocytes and thus not limited to vessels of the circulation with high blood pressure (43). A chronic 10-week inhibition of NF-κB (with pyrrolidine dithiocarbamate (PDTC) in the SHR reduces NF-κB and MMP-2 and MMP-9 activity in plasma, brain and kidney. This serves to normalize the elevated blood pressure. The level of the extracellular, but not intracellular domain density of the β2 adrenergic receptor is reduced in the kidney, and brain cortex, and hypothalamus of the SHR, revealing a receptor cleavage process that can be blocked by chronic PDTC treatment (78). The possible inhibition of membrane receptor cleavage in the SHR by NF-κB blockade and attenuation of MMP activity remain to be explored.
5.10. Evidence for Receptor Cleavage in Human Hypertensives
Enhanced MMP levels have been observed in human hypertensive, especially when subject to renal failure (79). The question arises whether there exists any evidence in human essential hypertension that proteolytic receptor cleavage mechanisms may be present. The coincidence of multiple cell and organ dysfunctions in hypertensives, other than just blood pressure elevation, may be in line with this possibility.
A case in point is the evidence for soluble receptors in plasma of diabetics. A number of receptor fragments have been detected and designated as “soluble receptors” although the basis of such solubility is uncertain. Examples include a soluble insulin receptor (80), soluble VEGFR2 (81–84), soluble angiopoietin receptor tie-2 (85), soluble IL-6 receptor (86), soluble receptor for advanced glycation end products (RAGE) (87,88) and soluble selectins (89). Several of the soluble receptors are in a fragmented form including the extracellular domains, which is expected after cleavage by an extracellular protease. Soluble receptors are present in general at low concentrations, so that it is difficult to assign a major physiological control function to such fragments, and instead they may have to be regarded as the consequence of receptor cleavage on cell surfaces that interfere with important cell functions.
Patients with essential hypertension have been shown to have elevated levels of soluble VEGFR2 (90), soluble junctional adhesion molecule A (JAM-A) (91), and soluble RAGE (92). The plasma of hypertensive patients contains soluble leukocyte adhesion molecules (P-selectin, E-selectin, ICAM-1, VCAM-1) (89,93–100). The soluble P-selectin receptor serves as a risk predictor for myocardial (but less for cerebral) infarction in hypertensive with no prior cardiovascular events (101).
Soluble VEGFR1 has been detected in pregnancy-induced hypertension (preeclampsia) with a tendency for increased levels of soluble receptor concentrations with increasing blood pressure (102,103). It is notable that some studies also found significantly reduced soluble receptors in hypertensives (104). The evidence, however, has to be examined in light of the relatively low concentrations of these receptor fragments (picograms/ml) in plasma or serum and the technical limitations to detect cleaved protein fragments. Soluble receptor fragments may be subject to further enzymatic degradation in plasma so that traditional antibody based techniques, e.g. ELISA, and even mass spectrometric techniques, may be undermined.
Finally, it is interesting to note in this context that the accumulation of soluble VEGFR2 can be reduced with ACE inhibitors (105), protease inhibitors that not only attenuate the formation of angiotensin but also inhibit MMP activity (106–109).
5.11. Summary
We propose here for the first time a mechanism for the multifaceted and diverse cell and organ dysfunction that frequently accompany hypertension. In the SHR model of hypertension we find a relatively low level and possibly heterogeneous but uncontrolled degrading protease activity, derived in part from serine proteases and MMPs. This uncontrolled protease activity is detectable with in-vivo microzymography on SHR endothelial cells and parenchymal cells and in plasma. We regard this as a mild autodigestion process in which multiple surface receptor types are enzymatically cleaved. Consequently diverse cell functions facilitated by such cleaved receptors become compromised (Table 1). For example, cleavage of the β2 adrenergic receptor on arterioles leads to arteriolar constriction and as a result contributes to elevated central blood pressure; cleavage of the extracellular domain of the insulin receptor leads to insulin resistance; cleavage of VEGFR2 leads to endothelial apoptosis which in the microcirculation causes capillary rarefaction; cleavage of leukocyte adhesion receptors contributes to immune suppression and at the same time to a reduced leukocyte adhesion upon; cleavage of the FPR on leukocytes attenuates mechanotransduction triggered by fluid shear stress. Chronic treatment with an MMP inhibitor serves to attenuate cleavage of multiple receptor types and restore their function via a single treatment that targets the protease activity. There is indirect evidence in patients for receptor cleavage in the form of soluble receptor fragments in plasma. But proteolytic destruction of extracellular domains of membrane receptors as basis for multiple cell and organ dysfunction in patients remains to be examined.
Table I.
Extracellular Domain Receptor Cleavage and Cell Dysfunctions in the SHR
| Receptor Type | Cell - Organ Dysfunction |
|---|---|
| β2 Adrenergic Receptor | Arteriolar vasoconstriction and central blood pressure elevation – hypertension; (66) |
| Insulin Receptor -α | Insulin Resistance with reduced glucose transport - Type 2 diabetes mellitus; (43) |
| Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) | Endothelial apoptosis and elevated endothelial permeability - Capillary rarefaction; (46,48) |
| Formyl Peptide Receptor (FPR) | Reduced response to formyl peptide and fluid shear stress on leukocytes – Suppression of fluid shear response and elevated capillary hemodynamic resistance; (72) |
| β2 Integrin Adhesion Molecule (CD18) | Attenuated leukocyte-endothelial interaction - Immune suppression and also resistance to inflammatory stimuli (43) |
| Tight Junction Proteins: Occludin and Claudin - 5 | Increased endothelial permeability; (74) |
| Inter-Cellular Adhesion Molecule 1 (ICAM-1, CD54) | Proteolytic cleavage of the extracellular domain of ICAM-1 and accumulation in kidney glomeruli; (63) |
| Serotonin 5HT-1A receptor | Reduced sleep quality – Insomnia; (77) |
5.12. Clinical Perspective
One of the implications of the current hypothesis is that there may be an opportunity to uncover in the future a more unified approach to the cluster of co-morbidities that accompany the metabolic syndrome X. Instead of treating one symptom at a time with a multitude of medications there may be an opportunity to reduce the number of medications by protease inhibition. Whatever approach is taken in the future, it has to be nuanced since proteases play an essential role in many physiological functions and inflammatory repair processes. Understanding the genetic and/or environmental cause of the uncontrolled protease activity will open the door to prevention.
Figure 4.
Schematic diagram of extracellular proteolytic enzyme activity with cleavage of the extracellular domain of membrane receptors resulting in cleaved extracellular receptor fragments (“soluble receptors”), lack of receptor signaling and consequently to cell, vascular and organ dysfunctions typical for the SHR. The diagram shows the case for three selected receptors; other receptors are also subject to proteolytic destruction. A similar proteolytic cleavage may affect the extracellular domain of other membrane receptors and consequently generate multiple cell, microvascular and organ dysfunctions encountered in the SHR.
Acknowledgment
The research summarized in this report was supported by NIH Grant HL 10881 and in part by a gift from Leading Ventures, San Diego California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Schmid-Schönbein is a scientific advisor to Leading Ventures.
References
- 1.Rizzo M, et al. The therapeutic modulation of atherogenic dyslipidemia and inflammatory markers in the metabolic syndrome: what is the clinical relevance? Acta Diabetol. 2009;46:1–11. doi: 10.1007/s00592-008-0057-4. [DOI] [PubMed] [Google Scholar]
- 2.Mule G, et al. Metabolic syndrome in subjects with essential hypertension: relationships with subclinical cardiovascular and renal damage. Minerva Cardioangiol. 2006;54:173–194. [PubMed] [Google Scholar]
- 3.Serne EH, et al. Microvascular dysfunction: causative role in the association between hypertension, insulin resistance and the metabolic syndrome? Essays Biochem. 2006;42:163–176. doi: 10.1042/bse0420163. [DOI] [PubMed] [Google Scholar]
- 4.Fontana L. Calorie restriction and cardiometabolic health. Eur J Cardiovasc Prev Rehabil. 2008;15:3–9. doi: 10.1097/HJR.0b013e3282f17bd4. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto K. Spontaneous hypertension in rats. Int. Rev. Exp. Pathol. 1969;7:227–270. [PubMed] [Google Scholar]
- 6.DeLano FA, Zweifach BW. Anesthesia and microvascular dynamics in spontaneously hypertensive rats. Am. J. Physiol. 1981;241:H821–H828. doi: 10.1152/ajpheart.1981.241.6.H821. [DOI] [PubMed] [Google Scholar]
- 7.Bursztyn M, et al. Insulin resistance in spontaneously hypertensive rats but not in deoxycorticosterone-salt or renal vascular hypertension. Journal of Hypertension. 1992;10:137–142. doi: 10.1097/00004872-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Melancon S, et al. Effects of high-sucrose feeding on insulin resistance and hemodynamic responses to insulin in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2006;290:H2571–H2581. doi: 10.1152/ajpheart.01002.2005. [DOI] [PubMed] [Google Scholar]
- 9.Hulman S, et al. Insulin resistance in the conscious spontaneously hypertensive rat: euglycemic hyperinsulinemic clamp study. Metabolism: Clinical and Experimental. 1993;42:14–18. doi: 10.1016/0026-0495(93)90165-k. [DOI] [PubMed] [Google Scholar]
- 10.Zecchin HG, et al. Insulin signalling pathways in aorta and muscle from two animal models of insulin resistance--the obese middle-aged and the spontaneously hypertensive rats. Diabetologia. 2003;46:479–491. doi: 10.1007/s00125-003-1073-0. [DOI] [PubMed] [Google Scholar]
- 11.Kuo TBJ, et al. Sleep-related sympathovagal imbalance in SHR. Am J Physiol Heart Circ Physiol. 2004;286:H1170–H1176. doi: 10.1152/ajpheart.00418.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kuo TBJ, et al. Changes in sleep patterns in spontaneously hypertensive rats. Sleep. 2004;27:406–412. doi: 10.1093/sleep/27.3.406. [DOI] [PubMed] [Google Scholar]
- 13.Kuo TBJ, Yang CC. Sleep-related changes in cardiovascular neural regulation in spontaneously hypertensive rats. Circulation. 2005;112:849–854. doi: 10.1161/CIRCULATIONAHA.104.503920. [DOI] [PubMed] [Google Scholar]
- 14.Legramante JM, Galante A. Sleep and hypertension: a challenge for the autonomic regulation of the cardiovascular system. Circulation. 2005;112:786–788. doi: 10.1161/CIRCULATIONAHA.105.555714. [DOI] [PubMed] [Google Scholar]
- 15.Chen IIH, et al. Microvascular rarefaction in spontaneously hypertensive rat cremaster muscle. Am. J. Physiol. 1981;241:H306–H310. doi: 10.1152/ajpheart.1981.241.3.H306. [DOI] [PubMed] [Google Scholar]
- 16.Hutchins PM, Darnell AE. Observations of a decreased number of small arterioles in spontaneously hypertensive rats. Circ. Res. 1974;34–35:161–165. [Google Scholar]
- 17.Prewitt RL, et al. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am. J. Physiol. 1982;243:H243–H251. doi: 10.1152/ajpheart.1982.243.2.H243. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi N, et al. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2005;25:2114–2121. doi: 10.1161/01.ATV.0000178993.13222.f2. [DOI] [PubMed] [Google Scholar]
- 19.Suematsu M, et al. The inflammatory aspect of the microcirculation in hypertension: oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation. 2002;9:259–276. doi: 10.1038/sj.mn.7800141. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, et al. Enhanced DNA fragmentation in the thymus of spontaneously hypertensive rats. Am J Physiol. 1999;276:H2135–H2140. doi: 10.1152/ajpheart.1999.276.6.H2135. [DOI] [PubMed] [Google Scholar]
- 21.Pascual DW, et al. Spontaneously hypertensive rat: cholera toxin converts suppression to immunity through a Th2 cell-IL-4 pathway. Am J Physiol. 1997;273:R1509–R1518. doi: 10.1152/ajpregu.1997.273.4.R1509. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka T. Age-related changes of immune reactivities and antitumor effects in spontaneously hypertensive rats (SHR) with T-cell depression. Hokkaido Igaku Zasshi. 1989;64:551–557. [PubMed] [Google Scholar]
- 23.Takeichi N, et al. Immunologic suppression of carcinogenesis in spontaneously hypertensive rats (SHR) with T cell depression. J Immunol. 1983;130:501–505. [PubMed] [Google Scholar]
- 24.Khraibi AA, et al. Thymectomy delays the development of hypertension in Okamoto spontaneously hypertensive rats. J Hypertens. 1987;5:537–541. doi: 10.1097/00004872-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Suematsu M, et al. Impairment of selectin-mediated leukocyte adhesion to venular endothelium in spontaneously hypertensive rats. J Clin Invest. 1995;96:2009–2016. doi: 10.1172/JCI118248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arndt H, et al. Leukocyte-endothelial cell adhesion in spontaneously hypertensive and normotensive rats. Hypertension. 1993;21:667–673. doi: 10.1161/01.hyp.21.5.667. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, et al. Impaired leukocyte-endothelial cell interaction in spontaneously hypertensive rats. Hypertension. 1994;24:719–727. doi: 10.1161/01.hyp.24.6.719. [DOI] [PubMed] [Google Scholar]
- 28.Schmid-Schönbein GW, et al. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension. 1991;17:323–330. doi: 10.1161/01.hyp.17.3.323. [DOI] [PubMed] [Google Scholar]
- 29.Pourageaud F, Freslon JL. Impaired endothelial relaxations induced by agonists and flow in spontaneously hypertensive rat compared to Wistar-Kyoto rat perfused coronary arteries. J Vasc Res. 1995;32:190–199. doi: 10.1159/000159093. [DOI] [PubMed] [Google Scholar]
- 30.Huang A, Koller A. Both nitric oxide and prostaglandin-mediated responses are impaired in skeletal muscle arterioles of hypertensive rats. J. Hypertens. 1996;14:887–895. doi: 10.1097/00004872-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda S, et al. Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ Res. 2004;95:100–108. doi: 10.1161/01.RES.0000133677.77465.38. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H, et al. The multifaceted contribution of microvascular abnormalities to the pathophysiology of the hypertensive syndrome. In: Zanchetti A, Mancia G, editors. Handbook of Hypertension Vol. 17, Pathopysiology of Hypertension. Amsterdam: Elsevier Science B.V.; 1997. pp. 482–523. [Google Scholar]
- 33.Liao L, et al. Oxidized low-density lipoproteins and microvascular responses to ischemia-reperfusion. Am J Physiol. 1996;271:H2508–H2514. doi: 10.1152/ajpheart.1996.271.6.H2508. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, et al. Protective role of adrenal glucocorticoids for gastric mucosa in spontaneously hypertensive rats. J Gastroenterol Hepatol. 1999;14:376–383. doi: 10.1046/j.1440-1746.1999.01861.x. [DOI] [PubMed] [Google Scholar]
- 35.DeLano FA, et al. Control of oxidative stress in microcirculation of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;288:H805–H812. doi: 10.1152/ajpheart.00696.2004. [DOI] [PubMed] [Google Scholar]
- 36.Newaz MA, et al. Modulation of nitric oxide synthase activity in brain, liver, and blood vessels of spontaneously hypertensive rats by ascorbic acid: protection from free radical injury. Clin Exp Hypertens. 2005;27:497–508. doi: 10.1081/CEH-200067681. [DOI] [PubMed] [Google Scholar]
- 37.Hibino M, et al. Oxygen-derived free radical-induced vasoconstriction by thromboxane A2 in aorta of the spontaneously hypertensive rat. Journal of Cardiovascular Pharmacology. 1999;33:605–610. doi: 10.1097/00005344-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki H, et al. Measurement of oxidative stress in stroke-prone spontaneously hypertensive rat brain using in vivo electron spin resonance spectroscopy. Redox Rep. 2002;7:260–265. doi: 10.1179/135100002125000758. [DOI] [PubMed] [Google Scholar]
- 39.Lee MC. Biomedical application of electron spin resonance (ESR) spectroscopy--assessment of antioxidant property for development of drugs. Yakugaku Zasshi. 2008;128:753–763. doi: 10.1248/yakushi.128.753. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi N, Schmid-Schönbein GW. Intrinsic disturbance of cellular redox balance enhances blood lymphocyte apoptosis in the spontaneously hypertensive rat. Free Radic Biol Med. 2006;41:484–492. doi: 10.1016/j.freeradbiomed.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Fennell JP, et al. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther. 2002;9:110–117. doi: 10.1038/sj.gt.3301633. [DOI] [PubMed] [Google Scholar]
- 42.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 43.DeLano FA, Schmid-Schönbein GW. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension. 2008;52:415–423. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 45.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 46.Tran ED, et al. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res. 2010;47:423–431. doi: 10.1159/000281582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan AHW. Master Thesis. Department of Bioengineering, University of California San Diego; 2010. Autodigestion in chronic hypertension: abnormal pancreatic enzyme section. [Google Scholar]
- 48.Tran ED, et al. Matrix metalloproteinase activity causes VEGFR-2 cleavage and microvascular rarefaction in rat mesentery. Microcirculation. 2011 doi: 10.1111/j.1549-8719.2011.00082.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camp TM, et al. Mechanism of matrix accumulation and glomerulosclerosis in spontaneously hypertensive rats. J Hypertens. 2003;21:1719–1727. doi: 10.1097/00004872-200309000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Tostes RC, et al. Endothelin A receptor blockade decreases expression of growth factors and collagen and improves matrix metalloproteinase-2 activity in kidneys from stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002;39:892–900. doi: 10.1097/00005344-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Li H, et al. MMP/TIMP expression in spontaneously hypertensive heart failure rats: the effect of ACE- and MMP-inhibition. Cardiovasc Res. 2000;46:298–306. doi: 10.1016/s0008-6363(00)00028-6. [DOI] [PubMed] [Google Scholar]
- 52.Peterson JT, et al. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001;103:2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 53.Mujumdar VS, et al. Activation of matrix metalloproteinase dilates and decreases cardiac tensile strength. Int J Cardiol. 2001;79:277–286. doi: 10.1016/s0167-5273(01)00449-1. [DOI] [PubMed] [Google Scholar]
- 54.Seccia TM, et al. Extracellular matrix gene expression in the left ventricular tissue of spontaneously hypertensive rats. Blood Press. 1999;8:57–64. doi: 10.1080/080370599438400. [DOI] [PubMed] [Google Scholar]
- 55.Rizzoni D, et al. Effects of losartan and enalapril at different doses on cardiac and renal interstitial matrix in spontaneously hypertensive rats. Clin Exp Hypertens. 2003;25:427–441. doi: 10.1081/ceh-120024986. [DOI] [PubMed] [Google Scholar]
- 56.Spiers JP, et al. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade. J Hypertens. 2005;23:1717–1724. doi: 10.1097/01.hjh.0000176787.04753.ee. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg GA, et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg GA, et al. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Sironi L, et al. Endogenous proteolytic activity in a rat model of spontaneous cerebral stroke. Brain Res. 2003;974:184–192. doi: 10.1016/s0006-8993(03)02578-2. [DOI] [PubMed] [Google Scholar]
- 60.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 61.Tsuji K, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 62.Shen K, et al. Properties of circulating leukocytes in spontaneously hypertensive rats. Biochem Cell Biol. 1995;73:491–500. doi: 10.1139/o95-054. [DOI] [PubMed] [Google Scholar]
- 63.Tong S, et al. Constitutive expression and enzymatic cleavage of ICAM-1 in the spontaneously hypertensive rat. J Vasc Res. 2010 doi: 10.1159/000323474. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmid-Schönbein GW, et al. Microvascular tone in a skeletal muscle of spontaneously hypertensive rats. Hypertension. 1987;9:164–171. doi: 10.1161/01.hyp.9.2.164. [DOI] [PubMed] [Google Scholar]
- 65.Zweifach BW, Lipowsky HH. Pressure-flow relations in blood and lymph microcirculation. In: Renkin EM, Michel CC, editors. Handbook of Physiology, Section 2: The Cardiovascular System. American Physiological Society, Bethesda, M.D., Vol. 4. Microcirculation I. 1984. pp. 251–307. [Google Scholar]
- 66.Rodrigues SF, et al. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H25–H35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheng T, et al. Constitutive expression and enzymatic cleavage of ICAM-1 in the spontaneously hypertensive rat. J. Vasc. Res. 2011 doi: 10.1159/000323474. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim HH, et al. Life and death cell labeling in the microcirculation of the spontaneously hypertensive rat. J Vasc Res. 2001;38:228–236. doi: 10.1159/000051051. [DOI] [PubMed] [Google Scholar]
- 69.Tran ED, Schmid-Schönbein GW. An in-vivo analysis of capillary stasis and endothelial apoptosis in a model of hypertension. Microcirculation. 2007;14:793–804. doi: 10.1080/10739680701419992. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, et al. Impaired angiogenesis in SHR is associated with decreased KDR and MT1-MMP expression. Biochem Biophys Res Commun. 2004;315:363–368. doi: 10.1016/j.bbrc.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 71.Makino A, et al. G Protein-coupled Receptors Serve as Mechanosensors for Fluid Shear Stress in Neutrophils. Am J Physiol Cell Physiol. 2006;290:C1633–C1639. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 72.Chen AY, et al. Receptor cleavage reduces the fluid shear response in neutrophils of the spontaneously hypertensive rat. Am J Physiol Cell Physiol. 2010;299:C1441–C1449. doi: 10.1152/ajpcell.00157.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukuda S, Schmid-Schönbein GW. Centrifugation attenuates the fluid shear response of circulating leukocytes. J Leukoc Biol. 2002;72:133–139. [PubMed] [Google Scholar]
- 74.Yang Y, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 75.del Zoppo GJ, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 76.Kuo TB, Yang CC. Sleep-related changes in cardiovascular neural regulation in spontaneously hypertensive rats. Circulation. 2005;112:849–854. doi: 10.1161/CIRCULATIONAHA.104.503920. [DOI] [PubMed] [Google Scholar]
- 77.Valdez SR. MS, Bioengineering. University of California San Diego; 2010. Serotonin 5HT-1A Receptor Density in the Brain of the Spontaneously Hypertensive Rats. [Google Scholar]
- 78.Wu K-IS, Schmid-Schönbein GW. NF kappaB and matrix metalloproteinase induced receptor cleavage in the spontaneously hypertensive rat. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.110.158709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friese RS, et al. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31:521–533. doi: 10.3109/10641960802668730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Group TSRS. Soluble insulin receptor ectodomain is elevated in the plasma of patients with diabetes. Diabetes. 2007;56:2028–2035. doi: 10.2337/db07-0394. [DOI] [PubMed] [Google Scholar]
- 81.Hernandez C, et al. Effect of intensive insulin therapy on macular biometrics, plasma VEGF and its soluble receptor in newly diagnosed diabetic patients. Diabetes Metab Res Rev. 2010;26:386–392. doi: 10.1002/dmrr.1093. [DOI] [PubMed] [Google Scholar]
- 82.Matsunaga N, et al. Role of soluble vascular endothelial growth factor receptor-1 in the vitreous in proliferative diabetic retinopathy. Ophthalmology. 2008;115:1916–1922. doi: 10.1016/j.ophtha.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 83.Kim NH, et al. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int. 2005;67:167–177. doi: 10.1111/j.1523-1755.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 84.Hazarika S, et al. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circulation Research. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 85.Lip PL, et al. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol. 2004;88:1543–1546. doi: 10.1136/bjo.2004.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawashima M, et al. Soluble IL-6 receptor in vitreous fluid of patients with proliferative diabetic retinopathy. Jpn J Ophthalmol. 2007;51:100–104. doi: 10.1007/s10384-006-0411-4. [DOI] [PubMed] [Google Scholar]
- 87.Nin JW, et al. Levels of soluble receptor for AGE are cross-sectionally associated with cardiovascular disease in type 1 diabetes, and this association is partially mediated by endothelial and renal dysfunction and by low-grade inflammation: the EURODIAB Prospective Complications Study. Diabetologia. 2009;52:705–714. doi: 10.1007/s00125-009-1263-5. [DOI] [PubMed] [Google Scholar]
- 88.Nakamura K, et al. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes Metab Res Rev. 2007;23:368–371. doi: 10.1002/dmrr.690. [DOI] [PubMed] [Google Scholar]
- 89.Roldan V, et al. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost. 2003;90:1007–1020. doi: 10.1160/TH02-09-0083. [DOI] [PubMed] [Google Scholar]
- 90.Belgore FM, et al. Plasma levels of vascular endothelial growth factor and its soluble receptor (SFlt-1) in essential hypertension. Am J Cardiol. 2001;87:805–807. A9. doi: 10.1016/s0002-9149(00)01512-5. [DOI] [PubMed] [Google Scholar]
- 91.Ong KL, et al. Elevated plasma level of soluble F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) in hypertension. Am J Hypertens. 2009;22:500–505. doi: 10.1038/ajh.2009.23. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura K, et al. Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvascular Research. 2005;70:137–141. doi: 10.1016/j.mvr.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 93.Glowinska B, et al. Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism: Clinical and Experimental. 2005;54:1020–1026. doi: 10.1016/j.metabol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 94.Mysliwiec J, et al. CD11a expression and soluble ICAM-1 levels in peripheral blood in high-risk and overt type 1 diabetes subjects. Immunol Lett. 1999;70:69–72. doi: 10.1016/s0165-2478(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 95.Spencer CG, et al. Von Willebrand factor, soluble P-selectin, and target organ damage in hypertension: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Hypertension. 2002;40:61–66. doi: 10.1161/01.hyp.0000022061.12297.2e. [DOI] [PubMed] [Google Scholar]
- 96.De Caterina R, et al. Soluble E-selectin in essential hypertension: a correlate of vascular structural changes. Am J Hypertens. 2001;14:259–266. doi: 10.1016/s0895-7061(00)01276-0. [DOI] [PubMed] [Google Scholar]
- 97.Semenov AV, et al. Soluble P-selectin - a marker of platelet activation and vessel wall injury: increase of soluble P-selectin in plasma of patients with myocardial infarction, massive atherosclerosis and primary pulmonary hypertension. Ter Arkh. 2000;72:15–20. [PubMed] [Google Scholar]
- 98.Blann AD, Waite MA. von Willebrand factor and soluble E-selectin in hypertension: influence of treatment and value in predicting the progression of atherosclerosis. Coron Artery Dis. 1996;7:143–147. doi: 10.1097/00019501-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 99.Lip GY, et al. Soluble adhesion molecule P-selectin and endothelial dysfunction in essential hypertension: implications for atherogenesis? A preliminary report. J Hypertens. 1995;13:1674–1678. [PubMed] [Google Scholar]
- 100.Blann AD, et al. Increased levels of the soluble adhesion molecule E-selectin in essential hypertension. J Hypertens. 1994;12:925–928. [PubMed] [Google Scholar]
- 101.Varughese GI, et al. Prognostic value of plasma soluble P-selectin and von Willebrand factor as indices of platelet activation and endothelial damage/dysfunction in high-risk patients with hypertension: a sub-study of the Anglo-Scandinavian Cardiac Outcomes Trial. J Intern Med. 2007;261:384–391. doi: 10.1111/j.1365-2796.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 102.Vuorela P, et al. Amniotic fluid--soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol. 2000;95:353–357. doi: 10.1016/s0029-7844(99)00565-7. [DOI] [PubMed] [Google Scholar]
- 103.Li Y, et al. Determination of serum soluble interleukin-6 receptor and soluble gp130 levels in patient with pregnancy induced hypertension and its significance. Zhonghua Fu Chan Ke Za Zhi. 2001;36:18–19. [PubMed] [Google Scholar]
- 104.Geroldi D, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23:1725–1729. doi: 10.1097/01.hjh.0000177535.45785.64. [DOI] [PubMed] [Google Scholar]
- 105.Forbes JM, et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16:2363–2372. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 106.Liebetrau M, et al. ACE inhibition reduces activity of the plasminogen/plasmin and MMP systems in the brain of spontaneous hypertensive stroke-prone rats. Neurosci Lett. 2005;376:205–209. doi: 10.1016/j.neulet.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 107.Kojima C, et al. MMP-9 inhibition by ACE inhibitor reduces oxidized LDL-mediated foam-cell formation. J Atheroscler Thromb. 2010;17:97–105. doi: 10.5551/jat.1685. [DOI] [PubMed] [Google Scholar]
- 108.Yamamoto D, et al. Molecular mechanism of imidapril for cardiovascular protection via inhibition of MMP-9. J Mol Cell Cardiol. 2007;43:670–676. doi: 10.1016/j.yjmcc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 109.Sakata Y, et al. Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of Angiotensin-converting enzyme inhibitor. Circulation. 2004;109:2143–2149. doi: 10.1161/01.CIR.0000125741.88712.77. [DOI] [PubMed] [Google Scholar]