Abstract
Eye tracking dysfunction (ETD) is one of the most widely replicated behavioral deficits in schizophrenia and is over-represented in clinically unaffected first-degree relatives of schizophrenia patients. Here, we provide an overview of research relevant to the characterization and pathophysiology of this impairment. Deficits are most robust in the maintenance phase of pursuit, particularly during the tracking of predictable target movement. Impairments are also found in pursuit initiation and correlate with performance on tests of motion processing, implicating early sensory processing of motion signals. Taken together, the evidence suggests that ETD involves higher-order structures, including the frontal eye fields, which adjust the gain of the pursuit response to visual and anticipated target movement, as well as early parts of the pursuit pathway, including motion areas (the middle temporal area and the adjacent medial superior temporal area). Broader application of localizing behavioral paradigms in patient and family studies would be advantageous for refining the eye tracking phenotype for genetic studies.
Keywords: Eye tracking dysfunction, Smooth pursuit eye movements, Motion processing, Extraretinal processes, Schizophrenia, Genetics, Endophenotypes
1 Introduction
In 1908, Allen Diefendorf, a psychiatrist, and Raymond Dodge, an experimental psychologist, collaborated on the first study of ocular motor function in psychiatric patients (Diefendorf and Dodge 1908). Dodge’s development of a method for photographically recording eye movements (e.g., the photochronograph) allowed objective quantification of certain eye movement metrics and made experimental studies feasible. They reasoned that because eye movements were a ubiquitous aspect of everyday functioning, patients and controls would have comparable degrees of acquired proficiency. Diefendorf and Dodge chose to study smooth pursuit and reflexive saccades in order to capitalize on over-learned visual behaviors and to avoid the confounding effects of tasks that were “too complicated” or had “too unusual demands” for chronically ill patients to perform. In this way, any deficits found would suggest disease-related dysfunction in a potentially informative neural system. Thus, from both the scientific and methodological vantage points, Diefendorf and Dodge’s landmark study of eye movements in psychiatric patients laid the foundation for investigations that continue to this day.
That first empirical study compared patients with dementia praecox (now schizophrenia), manic-depressive psychosis (now bipolar disorder), and various organic conditions (e.g., epilepsy, neurosyphilis) with controls on simple pursuit and saccade tasks. They found such a strong and selective association between impaired smooth pursuit eye movements and dementia praecox that they described it as “praecox pursuit.” Surprisingly, the finding of a specific psychophysiological abnormality that differentiated one major psychosis from other functional and organic psychotic conditions was pursued only twice in the ensuing six decades;1 it was after this long fallow period that the modern era of research on ocular motor function in schizophrenia began.
The independent rediscovery of smooth pursuit eye movement impairment, otherwise known as eye tracking dysfunction (ETD), by Holzman and colleagues (Holzman et al. 1973, 1974a) was a serendipitous byproduct of an empirical study designed to assess the integrity of the vestibular system in schizophrenia. A consistent finding in schizophrenia at that time was vestibular hyporeactivity (Holzman 1969). Tests of vestibular function routinely include vestibularly induced eye movements (e.g., nystagmus, a slow eye movement in one direction followed by a fast eye movement in the opposite direction) as well as smooth pursuit and saccadic eye movements (Baloh and Honrubia 1990). Unexpectedly, the vestibulo-ocular reflex of schizophrenia patients was found to function normally (Levy et al. 1978).2 However, smooth pursuit eye movements (or “eye tracking patterns”) were abnormal, not only in patients but also in their clinically unaffected first-degree biological relatives (Holzman et al. 1973, 1974a). Unbeknownst to Holzman and colleagues, they had replicated and extended the findings of Diefendorf and Dodge from six and a half decades earlier (Stevens 1974; Holzman et al. 1974b).
Within 20 years of Holzman and colleagues’ first two eye tracking papers (Holzman et al. 1973, 1974a), over 80 replications of the finding of ETD in schizophrenia patients were published. Issues of specificity, psychotropic medication effects, stage of illness, temporal stability, and effects of clinical state and attention were addressed by independent groups all over the world. Multiple replications of the familial aggregation of ETD in relatives of schizophrenia patients also followed, suggesting that it might be heritable. Studies of twins discordant for schizophrenia as well as healthy twins supported the idea that eye tracking performance was under genetic control (Holzman et al. 1977, 1988; Iacono and Lykken 1979; Bell et al. 1994; Katsanis et al. 2000). The elevated rate of ETD in clinically unaffected relatives and in clinically discordant co-twins provided evidence that ETD could not be attributed to treatment, hospitalization, or other confounding factors. Rather, it raised the possibility that ETD might be an alternative manifestation of genetic liability for schizophrenia. The significantly higher rate of ETD than recurrence risk for schizophrenia in first-degree relatives of schizophrenia patients suggested that ETD might be a more penetrant, pleiotropic expression of the same genes that were risk factors for the clinical disorder (Holzman et al. 1988; Holzman and Matthysse 1990; Matthysse and Parnas 1992). This research also demonstrated the value of studying clinically unaffected relatives of patients, a once neglected resource that is now widely utilized in psychopathology research to unravel the pattern of genetic transmission of a schizophrenia-endophenotype complex.
The dedication of an entire recent issue of Brain & Cognition [volume 68(3), 2008] to eye movement research in psychiatry, coinciding with the 100th anniversary of Diefendorf and Dodge’s seminal paper, attests to the importance of eye movement research in psychopathology research. Although schizophrenia has tended to be the primary focus of this research, ocular motor function has been studied in many other psychiatric conditions as well – bipolar, major depressive and obsessive-compulsive disorders, anorexia nervosa, schizophrenia-related personality disorders, substance use (including nicotine effects), schizotypal traits, and childhood and adolescent-onset disorders [e.g., (Iacono et al. 1982; Clementz et al. 1996; Jacobsen et al. 1996; Pallanti et al. 1996, 1998; Thaker et al. 1996a; Bauer 1997; Farber et al. 1997; O’Driscoll et al. 1998; Sweeney et al. 1998b; Gooding et al. 2000; Larrison et al. 2000, 2004; Ross et al. 2000; Kumra et al. 2001; Depatie et al. 2002; Kathmann et al. 2003; Ceballos and Bauer 2004; Lenzenweger and O’Driscoll 2006; Sereno et al. 2009)]. Further, oculomotor control in psychiatric populations has now been studied with a range of tasks much broader than the standard pursuit and reflexive saccade paradigms. Researchers have employed tasks that include smooth pursuit during sudden changes in predictable target motion (Allen et al. 1990; Clementz et al. 1996; Thaker et al. 1998, 1999; Trillenberg et al. 1998; Hong et al. 2005a; Avila et al. 2006) and pursuit on textured backgrounds (Yee et al. 1987; Schlenker et al. 1994; Arolt et al. 1998; Hutton et al. 2000). In addition, several different voluntary saccade paradigms have been used, including saccades to predictable targets (Levin et al. 1982; Abel et al. 1992; Clementz et al. 1994; Crawford et al. 1995a, b; Karoumi et al. 1998; Hutton et al. 2001; Krebs et al. 2001; O’Driscoll et al. 2005; Spengler et al. 2006; Sailer et al. 2007) [see also review by (Gooding and Basso 2008); saccades away from targets (antisaccades) (Thaker et al. 1989; Fukushima et al. 1990; Clementz et al. 1994; Sereno and Holzman 1995; Katsanis et al. 1997; McDowell and Clementz 1997; Rosenberg et al. 1997; Hutton et al. 1998; Maruff et al. 1998; O’Driscoll et al. 1998; Gooding 1999; Castellanos et al. 2000; Curtis et al. 2001; Gooding and Tallent 2001; Mostofsky et al. 2001; Barton et al. 2002; Sweeney et al. 2002; Brownstein et al. 2003; Calkins et al. 2003; Munoz et al. 2003; Ettinger et al. 2004; Levy et al. 2004; Radant et al. 2007; Barton et al. 2008); saccades to remembered or attended targets (Park and Holzman 1992; Ross et al. 1994; Park et al. 1995; Everling et al. 1996; McDowell and Clementz 1996; Sweeney et al. 1998a; Muller et al. 1999; Larrison-Faucher et al. 2002; Winograd-Gurvich et al. 2006)]; and saccades to target sequences (Biscaldi et al. 1998; LeVasseur et al. 2001; Ram-Tsur et al. 2006). Fixation (Amador et al. 1991; Gooding et al. 2000; Munoz et al. 2003; Smyrnis et al. 2004; Barton et al. 2008), the oculocephalic reflex (Lipton et al. 1980), and optokinetic and vestibular responses (Levy et al. 1978, 1983; Latham et al. 1981; Jones and Pivik 1983; Yee et al. 1987; Cooper and Pivik 1991; Warren and Ross 1998) have been studied as well.
The rationale for Diefendorf and Dodge’s study implicitly acknowledged a fundamental connection between schizophrenia and brain dysfunction that might be elucidated by the investigation of eye movements. Much of the work by modern investigators is based on the same assumption. Indeed, one reason that the study of eye movements has become so widely adopted in psychopathology laboratories is that they can be mapped to specific neural structures [for overviews see (Thier and Ilg 2005; Leigh and Zee 2006)]. Investigations of the pathophysiology of ocular motor dysfunction using neurologically informative behavioral paradigms hold the potential to clarify aspects of normal and disrupted brain circuitry in schizophrenia. In this chapter, we present an overview of selected topics relevant to the characterization and pathophysiology of smooth pursuit ETD in schizophrenia.
2 Components of the Smooth Pursuit Eye Tracking Response
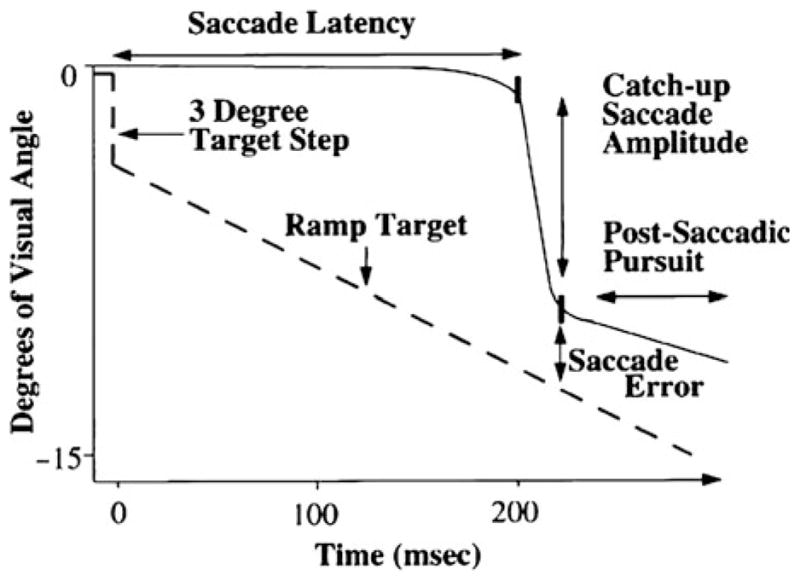
Smooth pursuit eye movements are slow movements of the eye (less than about 100 deg/s) that function to keep a small moving target on the fovea (the retinal area that has the greatest visual acuity) by matching eye velocity to target velocity (Lisberger et al. 1987). Saccadic eye movements, on the other hand, rapidly shift gaze (up to 900 deg/s) to bring a new target onto the fovea. In general, pursuit begins first (latency around 100–150 ms) and is interrupted by an initial catch-up saccade (CUS) (latency around 200–250 ms) that brings the target onto the fovea (Sereno et al. 2009), after which the two systems work together to maintain it there.
Pursuit has been divided into two phases, an initiation phase and a maintenance phase, which differ in terms of the principal processes driving pursuit. When the pursuit system is initially stimulated by the perception of motion across the retina, the eye begins to accelerate after a latency of about 100 ms (Lisberger and Westbrook 1985; Barnes et al. 1987). The first 100 ms of the pursuit response is called pursuit initiation or “open-loop pursuit.” It is driven primarily by the perception of a target moving slowly across the retina and reflects an initial estimate of the target speed. In this first 100 ms, no feedback from the retina influences the motor response, as the delay of information from the retina to the brainstem is approximately 100 ms (Krauzlis and Lisberger 1994). However, after 100 ms of pursuit, the relevant structures receive feedback from the retina regarding residual velocity and position error; at this point, the loop is closed, and the maintenance phase of pursuit begins. Pursuit maintenance uses velocity and position information from the retina as well as extraretinal information, such as corollary discharge from the motor system to sensory regions regarding the pursuit commands being issued, information about the position of the eyes in the head and the head in space, and accumulating experience with the target.
To study the smooth pursuit response in the initiation phase without the contribution of an orienting saccade that brings the target on to the fovea, researchers often use the “Rashbass” paradigm (Rashbass 1961). In the Rashbass paradigm (illustrated in Fig. 1), the central target steps off the fovea and then ramps (i.e., slides) back toward the fovea at a speed that returns it to center in less than 200 ms. Since the latency of a saccade is about 200 ms, and the target is back on the fovea at this point, pursuit begins without being interrupted by a saccade. Thus, by using the Rashbass paradigm, it is possible to isolate the smooth component of pursuit initiation. The integrity of pursuit initiation is quantified using measures of eye velocity or acceleration during the first 100 ms of pursuit as well as pursuit latency.
Fig. 1.
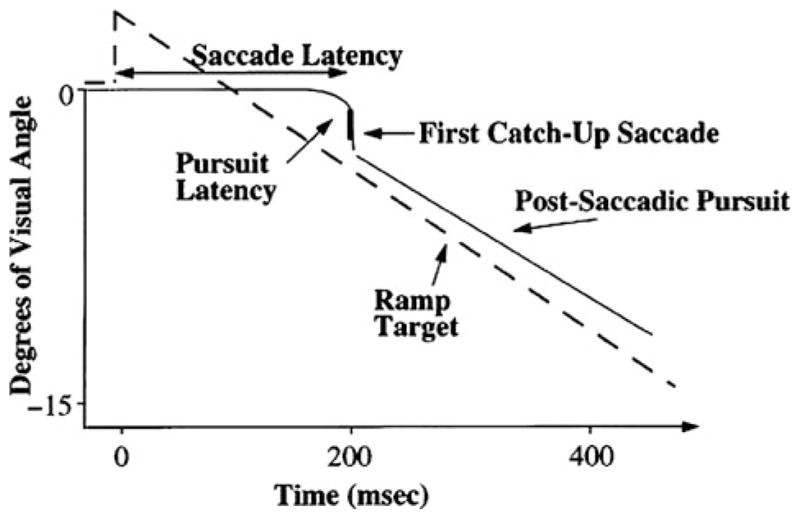
Schematic presentation of a foveopetal (Rashbass type) step-ramp task used to assess pursuit initiation and pursuit gain. Reprinted with permission from Sweeney et al. (1998a)
The adequacy of the pursuit response during the maintenance phase is often quantified by “pursuit gain” (the ratio of eye velocity to target velocity). The closer pursuit gain is to 1.0, the greater is the correspondence between the eye velocity and target velocity, and the more stable the target is on the fovea.3 When pursuit gain is less than 1.0, the eyes are moving slower than the target, and compensatory CUSs can be used to reposition the eyes on the target (see Fig. 2, top tracing). Conversely, when gain is greater than 1.0, the eyes are moving faster than the target, and compensatory back-up saccades bring the eyes back to the target. For predictable target trajectories, such as sinusoidal waveforms (e.g., Figs. 2 and 4) and constant velocity ramps (e.g., Fig. 3), the match between eye velocity and target velocity can be quantified either as average gain across the trace or, in the case of sinusoidal targets, “peak gain” (gain during a brief period when target velocity is highest).
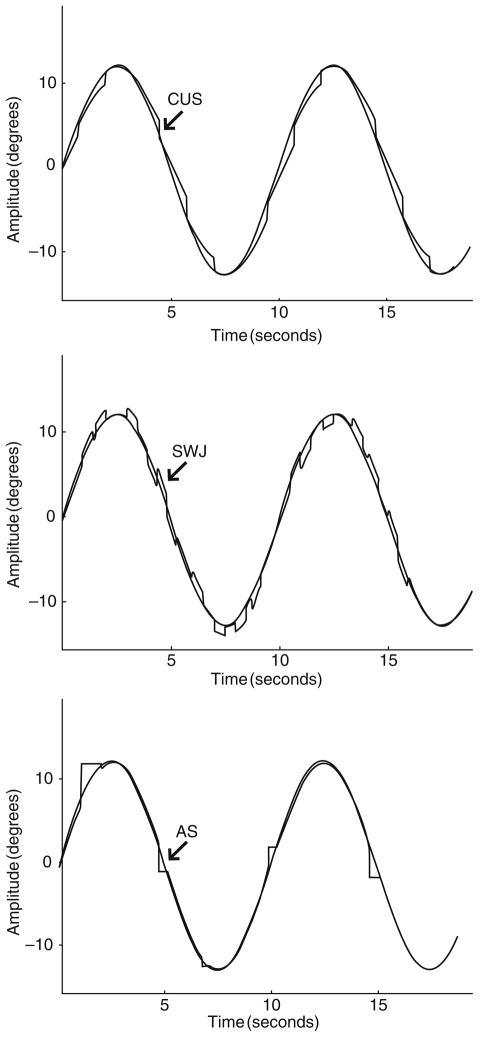
Fig. 2.
A 0.1 Hz sinusoidal target (lighter gray) and simulations of low gain pursuit and catch-up saccades (CUS) (top), square wave jerks (SWJ) (middle), and anticipatory saccades (AS) (bottom). Adapted with permission from Abel and Ziegler (1988)
Fig. 4.
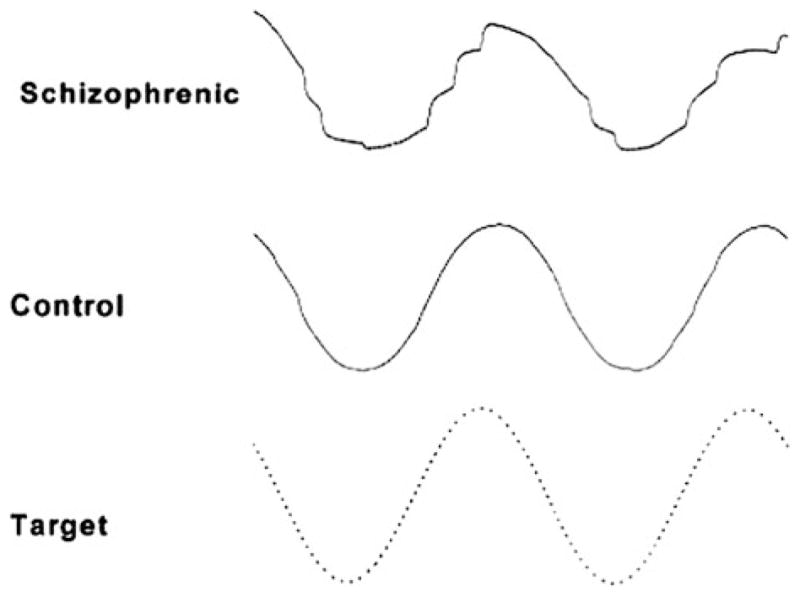
Illustrative tracings of smooth pursuit eye movements of a schizophrenia patient (top panel) and of a normal control (middle panel). The target is a 0.4 Hz sine wave (bottom panel, dotted line). The record of the schizophrenia patient shows many irregularities that suggest low gain pursuit with frequent catch-up saccades. The record of the normal control shows an occasional small catch-up saccade. Reprinted with permission from Holzman (2000)
Fig. 3.
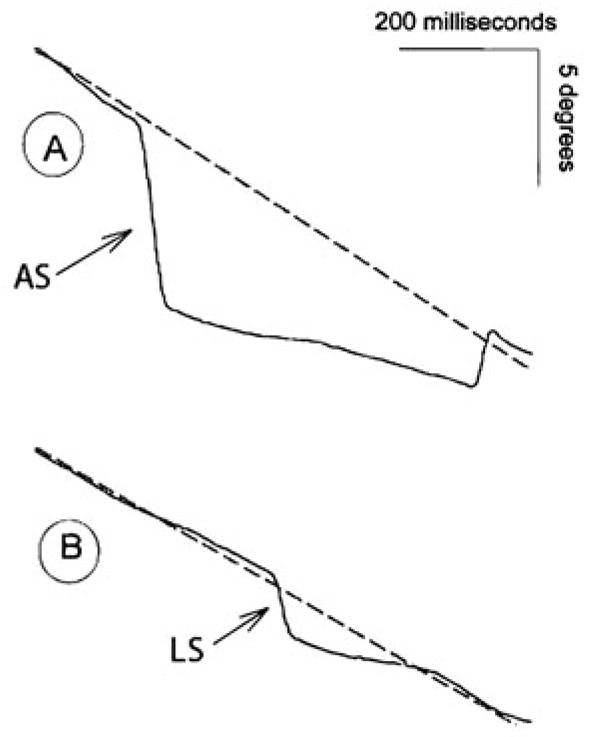
Two segments of eye movement tracing. Dotted lines represent target motion as it moves from right (top) to left (bottom) at 16.7 deg/s. Seven hundred milliseconds are presented in each tracing. Arrows identify anticipatory saccades. Panel A: A large anticipatory saccade with an amplitude of 6.9°, followed by 312 ms of slowed smooth pursuit at 6 deg/s, then 110 ms of slowed smooth pursuit at 8 deg/s, followed by a saccade to return gaze to target location. Panel B: A small anticipatory saccade (or leading saccade, LS) with an amplitude of 2.7°, followed by 210 ms of slowed smooth pursuit at 7 deg/s. Reprinted with permission from Ross et al. (1999)
Saccades that occur during pursuit can be classified as compensatory or intrusive. Compensatory saccades include catch-up and back-up saccades that reposition the eyes on the target and thus reduce position error. Intrusive saccades, in contrast, disrupt the correspondence between the eye and target position and increase position error. Three types of intrusive saccades have been included in the quantitative characterization of ETD in psychiatric populations. Square wave jerks (SWJ) consist of oppositely directed pairs of small (1–5°) saccades in which the first saccade takes the eyes off the target and the second saccade returns the eyes to the target. The intersaccadic interval is ~130–450 ms, during which pursuit continues (Fig. 2, middle tracing). Anticipatory saccades (AS) are large amplitude (>4–5°) saccades that move the eyes ahead of the target and are followed by periods of low gain pursuit (Fig. 2, bottom tracing; Fig. 3a) (Abel and Ziegler 1988; Leigh and Zee 2006). Leading saccades are saccades that take the eyes ahead of the target but have no minimum amplitude criterion, and are generally in the 1–4° range (Fig. 3b) (Ross et al. 1999). Other types of saccadic intrusions are found in certain neurological populations, but have not been studied in psychiatric populations (e.g., macro-SWJ, macrosaccadic oscillations, ocular flutter, and opsoclonus) (Leigh and Zee 2006).
3 Characterization of ETD
The early years of modern studies of ETD used global ratings that were either qualitative or quantitative. Qualitative ratings were judgments of how closely the eye position trace corresponded to the target position trace, either by dichotomizing the degree of correspondence as “normal” or “abnormal” (Fig. 4), or by using an ordinal scale to reflect varying degrees of deviation from the position trace. Quantitative measures included frequency of velocity arrests, the natural logarithm of the signal-to-noise ratio, root mean square error, and total saccade frequency, among others [for a review see (Levy et al. 1993)]. These measures consistently established the presence of an eye tracking abnormality in schizophrenia patients and their relatives. Indeed, in two recent meta-analyses, global measures such as these had among the largest effect sizes (Calkins et al. 2008; O’Driscoll and Callahan 2008).
Although global measures are effective in identifying deviance, a disadvantage of these measures is that they cannot specify what is abnormal about the eye tracking. As Abel and Ziegler pointed out, global measures do not distinguish between “abnormalities of pursuit” and “abnormalities during pursuit” (Abel and Ziegler 1988). Specifically, global measures could not distinguish among abnormalities of the smooth pursuit system, disinhibition of the saccadic system, or some combination (Levin 1984). Thus, they cannot provide insight into the processes or physiological substrates of eye tracking deviance.
Specific measures of pursuit, however, can help to clarify the nature of the deficit. For example, saccadic intrusions in the context of normal gain suggest disinhibition of the saccadic system. Reduced gain in the context of increased CUS implicates a disturbance in the pursuit system for which CUS are compensating. Decreased gain with no increase in CUS suggests a pursuit disturbance as well as increased tolerance for position error. The converse, normal gain in the context of increased compensatory saccades, indicates reduced tolerance for position error (Levy et al. 1993). As these various scenarios make clear, parsing ETD into its specific components is an essential step both toward identifying the specific processes that underlie ETDs and identifying the pathophysiological substrates of the deficits.
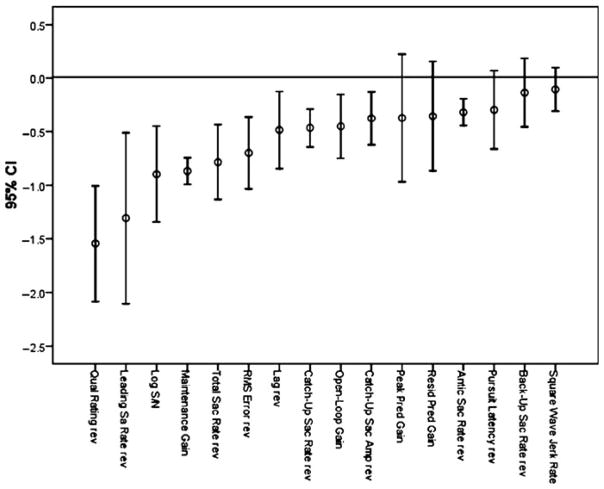
A recent meta-analysis of ETD in schizophrenia quantified the results of studies that used global and specific measures (O’Driscoll and Callahan 2008). The analysis included studies comparing pursuit in schizophrenia patients and controls published subsequent to a 1993 review (Levy et al. 1993). Fifty-nine studies met criteria for inclusion and involved 2,107 schizophrenia patients and 1,965 controls. A summary of mean effect sizes and 95% confidence intervals for different eye tracking measures is shown in Fig. 5 (from O’Driscoll and Callahan (2008) with permission). The analysis confirmed strong differences between schizophrenia patients and controls in eye tracking performance for global and certain specific measures. The effect sizes (Cohen’s d) for global variables were large; indeed, the largest effect size was obtained for qualitative ratings (d = 1.55). The latter finding is consistent with several reports indicating that qualitative ratings discriminate patients and relatives from controls better than specific quantitative measures [e.g., (Friedman et al. 1995; Keefe et al. 1997; Levy et al. 2000)]. Two of the specific indices, maintenance gain and leading saccade rate (i.e., anticipatory saccades with no minimum amplitude criterion) had large effect sizes (d = −0.87 and d = 1.31, respectively)4 as well as the smallest and largest 95% confidence intervals, respectively. The effect size for total saccade rate was also large. Effect sizes in the medium range were found for CUS, open-loop gain, and predictive gain measures (the latter variables are discussed below). O’Driscoll and Callahan concluded that the results did “not yield a clear-cut distinction between involvement of the pursuit or saccade system in the eye tracking deficit in schizophrenia; both pursuit and intrusive saccade measures yield at least one large effect size. It is also clear… that global measures generally yield larger effect sizes than specific measures” (p. 366). These findings notwithstanding, the authors correctly recognized that “in terms of neurophysiological informativeness, specific measures … allow precise hypotheses to be generated … in relation to areas in the pursuit pathway” (p. 366). They also noted several important caveats in interpreting the results of the meta-analysis. First, the amount of the recording on which a dependent measure is based seemed to be positively correlated with effect size. Qualitative ratings and maintenance gain, for example, are based on a larger proportion of the record than variables that, of necessity, are based on smaller segments (e.g., open-loop gain, predictive gain). As the reliability of a variable increases with the amount of data used to measure it, variables that are measured for longer periods of time may produce stronger results because of their enhanced statistical properties. Second, effect sizes for maintenance gain and CUS varied as a function of matching for sex in patients and controls, with larger effect sizes when the groups were matched than when they were not matched. This finding reflects a minor tendency for men to have higher maintenance gain than women (Lenzenweger and O’Driscoll 2006) and for men to be over-represented in patient samples.
Fig. 5.
Mean effect size and confidence intervals for patient-control differences in 16 measures of eye tracking performance. To allow a visual comparison of the magnitude of the effects, all ds have been made negative. Positive ds that have been reversed for the figure have “rev” appended to the variable name. The actual sign of the d based on the formula meanSz-MEANCOntrol/(Pooled SD) is shown in Table 3 of the published paper. Reprinted with permission from O’Driscoll and Callahan (2008)
In a recent complementary meta-analysis of studies on first-degree relatives of schizophrenia patients, Calkins and colleagues reported very similar results to those of O’Driscoll and Callahan. They found the largest effect sizes for global measures and for the specific measures, maintenance gain, and anticipatory saccades (a subset of leading saccades) (Calkins et al. 2008).
One possible reason for the apparent superiority of global ratings in terms of differentiating patients from controls is that global measures sum across different types of deficits in much the same way that in a depression questionnaire, the global question “Have you been been feeling down, depressed or hopeless?” will identify more individuals who subsequently meet criteria for depression than specific items like “Do you have trouble sleeping?” Global ratings average across different kinds of deviance that express or present in different severities in different individuals, while specific measures do not have this flexibility.
Thus, an advantage of global measures of ETD, in addition to their greater sensitivity to between-group differences, is that they can be used to take into account the within-group heterogeneity in ways that specific measures often do not or cannot [see (Gibbons et al. 1984; Levy et al. 1993) for detailed discussions of the use of mixture analysis to resolve within-group heterogeneity; see (Levy et al. 2000) for an example of how global and specific measures can be used in tandem to clarify the nature of within-group heterogeneity; see (Buchsbaum and Rieder 1979) for a discussion of the impact of heterogeneity on traditional between-group comparisons].
In both the above meta-analyses, it is important to note that the amount of research devoted to different specific measures varied widely (e.g., from five studies of schizophrenia for predictive gain to 42 for maintenance gain, and generally fewer for each variable in relatives). Thus, for some of the newest measures where there are not enough data currently to draw firm conclusions, there should be some caution in interpretation.
4 Pathophysiology of ETD
Below we discuss several different approaches to identifying the neural substrates of ETD, each of which draws heavily on the effects of spontaneously occurring lesions in humans and experimental lesions and single-cell recordings in nonhuman primates. We begin with investigations of motion processing, a sensory function mediated in extrastriatal regions, and proceed to investigations of higher-order cognitive contributions that implicate regions later in the pursuit pathway.
4.1 Behavioral Evaluations of the Contribution of Motion Processing to ETD
A key component of the pursuit response is the processing of target velocity. This component contributes more to pursuit initiation, or “open-loop” pursuit, than to pursuit maintenance (Lisberger et al. 1987). This is because, generally, the stimulus for pursuit initiation is the movement of a novel target across the retina, the velocity of which must initially be estimated entirely perceptually. Once the maintenance phase of pursuit begins, other components of the pursuit response – predictions regarding target movement based on velocity memory, corollary discharge of the motor command to sensory areas regarding movement of the eyes in the head and the head in space, etc. – begin to contribute; at the same time, motion changes on the retina (i.e., retinal slip) decrease as the eye and target are now moving at approximately the same speed in the same direction.
Two regions of the extrastriate cortex, the middle temporal (MT) area and adjacent medial superior temporal (MST) area (in humans V5/V5a), play a critical role in the processing of visual motion. These regions respond to the passive perception of moving stimuli during smooth pursuit (Zeki 1974; Van Essen and Maunsell 1983). When these motion-sensitive regions of the brain are damaged, initial pursuit eye velocity is reduced, pursuit latency is increased, and motion perception is temporarily impaired (Wurtz et al. 1990).
Psychophysical studies investigating the potential contribution of motion processing deficits to ETD have taken several approaches. The first approach requires participants to make judgments about the velocity or direction of a motion stimulus (e.g., Fig. 6). The second approach requires participants to generate saccades to moving targets based on their velocity and direction (Figs. 1 and 7). Both approaches have been shown to index the integrity of extrastriate motion areas in nonhuman primates and in neurological populations. Nonhuman primates with lesions of MT (but not with lesions of the frontal eye fields) generate saccades that underestimate target speed, suggesting that the accuracy of saccades to moving targets is sensitive and somewhat specific to the integrity of extrastriate motion areas (Newsome et al. 1985; Thurston et al. 1988). The third approach involves evaluating the integrity of open-loop pursuit vs. closed-loop pursuit with the expectation that open-loop would be more compromised than closed-loop if motion processing were the major contributor to tracking deficits. The reason is that prediction is the predominant driver of closed-loop pursuit (Vandenberg 1988), while motion perception is the predominant driver of open-loop pursuit (Lisberger et al. 1987). In the two oculomotor approaches, the contribution of prediction to performance (which can compensate for motion perception deficits) can be controlled by varying target velocity, direction, and timing on a trial-by-trial basis (see Figs. 1 and 7).
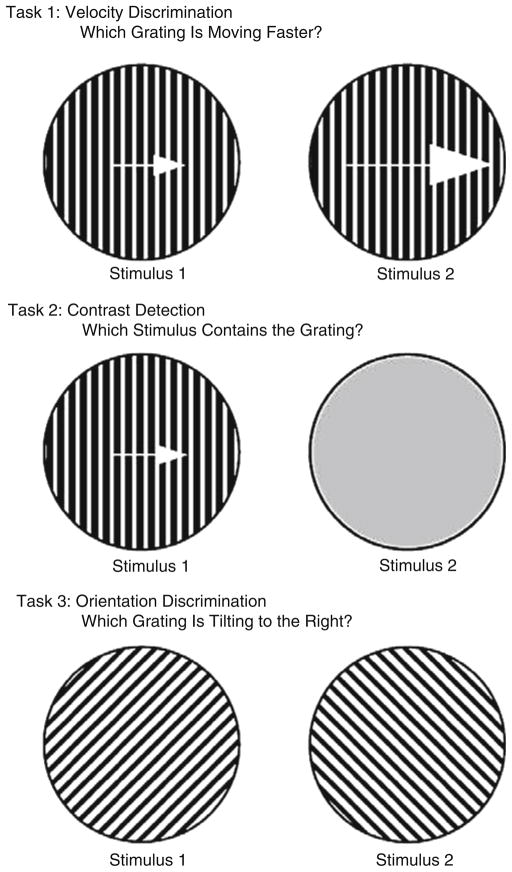
Fig. 6.
A schematic representation of the stimuli used for the velocity discrimination, contrast detection, and orientation discrimination tasks. Reprinted with permission from Chen et al. (1999c)
Fig. 7.
Schematic presentation of a foveofugal step-ramp task used to assess the use of motion information by the pursuit and saccadic eye movement systems. Reprinted with permission from Sweeney et al. (1998a)
4.1.1 Psychophysical Judgment Studies of Motion Perception
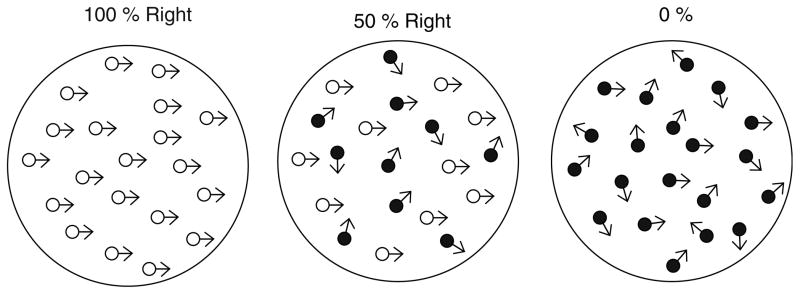
Using a standard motion perception task, one early study addressed the question of whether motion perception contributed to ETD in schizophrenia (Stuve et al. 1997). This study used a direction discrimination paradigm to assess motion perception in patients with schizophrenia and controls. In this task, participants watch a screen in which hundreds of dots move in random directions (illustrated in Fig. 8). The proportion of dots that move in a fixed direction (i.e., “motion coherence”) is varied, and the level of coherence that is needed to correctly identify the direction is the individual’s motion perception threshold (Newsome and Pare 1988). This task has been extensively used in single-unit recordings from nonhuman primates and has also been used in studies of neurological populations with lesions to MT/MST. Neuronal firing in this region significantly predicts the direction the monkey will choose on a trial-by-trial basis (Britten et al. 1996); stimulation of neurons in MT biases the monkey’s judgment in the preferred direction of the stimulated neurons (Salzman et al. 1992). Lesions to MT/MST significantly increase direction discrimination thresholds in nonhuman primates (Newsome and Pare 1988) and in a patient with a V5 (MT) lesion (Baker et al. 1991). Stuve and colleagues found that patients with schizophrenia had significantly elevated motion thresholds that were correlated with pursuit deficits but not with performance on a sustained attention task. Accumulating research has provided consistent evidence that schizophrenia patients have a higher threshold for detecting the direction of coherent motion than controls (Wertheim et al. 1985; Stuve et al. 1997; Li 2002; Chen et al. 2003; Slaghuis et al. 2005, 2007a; Kim et al. 2006) and three of these studies found that the magnitude of the deficit correlated with closed-loop gain (Stuve et al. 1997; Slaghuis et al. 2005, 2007b).
Fig. 8.
Schematic representation of coherent motion at 100, 50, and 0% movement in a rightward direction. In the actual stimulus display, the dots moving coherently and those moving at random (i.e., noise) are the same color. Reprinted with permission from Slaghuis et al. (2007b)
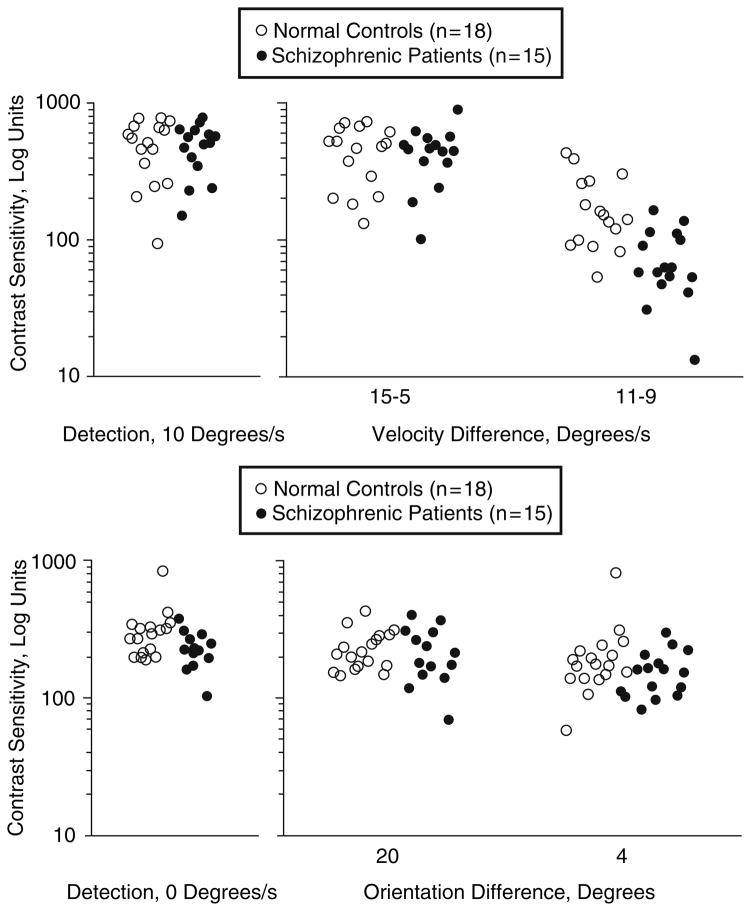
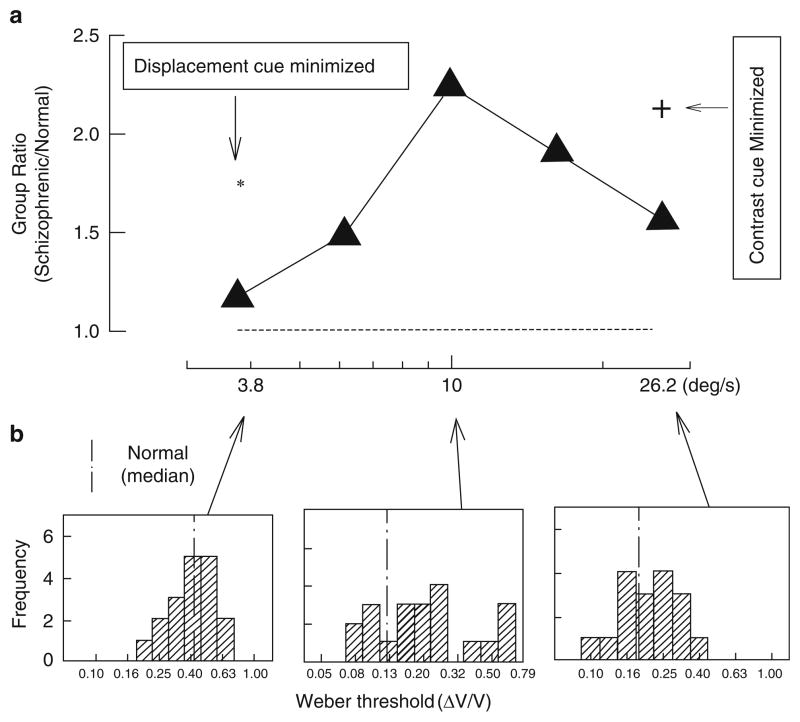
Another method of assessing the functional integrity of the motion processing system is to measure the amount of contrast necessary to perform a velocity discrimination task. When the processing of visual signals is impaired, higher levels of contrast are necessary (Plant and Nakayama 1993; Pasternak and Merrigan 1994). Thus, measuring contrast sensitivity during velocity discrimination can index the integrity of the motion processing system. Contrast sensitivity measured independent of movement, such as in pure contrast detection tasks or in orientation discrimination tasks (shown in Fig. 6), provide valuable control conditions for movement. Chen and colleagues used this approach to establish a selective deficit in motion processing in schizophrenia that correlated with pursuit performance. They found that non-hospitalized schizophrenia patients needed higher amounts of contrast than controls to detect small differences in velocity (11 vs. 9 deg/s), but not to detect large differences in velocity (15 vs. 5 deg/s) (Fig. 9, top). The groups did not differ in detecting contrast or orientation (Fig. 9, bottom) (Chen et al. 1999c). Another study showed that the deficits were found in patients (Fig. 10) and in their clinically unaffected relatives (Fig. 11) at intermediate velocities (e.g., 10 deg/s), but not at slow (3.8 deg/s) and fast (26.2 deg/s) velocities (Chen et al. 1999b). At slow and fast velocities, non-velocity cues can be used to help make velocity discriminations – position information at slow velocities (McKee 1981; Nakayama and Tyler 1981) and contrast differences at fast velocities (Pantle 1978). Manipulations to remove these non-velocity cues raised the velocity thresholds of both patients and relatives, indicating that the deficit was velocity-specific and could be partially compensated for by reliance on non-velocity cues (Chen et al. 1999b).
Fig. 9.
Top panel: Contrast sensitivity for contrast detection (left panel) and for velocity discrimination (right panel). The groups differed significantly only on velocity discriminations of 11 vs. 9 deg/s. Bottom panel: Contrast sensitivity for detection (left panel) and for orientation discrimination (right panel). Patients and normal controls performed similarly. Reprinted with permission from Chen et al. (1999c)
Fig. 10.
Comparison of velocity discrimination of schizophrenia and normal control groups. (a) Group ratios (schizophrenia/normal control) of Weber thresholds plotted as a function of base velocity. The Weber fraction (ΔV/V) is the just-noticeable differences between the velocities of the targets being compared. A ratio of unity, shown in the dotted horizontal line, indicates equivalent performance by the two groups. The larger the ratio is, the higher the velocity discrimination threshold of the patients relative to the normal controls. The asterisk and cross sign represent the group ratios after exposure time for the 3.8 deg/s target (asterisk), and the amount of contrast for the 26.2 deg/s target (cross sign) was randomized. (b) Histograms in the three panels (from left to right) represent distributions of individual patients’ thresholds at the slowest (3.8 deg/s), middle (10 deg/s), and fastest (26.2 deg/s) base velocities. The vertical line in each panel indicates the median threshold of the normal control group. Reprinted with permission from Chen et al. (1999b)
Fig. 11.
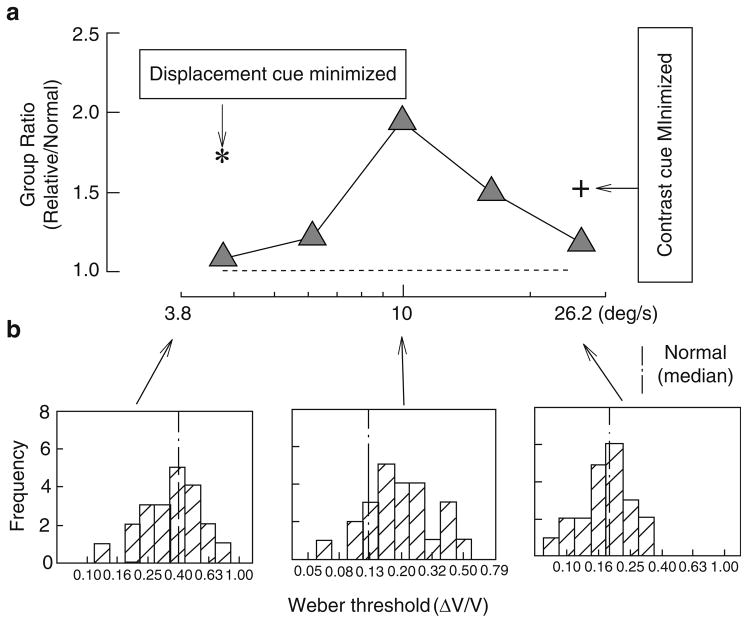
Comparison of velocity discrimination between first-degree relatives of schizophrenia patients and normal controls. (a) Group ratio (as in Fig. 10, but here for relatives/normal controls) of Weber fraction thresholds plotted as a function of base velocity. The asterisk and cross sign represent group ratios after exposure time and amounts of contrast of the two velocity comparison targets were randomized. (b) Histograms in the three panels represent, from left to right, the distributions of individual relatives’ thresholds at the slowest, middle, and fastest velocities. Other details are similar to those in Fig. 10. Reprinted with permission from Chen et al. (1999b)
A subsequent study isolated the motion deficit to later stages of visual processing (Chen et al. 2004). However, studies done by other laboratories have suggested deficits in early visual processing as well (Schwartz et al. 1987; Slaghius 1998; Butler et al. 2001; Green et al. 2003; Coleman et al. 2009; also see Slaghuis et al. 2007a).
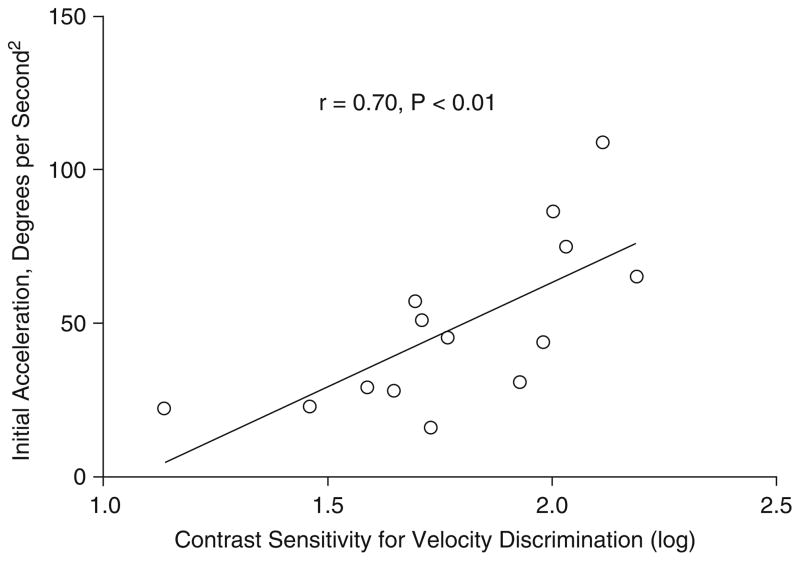
We could find only one study that examined the relationship between open-loop gain (Fig. 12) and motion perception measures (Chen et al. 1999a). These authors found an association between both open- and closed-loop gain and reduced sensitivity to velocity information, supporting a connection between impaired motion processing and deficits in both the initiation and maintenance of pursuit (Chen et al. 1999a). The stronger association with open-loop gain (r = 0.70, p < 0.01, n = 15; Fig. 13), which depends on sensory input without feedback about target position, than for closed-loop gain (r = 0.53, p < 0.05, n = 15) is expected, given the primacy of motion processing in driving pursuit in the open-loop phase.
Fig. 12.
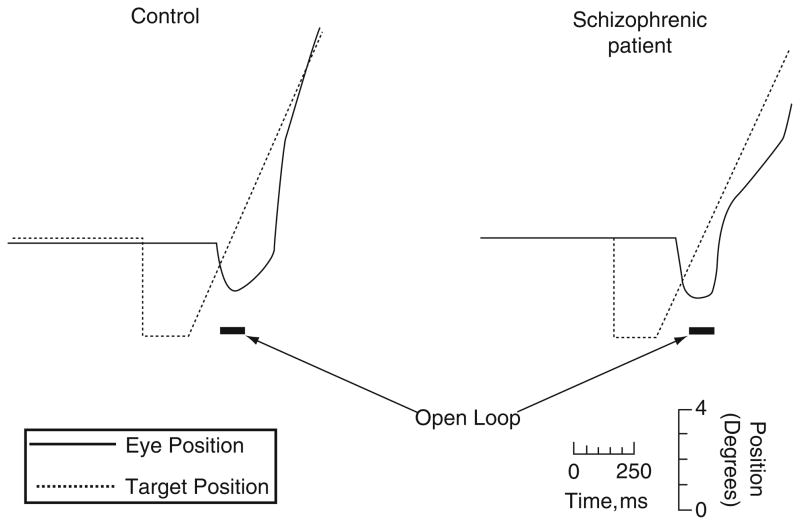
Step-ramp pursuit of a normal control (left) and a schizophrenia patient (right). The target (dotted line) steps abruptly to the left and remains stationary for 200 ms before beginning a 20 deg/s ramp trajectory to the right. The open-loop period, denoted by the black horizontal bars, begins 130 ms after the target starts its ramp and continues for 100 ms. In response, at about 150 ms after the start of the ramp, the normal control begins a smooth eye movement that accelerates at a rate that is discernibly faster than that of the schizophrenia patient, whose initial eye movement barely accelerates. Reprinted with permission from Chen et al. (1999a)
Fig. 13.
Scatter diagram of the relationship within the schizophrenia group (n = 15) between open-loop acceleration for the 10 deg/s target and velocity discrimination between two targets (11 deg/s vs. 9 deg/s). Reprinted with permission from Chen et al. (1999a)
4.1.2 Saccadic Studies of Motion Perception
Several groups have assessed motion processing in schizophrenia by evaluating the accuracy of saccades to moving targets (Clementz 1996; Thaker et al. 1996b; Sweeney et al. 1998a, 1999; Lencer et al. 2004). This paradigm originated in the nonhuman primate literature and involves targets that step off the fovea and then ramp either away from the fovea (foveofugal) or toward the fovea (foveopetal) at different speeds (Newsome et al. 1985) (Figs. 1 and 7, respectively). MT lesions increase saccade latency and reduce the sensitivity of saccade amplitude to differences in ramp speed and ramp direction (i.e., foveofugal vs. foveopetal) (Newsome et al. 1985). All studies of schizophrenia have found that patients adjust saccadic amplitude according to ramp speed and direction to the same extent as controls and have normal saccade latencies (Clementz 1996; Thaker et al. 1996b; Sweeney et al. 1998a, 1999; Lencer et al. 2004) regardless of medication status and chronicity (Sweeney et al. 1998a, 1999). These studies suggest that saccadic motion estimates are unaffected in schizophrenia (Sweeney et al. 1998a, 1999), a conclusion that is inconsistent with patients’ performance on motion perception tests. One possible explanation for this inconsistency is that motion perception studies have found impairments in fine velocity discriminations (e.g., 9 vs. 11 deg/s target speeds) but not in gross velocity discriminations (e.g., 5 vs. 15 deg/s) (Chen et al. 1999c). Studies that used saccades-to-moving-target paradigms in schizophrenia have generally used ramp speeds that differ widely (e.g., 8 vs. 16 deg/s, and even 8 vs. 24 deg/s, 9 vs. 27 deg/s), partly because saccadic endpoints to moving targets have some scatter, and larger differences in target speeds allow clearer distinctions between endpoints. However, the large differences in target speeds may reduce the difficulty of the motion component of the task and allow non-velocity cues (for example, changes in contrast and position) to aid saccade targeting.
4.1.3 Pursuit Initiation Studies
Several studies have used pursuit initiation in schizophrenia to examine the contribution of motion processing to pursuit deficits. Larger deficits in pursuit initiation (open-loop pursuit) than in pursuit maintenance (closed-loop pursuit) would be consistent with an impairment in motion processing. Deficits similar in magnitude in the two phases, or larger in the pursuit maintenance phase, suggest deficits in other functions (prediction, corollary discharge) that play a greater role in closed-loop pursuit (see Sect. 2, Components of the Smooth Pursuit Eye Tracking Response). Pursuit initiation has been studied both subsequent to the initial saccade (Feil 1997; Sweeney et al. 1999; Chen et al. 1999a; Sherr et al. 2002; Lencer et al. 2004; Avila et al. 2006) and without an initial saccade using the Rashbass paradigm (Clementz 1996; Ross et al. 1996; Farber et al. 1997; Radant et al. 1997; Hong et al. 2003). The schizophrenia-control difference in average effect size for studies that eliminate the saccade (d = −0.54 ± 0.28) vs. those that do not (d = −0.36 ± 0.62) is modest, and the average effect size across studies of open-loop pursuit is medium (see Fig. 5). Eight studies measured open- and closed-loop pursuit in the same patients (Clementz and McDowell 1994; Farber et al. 1997; Feil 1997; Radant et al. 1997; Sweeney et al. 1999; Chen et al. 1999a; Sherr et al. 2002; Lencer et al. 2004). Five of these studies found larger effects for open-loop than for closed-loop pursuit (Clementz and McDowell 1994; Radant et al. 1997; Sweeney et al. 1999; Chen et al. 1999a; Lencer et al. 2004),5 two studies found larger effects for closed-loop than for open-loop pursuit (Sherr et al. 2002; Hong et al. 2003), and one study found no deficits in closed-loop pursuit or in pursuit acceleration during the first 100 ms (Farber et al. 1997).6 However, across all studies published since 1993 (which include all open-loop studies and a large subset of closed-loop studies), open-loop pursuit measures have yielded a medium effect size, d of −0.45 (±0.47, n = 12), whereas closed-loop pursuit gain has yielded a large effect size, d, of −0.87 (±0.42, n = 42). For measures of both open- and closed-loop pursuit, deficits have been found even in neuroleptic naïve and unmedicated patients (Hutton et al. 1998; Sweeney et al. 1998a, 1999; Thaker et al. 1999; Lencer et al. 2008). These findings suggest that if motion processing deficits contribute to ETD, higher-order processes that would normally compensate for motion processing deficits are affected as well. In the studies by Sweeney and colleagues (Sweeney et al. 1998a, 1999), schizophrenia patients had delayed pursuit initiation and decreased closed-loop gain, normal CUS latency and amplitude, and reduced gain of postsaccadic pursuit compared with controls. The authors concluded that the pattern of deficits was consistent with involvement of the frontal eye fields (FEFs) (Sharpe and Morrow 1991; Keating 1993). The pattern seen after MT lesions –which is similar but includes dysmetric saccades to moving targets (Newsome et al. 1985; Thurston et al. 1988) – was not observed and seemed to militate against a motion processing explanation of pursuit deficits (but see caveat in Sect. 4.1.2).
4.2 Extraretinal Processes in Pursuit
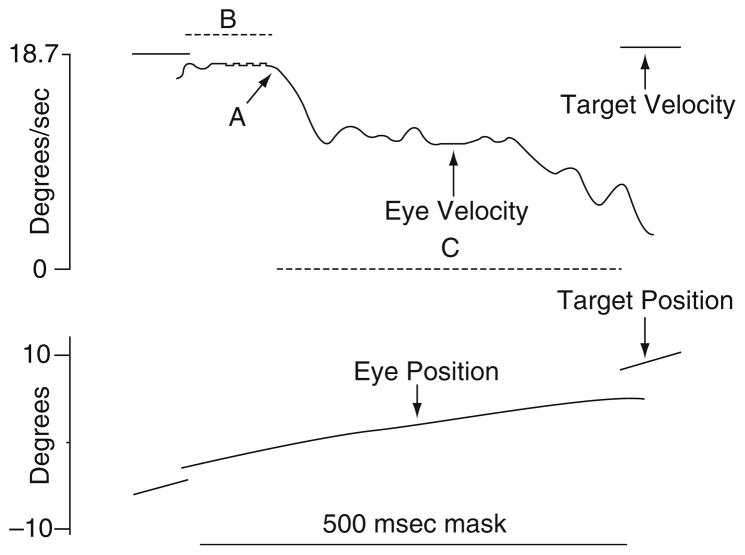
The robust deficits in maintenance pursuit in schizophrenia [see (O’Driscoll and Callahan 2008)] could reflect impairments in extraretinal processes, rather than or as well as deficits in motion processing. Recent studies have focused on whether the predictive component of pursuit is impaired in schizophrenia as prediction of target movement is critical to high-gain closed-loop pursuit (Vandenberg 1988). An early psychophysical study addressed this question by having patients and controls watch a smoothly moving target disappear behind a screen and press a button at the moment they expected the target to reappear (Hooker and Park 2000). Patients had larger timing errors than controls, consistent with a deficit in motion prediction and the finding could not be attributed to motor slowing. Other studies of prediction have analyzed the speed of pursuit during brief periods when the target disappears. Figure 14 shows an example of a paradigm used to evaluate the predictive component of pursuit. Masking the trajectory of the pursuit target for short periods (i.e., 500 ms) eliminates retinal feedback and requires that extraretinal information, such as corollary discharge, velocity memory, and predictions regarding the target movement, drive pursuit (Lisberger et al. 1987; Newsome et al. 1988). The ratio of eye velocity to target velocity during epochs when the target is masked (i.e., predictive gain) indexes the efficacy of extraretinal signals in sustaining pursuit. A few studies have reported that schizophrenia patients (Thaker et al. 1999; Hong et al. 2003, 2005a), as well as their clinically unaffected relatives (Thaker et al. 1998, 2003; Hong et al. 2008), have lower predictive gain than controls.
Fig. 14.
The top panel shows eye and target velocity data, and the bottom panel shows corresponding position data from a 500-ms mask occurring during a ramp. Eye velocity remained unchanged for about 95 ms after the target was extinguished (B), presumably still influenced by the prior closed-loop response. After this initial period, the eye velocity stabilized to a lower level (58% of the closed-loop response) (C), arguably the response based on extraretinal motion signals. Residual predictive gain was calculated by dividing average eye velocity during C by expected target velocity. The transition point from closed-loop to extraretinal response (A) was identified by an algorithm. The program searched for the time point within the mask when the eye velocity first decreased by 50% of the premask value. From this point backwards, the algorithm searches for the local minimum or maximum value (depending on target direction) by analyzing the smoothed first (velocity) and second (acceleration) derivatives of position. This is identified as the transition point. Reprinted with permission from Thaker et al. (2003)
A decrease in eye velocity during target blanking could reflect a reduction of motion signals in memory or a reduction in the gain of the signals driving the smooth pursuit system (Orban de Xivry et al. 2008). The effect sizes for this deficit are in the medium range. However, as larger effect sizes are found for measures of closed-loop pursuit (Fig. 5) that combine prediction and retinal information (i.e., gain and leading saccades), ETD likely reflects impairments in both motion processing and in prediction, implicating motion areas and FEFs, or possibly other areas in which both motion signals and predictive signals are represented [e.g., MST (Newsome et al. 1988); ventral intraparietal area (Schlack et al. 2003)].
The FEF contribution to pursuit has been studied in both nonhuman primates and in neurological populations. The characteristic features of pursuit after damage to the FEFs in nonhuman primates and in neurological populations include low initial and maintenance gain7 (Keating 1991; MacAvoy et al. 1991; Rivaud et al. 1994; Morrow and Sharpe 1995; Heide et al. 1996; Lekwuwa and Barnes 1996; Shi et al. 1998) and impaired predictive pursuit (pursuit during target blanking) (Keating 1991, 1993; MacAvoy et al. 1991). In FEFs, the smooth velocity of the eye is rate-coded, such that increased eye velocity is associated with increased firing (Gottlieb et al. 1994). Microstimulation of FEF neurons increases smooth eye velocity (Gottlieb et al. 1993). Predictive pursuit, or pursuit during target blanking, is thought to depend on a neural representation of target motion. Neural correlates of internal representations of target motion, even changing target motion, have been found in FEFs, with neural activity coding target motion estimates during target blanking (Tanaka and Fukushima 1998; Barborica and Ferrera 2003, 2004; Xiao et al. 2003). Such a representation might be reconstructed from an efference copy of the pursuit motor command combined with retinal slip when the target is visible. The FEFs are also thought to play a critical role in controlling the “gain” of the signals driving pursuit (Tanaka and Lisberger 2001, 2002a, b). This notion of “gain” is distinct from pursuit gain, and describes the amplification of the pursuit response to visual or predictive signals driving pursuit. Tanaka and Lisberger showed that microstimulation of the pursuit area of the FEFs increases the gain of the pursuit system, that is, increases the magnitude of the pursuit response to retinal slip (Tanaka and Lisberger 2002c). In humans, transcranial magnetic stimulation of the FEFs also increases the magnitude of the pursuit response to predicted target motion (Gagnon et al. 2006).
Neurons in MST are sensitive to velocity and direction signals on the retina (Newsome et al. 1985), and also code extraretinal information, in that neurons in MST continue to fire during pursuit of a target that has briefly disappeared (Newsome et al. 1988; Bremmer et al. 1997). The extraretinal firing may code corollary discharge from motor areas (Newsome and Pare 1988; Komatsu and Wurtz 1989) or a representation of target movement in space (Thier and Erickson 1992). In nonhuman primates, lesions to MST do not affect saccades to moving targets (Fig. 1), but lesions to MST do reduce closed-loop pursuit gain (postsacca-dic pursuit in Figs. 1 and 7) (Dursteler and Wurtz 1988) and reduce eye acceleration during pursuit initiation (Fig. 12). Lesions to the lateral portion of MST reduce sensitivity to retinal slip during ongoing pursuit (Komatsu and Wurtz 1989).
4.3 Neuroimaging of Pursuit and Component Processes
Several neuroimaging studies have investigated the neural substrates of ETD in schizophrenia patients and in their first-degree relatives. Paradigms used have included closed-loop smooth pursuit and predictive pursuit as well as tasks tapping motion perception.
An early imaging study relating neural activation to ETD found that reduced FEF activation during an attentional task was correlated with measures of pursuit quality outside the scanner (Ross et al. 1995). Subsequent studies of ETD in patients have compared the activation observed during smooth pursuit in schizophrenia patients with that seen in controls. Results are somewhat difficult to summarize across studies because coordinates differ by up to 4 cm across studies for both putative MT/MST and for FEF. Setting these anatomical discrepancies aside, a few studies have reported lower activation in schizophrenia patients than in controls in MT/MST (Lencer et al. 2005; Keedy et al. 2006) and an adjacent anterior temporal region (Hong et al. 2005b), as well as in FEFs (Tregellas et al. 2004; Hong et al. 2005b; Keedy et al. 2006), supplementary eye fields (Hong et al. 2005b), parietal cortex (Keedy et al. 2006), and cingulate (Hong et al. 2005b; Keedy et al. 2006). Differences have also been found outside the traditional pursuit pathway, with replications of increased activity in patients in hippocampus (Tregellas et al. 2004; Tanabe et al. 2006), thalamus (Tregellas et al. 2004; Nagel et al. 2007), and right fusiform gyrus (Tregellas et al. 2004; Tanabe et al. 2006). The scatter in coordinates for canonical regions does not occur in comparing pursuit to fixation, but in comparing the pursuit-related activation in schizophrenia to pursuit-related activation in controls. These outlying activations, which fall in the periphery of a region of interest, could result from a comparison of two different size peaks (in controls vs. patients) centered on the same location. Higher peaks have wider peripheries (due to spatial smoothing), so two activations in the same location may yield maximal statistical differences in the periphery of the peaks where standard deviations for the group with the small peak will be very low.
There are several limitations in the interpretation of these studies. First, for most studies, differences in activations between groups may not be due to ETD, but rather to other factors associated with the diagnosis (e.g., medication, institutionalization) that could affect brain function. To minimize these differences, Keedy and colleagues (2006) included only first-episode, neuroleptic-naive patients; their study found extensive deficits in pursuit activation, and the authors concluded that there was a “system-wide” involvement of cortical oculomotor areas. Another limitation of most of the studies is that schizophrenia patients with pursuit deficits are compared with controls with no pursuit deficits. Since the groups differ in eye tracking performance, activation differences between the groups may simply reflect group differences in engagement in the task. Hong and colleagues attempted to minimize this problem by comparing patients and controls who were matched for average pursuit performance. Group differences in visual processing areas (increased activation), and in FEFs and supplementary eye fields (decreases in schizophrenia), were still found (Hong et al. 2005b). However, if there are no group differences in average pursuit performance, the extent to which the differences in activation are attributable to pursuit rather than to diagnosis remains unclear.
A more compelling design might involve comparing poor tracking and good tracking patients with each other and with controls [see (Levy et al. 2000)]. Such a comparison has the advantage of clarifying the neural correlates of ETD uncon-founded by neural differences that are specific to the diagnosis rather than to tracking. A study that used this type of approach to examine ETD in unaffected first-degree relatives of schizophrenia patients made a strong case for FEF dysfunction as a substrate of low gain pursuit (O’Driscoll et al. 1999). Controls and relatives with normal pursuit both significantly activated FEFs during smooth pursuit, whereas demographically similar relatives with ETD as a group did not (p > 0.9). A correlational analysis relating regional neural activation to pursuit gain in the relatives found the highest correlation to be in the FEFs (r = 0.74). The peak correlation was located only 3 mm from the site of maximum FEF activation in controls. No group differences in activation were found in motion perception areas.
The extraretinal component of pursuit was examined in one imaging study of schizophrenia (Nagel et al. 2007). Patients and controls were examined during predictive tracking of a target that was periodically blanked. There were no signi-ficant performance differences between groups during target blanking, although gain values during blanking dropped to the 0.2 range, suggesting that neither group was able to sustain predictive pursuit. The schizophrenia group was found to have reduced activation in cerebellum during predictive tracking compared with controls, and increased activation in right anterior cingulate and in an area referred to as FEFs, although the very posterior location, y = −20, suggests that this may correspond to motor strip eye field, [see (Tehovnik et al. 2000)], an area that has been implicated in oculomotor prediction (Gagnon et al. 2002).
The integrity of motion processing areas supporting pursuit has been assessed in several imaging studies. One study had schizophrenia patients and controls make speed discriminations and contrast discriminations in the scanner (Chen et al. 2008). Controls showed strong activation (BOLD signal changes) in area MT/MST during motion tasks, consistent with the known role of this region in sensory processing of motion stimuli. Schizophrenia patients showed significantly less activation than controls in MT/MST. The groups did not differ in activation patterns while processing nonmotion stimuli. During motion processing, patients activated the inferior convexity of the prefrontal cortex more than controls did, suggesting that cognitive processing may have been used to help compensate for deficient sensory processing. Another study compared activation in first-episode neuroleptic-naïve schizophrenia patients and controls during passive viewing of motion stimuli compared with fixation. Patients had widespread reductions in activation, including in lateral and medial geniculate nuclei of right thalamus, a ventral region of FEF, as well as in occipital cortex, temporal lobe, and inferior parietal lobe (Braus et al. 2002). Widespread abnormalities were also found in schizophrenia in a study investigating the integrity of magnocellular vs. parvocellular pathways (Martinez et al. 2008). Magnocellular pathways are preferentially involved in motion processing, and some studies have suggested that schizophrenia patients are selectively impaired on tasks that tap magnocellular function as opposed to parvocellular function [(Kéri et al. 2004; Delord et al. 2006), but see also (Skottun and Skoyles 2007)]. Patients and controls viewed sinusoidal gratings biased to preferentially activate magnocellular (low spatial frequency and low contrast) or parvocellular (high spatial frequency) pathways. Differences between groups emerged only in the magnocellular condition. Reduced activation was found throughout the magnocellular system, including visual cortex, temporal cortex, and the dorsal parietal pathway (Martinez et al. 2008).
In sum, neuroimaging studies of maintenance pursuit have reported reduced activation of FEFs and motion processing areas in schizophrenia, with some studies finding that the reductions are more widespread and others finding as well greater activation in some areas outside the traditional pursuit pathway. Studies of motion processing are similarly divided between findings of focal reduction in motion processing areas and in generalized reductions that include thalamus, visual cortex, parietal cortex, and other regions in the dorsal stream, with some evidence of compensatory activations outside the motion pathway. To date, studies comparing patients with and without pursuit deficits or with and without motion processing deficits have not been conducted.
5 Association Between Genetic Polymorphisms and ETD
When an endophenotype is a more penetrant, pleiotropic expression of the same genes that are risk factors for schizophrenia, it can increase power to detect linkage for schizophrenia susceptibility genes compared with the clinical disorder alone (Lander 1988; Holzman and Matthysse 1990; Matthysse and Parnas 1992; Holzman 1994; Freedman et al. 1999). Indeed, this is the primary rationale for incorporating endophenotypes (Gottesman and Gould 2003) into linkage studies of complex diseases. The reason for this improvement in power is that the endophenotype (in this case, ETD) would improve accurate identification of non-penetrant gene carriers (Matthysse and Parnas 1992; Botstein and Risch 2003).
The first effort to examine the usefulness of ETD measures in linkage studies was conducted by Arolt and colleagues (Arolt et al. 1996, 1999). Using a gain score dichotomized into normal or abnormal pursuit, they calculated two point linkage analyses between ETD and 16 microsatellite markers on chromosome 6p21–23. A maximum LOD (logarithm of the odds to the base 10) score of 3.51 was obtained for marker D6S271 (θ = 0.0); marker D6S282 yielded a maximum LOD score of 3.44 at θ = 0.05 (Arolt et al. 1996). The results were quite similar when the analyses were repeated on a slightly larger sample using additional markers in the same region. Independent support for these results was found in other studies that combined qualitative ratings of ETD and schizophrenia as part of a latent trait model (Matthysse and Holzman 1987; Holzman et al. 1988); a LOD score of 2.05 was found for a marker within 3 cm of the positive markers studied by Arolt and colleagues (Matthysse et al. 2004).
Several studies have examined the relation between the COMT (catechol-O-methyltransferase) genotype and ETD. Rybakowski and colleagues reported that the Met/Met genotype was significantly associated with better closed-loop gain in male schizophrenia patients (Rybakowski et al. 2002). A similar association between this genotype and predictive gain was found in controls in a study by Thaker and colleagues (Thaker et al. 2004). However, in that study, patients with this genotype did not differ in maintenance gain and had worse predictive gain than patients with the Val/Val or Val/Met genotypes. Haraldsson and colleagues recently reported no association between the rs4680 val158met COMT polymorphism and either schizophrenia or steady-state pursuit gain and saccade frequency (Haraldsson et al. 2009). Further studies are needed to clarify this assortment of different findings with respect to COMT. Polymorphisms in other genes have also been examined in several samples, with reported but unconfirmed associations between pursuit performance and genotype (Rybakowski et al. 2001; Bogacki et al. 2005).
6 Summary
ETD is a robust finding associated with schizophrenia and shows significant co-familiality. Using well-characterized paradigms that were developed in nonhuman primate single-unit work, researchers have attempted to link specific component processes of pursuit to specific neural substrates. Despite variability in quantitative measures and behavioral paradigms, there is general agreement that ETD seems to involve impairments in motion processing and in higher-order processes such as prediction and gain control of signals driving pursuit. Motion-sensitive regions (MT/MST) and the FEF have been implicated as neural substrates of ETD, although some neuroimaging studies suggest a more system-wide pattern of dysfunction in the dorsal stream. Genetic associations with ETD have not yet conclusively implicated any one chromosomal region or specific genes.
Acknowledgments
This work was supported in part by NIMH grants R01 MH071523 and MH31340, the Sidney R. Baer, Jr. Foundation, the Essel Foundation, the National Association for Research on Schizophrenia and Depression (NARSAD), an Essel Investigator NARSAD and NSF grant 0924636, a grant from the Canadian Institute of Health Research, a William Dawson Scholar Award, and a Stairs Memorial Foundation grant. The authors thank Dr. Larry Abel for making the original material for Fig. 2 available for adaptation and Joshua Ritz for formatting the figures.
Footnotes
Two studies explicitly followed up the Diefendorf and Dodge report (Couch and Fox 1934; White 1938). Both studies replicated the finding of impaired pursuit in schizophrenic patients, but questioned its specificity and independence from clinical state, especially in manic-depressive patients. Modern psychotropic drugs were not yet in use, but barbiturates were commonly used to control agitation. Impaired pursuit was found during periods of clinical exacerbation, corresponding to periods of barbiturate treatment, whereas pursuit normalized during periods of remission, corresponding to barbiturate discontinuation. Only later were barbiturates discovered to impair pursuit (Rashbass and Russell 1961; Schalen et al. 1988), suggesting that what appeared at the time to be an association between clinical state and pursuit performance was actually a drug-induced epiphenomenon.
A discussion of possible reasons for the difference between these results and those of earlier investigators as well as a critical review of the literature on vestibular function in psychopatho-logical conditions can be found elsewhere (Levy et al. 1983). The status of visual–vestibular interaction remains unclear, with some data supporting normal responses in schizophrenic patients (Levy et al. 1978) and other data supporting abnormal responses (Jones and Pivik 1983; Yee et al. 1987; Warren and Ross 1998).
This function of gain was discovered by the same Dodge who collaborated with Diefendorf in the first study of oculomotor function in schizophrenia (Dodge 1903).
Positive and negative values for effect sizes correspond to whether patients had higher or lower mean scores than controls, respectively.
Larger for 10 deg/s targets, no difference for 20 deg/s targets.
Differences were found in the last 40 ms of pursuit initiation, but not in the first 60 ms. Other investigators averaged across these epochs.
If lesion is unilateral, deficits may be for ipsiversive pursuit only (Morrow and Sharpe 1995) or may affect pursuit in both directions (Lekwuwa and Barnes 1996).
Contributor Information
Deborah L. Levy, Email: dlevy@mclean.harvard.edu, Psychology Research Laboratory, McLean Hospital, 115 Mill Street, Belmont, MA 02478, USA
Anne B. Sereno, Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, Houston, TX, USA
Diane C. Gooding, Department of Psychology, University of Wisconsin-Madison, Madison, WI, USA
Gilllian A. O’Driscoll, Department of Psychology, McGill University, Montreal, QC, Canada
References
- Abel L, Ziegler A. Smooth pursuit eye movements in schizophrenics – what constitutes quantitative assessment? Biol Psychiatry. 1988;24:747–761. doi: 10.1016/0006-3223(88)90250-8. [DOI] [PubMed] [Google Scholar]
- Abel LA, Levin S, Holzman PS. Abnormalities of smooth pursuit and saccadic control in schizophrenia and affective disorders. Vision Res. 1992;32:1009–1014. doi: 10.1016/0042-6989(92)90002-z. [DOI] [PubMed] [Google Scholar]
- Allen JS, Matsunaga K, Hacisalihzade S, Stark L. The smooth pursuit eye movements of normal and schizophrenic subjects tracking an unpredictable target. Biol Psychiatry. 1990;28:795–720. doi: 10.1016/0006-3223(90)90457-d. [DOI] [PubMed] [Google Scholar]
- Amador XF, Sackheim HA, Mukherjee S, Halperin R, Neeley P, Maclin E, Schnur D. Specificity of smooth pursuit eye movement and visual fixation abnormalities in schizophrenia: comparison to mania and normal controls. Schizophr Res. 1991;5:135–144. doi: 10.1016/0920-9964(91)90040-x. [DOI] [PubMed] [Google Scholar]
- Arolt V, Lencer R, Nolte A, Muller-Myhsok B, Purmann S, Schurmann M, Leutelt J, Pinnow M, Schwinger E. Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am J Med Genet. 1996;67:564–579. doi: 10.1002/(SICI)1096-8628(19961122)67:6<564::AID-AJMG10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Arolt V, Teichert H-M, Steege D, Lencer R, Heide W. Distinguishing schizophrenic patients from healthy controls by quantitative measurement of eye movement parameters. Biol Psychiatry. 1998;44:448–458. doi: 10.1016/s0006-3223(97)00479-4. [DOI] [PubMed] [Google Scholar]
- Arolt V, Lencer R, Purmann S, Schurmann M, Muller-Myhsok B, Krecker K, Schwinger E. Testing for linkage of eye tracking dysfunction to markers on chromosomes 6, 8, 9, 20, 22 in families multiply affected with schizophrenia. Am J Med Genet. 1999;88:603–606. [PubMed] [Google Scholar]
- Avila MT, Hong LE, Moates A, Turano KA, Thaker GK. Role of anticipation in schizophrenia-related pursuit initiation deficits. J Neurophysiol. 2006;95:593–601. doi: 10.1152/jn.00369.2005. [DOI] [PubMed] [Google Scholar]
- Baker CL, Hess RF, Zihl J. Residual motion perception in a “motion blind” patient assessed with limited-lifetime random dot stimuli. J Neurosci. 1991;11:451–461. doi: 10.1523/JNEUROSCI.11-02-00454.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RW, Honrubia V. Clinical neurophysiology of the vestibular system. F.A. Davis Company; Philadelphia: 1990. [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Estimating invisible target speed from neuronal activity in monkey frontal eye field. Nat Neurosci. 2003;6:66–74. doi: 10.1038/nn990. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Modification of saccades evoked by stimulation of frontal eye field during invisible target tracking. J Neurosci. 2004;24:3260–3267. doi: 10.1523/JNEUROSCI.4702-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Donnelly SF, Eason RD. Predictive velocity estimation in the pursuit reflex response to pseudo-random and step displacement stimuli in man. J Physiol. 1987;389:111–136. doi: 10.1113/jphysiol.1987.sp016649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton JJS, Cherkasova M, Lindgren K, Goff DC, Intriligator JM, Manoach DS. Anti-saccades and task-switching: studies of control processes in saccadic function in normal subjects and schizophrenic patients. Neurobiology of eye movements: from molecules to behavior. Ann N Y Acad Sci. 2002;956:250–263. doi: 10.1111/j.1749-6632.2002.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Pandita M, Thakkar K, Goff DC, Manoach DS. The relation between anti-saccade errors, fixation stability and prosaccade errors in schizophrenia. Exp Brain Res. 2008;186:273–282. doi: 10.1007/s00221-007-1235-2. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Smooth pursuit eye movement dysfunction in abstinent cocaine abusers. Clin Exp Res. 1997;21:910–915. [PubMed] [Google Scholar]
- Bell BB, Abel LA, Li W, Christian JC, Yee RD. Concordance of smooth pursuit and saccadic measures in normal monozygotic twins. Biol Psychiatry. 1994;36:522–526. doi: 10.1016/0006-3223(94)90616-5. [DOI] [PubMed] [Google Scholar]
- Biscaldi M, Gezeck S, Stuhr V. Poor saccadic control correlates with dyslexia. Neuropsy-chologia. 1998;36:1189–1202. doi: 10.1016/s0028-3932(97)00170-x. [DOI] [PubMed] [Google Scholar]
- Bogacki PA, Borkowska A, Wojanowska-Bogacka M, Rybakowski JK. Relationship between class I and II HLA antigens in schizophrenia and eye movement disturbances: a preliminary study. Neuropsychobiology. 2005;51:204–210. doi: 10.1159/000085595. [DOI] [PubMed] [Google Scholar]
- Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for Mendelian disease, future approaches for complex disease. Nat Genet. 2003;33(Suppl):228–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients. Arch Gen Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Ilg UJ, Thiele A, Distler C, Hoffmann KP. Eye position effects in monkey cortex. I. Visual and pursuit-related activity in extrastriate areas MT and MST. J Neurophsyiol. 1997;77:944–961. doi: 10.1152/jn.1997.77.2.944. [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual response of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- Brownstein J, Krastoshevsky O, McCollum C, Kundamal S, Matthysse S, Holzman PS, Mendell NR, Levy DL. Antisaccade performance is abnormal in schizophrenia patients but not in their biological relatives. Schizophr Res. 2003;63:13–25. doi: 10.1016/s0920-9964(02)00438-3. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Rieder RO. Biological heterogeneity and psychiatric research. Arch Gen Psychiatry. 1979;36:1163–1169. doi: 10.1001/archpsyc.1979.01780110017001. [DOI] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction in early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG, Curtis CE. Smooth pursuit and antisaccade performance evidence trait stability in schizophrenia patients and their relatives. Int J Psychophysiol. 2003;49:139–146. doi: 10.1016/s0167-8760(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG, Ones DS. Eye movement dysfunction in first-degree relatives of patients with schizophrenia: a meta-analytic evaluation of candidate endophenotypes. Brain Cogn. 2008;68:436–461. doi: 10.1016/j.bandc.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Marvasti FF, Ducharme JL, Walter JM, Israel ME, Krain A, Pavlovsky C, Hommer DW. Executive function oculomotor tasks in girls with ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39:644–650. doi: 10.1097/00004583-200005000-00019. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Bauer LO. Effects of antisocial personality, cocaine and opioid dependence, and gender on eye movement control. Psychol Rep. 2004;95:551–563. doi: 10.2466/pr0.95.2.551-563. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Nakayama K, Matthysse S, Holzman PS. Dependence of impaired eye tracking on deficient velocity discrimination of schizophrenia. Arch Gen Psychiatry. 1999a;56:155–161. doi: 10.1001/archpsyc.56.2.155. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci USA. 1999b;96:4724–4729. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Palafox G, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999c;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61:215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Holzman PS. Compromised late-stage motion processing in schizophrenia. Biol Psychiatry. 2004;55:834–841. doi: 10.1016/j.biopsych.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Chen Y, Grossman ED, Bidwell LC, Yurgelun-Todd D, Gruber SA, Levy DL, Nakayama K, Holzman PS. Altered posterior and prefrontal cortical activation during visual motion processing in schizophrenia. Cogn Affect Behav Neurosci. 2008;8:293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA. Saccades to moving targets in schizophrenia: evidence for normal posterior cortex functioning. Psychophysiology. 1996;33:650–654. doi: 10.1111/j.1469-8986.1996.tb02360.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE. Smooth-pursuit in schizophrenia: abnormalities of open-loop and closed-loop responses. Psychophysiology. 1994;31:79–86. doi: 10.1111/j.1469-8986.1994.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol. 1994;103:277–287. [PubMed] [Google Scholar]
- Clementz BA, Farber RH, Lam MN, Swerdlow NR. Ocular motor responses to unpredictable and predictable smooth pursuit stimuli among patients with obsessive-compulsive disorder. J Psychiatry Neurosci. 1996;21:21–28. [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Cestnick L, Krastoshevsky O, Krause V, Huang Z, Mendell NR, Levy DL. Schizophrenia patients show deficits in shifts of attention to different levels of global–local stimuli: evidence for magnocellular dysfunction. Schizophr Bull. 2009;35:1108–1116. doi: 10.1093/schbul/sbp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PM, Pivik RT. Abnormal visual–vestibular interaction and smooth pursuit tracking in psychosis. J Psychiatry Neurosci. 1991;16:30–40. [PMC free article] [PubMed] [Google Scholar]
- Couch FH, Fox JC. Photographic study of ocular movements in mental disease. Arch Neurol Psychiatry. 1934;34:556–578. [Google Scholar]
- Crawford TJ, Haegar B, Kennard C, Reveley MA, Henderson L. Saccadic abnormalities in psychotic patients. I. Neuroleptic-free psychotic patients. Psychol Med. 1995a;25:461–471. doi: 10.1017/s0033291700033389. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Haegar B, Kennard C, Reveley MA, Henderson L. Saccadic abnormalities in psychotic patients. II. The role of neuroleptic treatment. Psychol Med. 1995b;25:473–483. doi: 10.1017/s0033291700033390. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG. Saccadic disinhibition in patients with acute and remitted schizophrenia and their first-degree biological relatives. Am J Psychiatry. 2001;158:100–106. doi: 10.1176/appi.ajp.158.1.100. [DOI] [PubMed] [Google Scholar]
- Delord S, Ducato MG, Pins D, Devinck F, Thomas P, Boucart M, Knoblauch K. Psychophysical assessment of magno- and parvocellular function in schizophrenia. Vis Neurosci. 2006;23:645–650. doi: 10.1017/S0952523806233017. [DOI] [PubMed] [Google Scholar]
- Depatie L, O’Driscoll GA, Holahan A-LV, Atkinson V, Thavundayil JX, Kin NY, Lal S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Diefendorf AR, Dodge R. An experimental study of the ocular reactions of the insane from photographic records. Brain. 1908;31:451–489. [Google Scholar]
- Dodge R. Five types of eye movement in the horizontal meridian plane in the field of regard. Am J Physiol. 1903;8:307–329. [Google Scholar]
- Dursteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophsyiol. 1988;60:940–965. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Corr PJ, Das M, Zachariah E, Hughes C, Sumich AL, Rabe-Hesketh S, Sharma T. Smooth pursuit and antisaccade eye movements in siblings discordant for schizophrenia. J Psychiatr Res. 2004;38:177–184. doi: 10.1016/s0022-3956(03)00105-5. [DOI] [PubMed] [Google Scholar]
- Everling S, Krappman P, Pruess S, Brand A, Flohr H. Hypometric primary saccades of schizophrenics in a delayed response task. Exp Brain Res. 1996;111:289–295. doi: 10.1007/BF00227306. [DOI] [PubMed] [Google Scholar]
- Farber RH, Clementz BA, Swerdlow NR. Characteristics of open- and closed-loop smooth pursuit responses among obsessive-compulsive disorder, schizophrenia, and nonpsychiatric individuals. Psychophysiology. 1997;34:157–162. doi: 10.1111/j.1469-8986.1997.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Feil K. Smooth pursuit eye movement dysfunctions in remitted and acute schizophrenia: a failure to replicate. Department of Psychology, University of Minnesota; Minneapolis, MN: 1997. [Google Scholar]
- Freedman R, Adler LE, Leonard S. Alternative phenotypes for the complex genetics of schizophrenia. Biol Psychiatry. 1999;45:551–558. doi: 10.1016/s0006-3223(98)00321-7. [DOI] [PubMed] [Google Scholar]
- Friedman L, Jesberger J, Siever L, Thompson P, Mohs R, Meltzer H. Smooth pursuit performance in patients with affective disorders or schizophrenia and normal controls: analysis with specific oculomotor measures, RMS error and qualitative ratings. Psychol Med. 1995;25:387–403. doi: 10.1017/s003329170003628x. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Morita N, Fukushima K, Chiba T, Tanaka S, Yamashita I. Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. J Psychiatr Res. 1990;24:9–24. doi: 10.1016/0022-3956(90)90021-h. [DOI] [PubMed] [Google Scholar]
- Gagnon D, O’Driscoll GA, Petrides M, Pike GB. The effect of spatial and temporal information on saccades and neural activity in oculomotor structures. Brain. 2002;125:123–139. doi: 10.1093/brain/awf005. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Paus T, Grosbras M-H, Pike GB, O’Driscoll GA. Transcranial magnetic stimulation of frontal oculomotor regions during smooth pursuit. J Neurosci. 2006;26:458–466. doi: 10.1523/JNEUROSCI.2789-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RD, Dorus E, Ostrow D, Pandey GN, Davis JM, Levy DL. Mixture distributions in psychiatric research. Biol Psychiatry. 1984;19:935–961. [PubMed] [Google Scholar]
- Gooding DC. Antisaccade task performance in questionnaire-identified schizotypes. Schizophr Res. 1999;35:157–166. doi: 10.1016/s0920-9964(98)00120-0. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Basso MA. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn. 2008;68:371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. J Nerv Ment Dis. 2001;189:8–16. doi: 10.1097/00005053-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Miller MD, Kwapil TR. Smooth pursuit eye tracking and visual fixation in psychosis-prone individuals. Psychiatry Res. 2000;93:41–54. doi: 10.1016/s0165-1781(00)00113-x. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;130:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth pursuit eye movements elicited by micro-stimulation of the primate frontal eye field. J Neurophysiol. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- Green MF, Mintz J, Salveson D, Nuechterlein KH, Breitmeyer B, Light GA, Braff DL. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003;53:113–1119. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- Haraldsson HM, Ettinger U, Magnusdottir BB, Sigmundsson T, Sigurdsson E, Ingason A, Petursson H. COMT val158met genotype and smooth pursuit eye movements in schizophrenia. Psychiatry Res. 2009;169:173–175. doi: 10.1016/j.psychres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Heide W, Kurzidim K, Kompf D. Deficits of smooth pursuit eye movements after frontal and parietal lesions. Brain. 1996;119:1951–1969. doi: 10.1093/brain/119.6.1951. [DOI] [PubMed] [Google Scholar]
- Holzman PS. Perceptual aspects of psychopathology. In: Zubin J, Shagass C, editors. Neuro-biological aspects of psychopathology. Grune & Stratton; New York: 1969. pp. 144–178. [Google Scholar]
- Holzman PS. The role of psychological probes in genetic studies of schizophrenia. Schizophr Res. 1994;13:1–9. doi: 10.1016/0920-9964(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Holzman PS. Eye movements and the search for the essence of schizophrenia. Brain Res Rev. 2000;31:350–356. doi: 10.1016/s0165-0173(99)00051-x. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Matthysse S. The genetics of schizophrenia: a review. Psychol Sci. 1990;1:279–286. [Google Scholar]
- Holzman PS, Proctor LR, Hughes DW. Eye-tracking patterns in schizophrenia. Science. 1973;181:179–181. doi: 10.1126/science.181.4095.179. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW. Eye tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry. 1974a;31:143–151. doi: 10.1001/archpsyc.1974.01760140005001. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Yasillo NJ, Levy DL. Reply to Stevens. Science. 1974b;184:1203–1204. [Google Scholar]
- Holzman PS, Kringlen E, Levy DL, Proctor LR, Haberman S, Yasillo NJ. Abnormal-pursuit eye movements in schizophrenia: evidence for a genetic indicator. Arch Gen Psychiatry. 1977;34:802–805. doi: 10.1001/archpsyc.1977.01770190064005. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Kringlen E, Matthysse S, Flanagan SD, Lipton RB, Cramer G, Levin S, Lange K, Levy DL. A single dominant gene can account for eye tracking dysfunctions and schizophrenia in offspring of discordant twins. Arch Gen Psychiatry. 1988;45:641–647. doi: 10.1001/archpsyc.1988.01800310049006. [DOI] [PubMed] [Google Scholar]
- Hong LE, Avila MT, Adami H, Elliot A, Thaker GK. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophr Res. 2003;63:39–48. doi: 10.1016/s0920-9964(02)00388-2. [DOI] [PubMed] [Google Scholar]
- Hong LE, Avila MT, Thaker GK. Response to unexpected target changes during sustained visual tracking in schizophrenic patients. Exp Brain Res. 2005a;165:125–131. doi: 10.1007/s00221-005-2276-z. [DOI] [PubMed] [Google Scholar]
- Hong LE, Tagamets M, Avila MT, Wonodi I, Holcomb H, Thaker GK. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biol Psychiatry. 2005b;57:726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Hong LE, Turano KA, O’Neill J, Hao LIW, McMahon RP, Elliott A, Thaker GK. Refining the predictive pursuit endophenotype in schizophrenia. Biol Psychiatry. 2008;63:458–464. doi: 10.1016/j.biopsych.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C, Park S. Trajectory estimation in schizophrenia. Schizophr Res. 2000;45:83–92. doi: 10.1016/s0920-9964(99)00166-8. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Crawford TJ, Puri BK, Duncan LJ, Chapman M, Kennard C, Barnes TRE, Joyce EM. Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychol Med. 1998;28:685–692. doi: 10.1017/s0033291798006722. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Crawford TJ, Kennard C, Barnes TRE, Joyce EM. Smooth pursuit tracking over a structured background in first-episode schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2000;250:221–225. doi: 10.1007/s004060070011. [DOI] [PubMed] [Google Scholar]
- Hutton S, Cuthbert I, Crawford TJ, Kennard C, Barnes TRE, Joyce EM. Saccadic hypome-tria in drug-naïve and drug-treated schizophrenic patients: a working memory deficit? Psycho-physiology. 2001;38:125–132. [PubMed] [Google Scholar]
- Iacono W, Lykken D. Electro-oculographic recording and scoring of smooth pursuit and saccadic eye tracking: a parametric study using monozygotic twins. Psychophysiology. 1979;16:94–107. doi: 10.1111/j.1469-8986.1979.tb01451.x. [DOI] [PubMed] [Google Scholar]
- Iacono W, Peloquin LJ, Lumry AE, Valentine RH, Tuason VB. Eye tracking in patients with unipolar and bipolar affective disorders in remission. J Abnorm Psychol. 1982;91:35–44. doi: 10.1037//0021-843x.91.1.35. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Hong WL, Hommer DW, Hamburger SD, Castellanos FX, Frazier JA, Giedd JN, Gordon CT, Karp BI, McKenna K, Rapoport JL. Smooth pursuit eye movements in child onset schizophrenia: comparison with attention-deficit hyperactivity disorder and normal controls. Biol Psychiatry. 1996;40:1144–1154. doi: 10.1016/S0006-3223(95)00630-3. [DOI] [PubMed] [Google Scholar]
- Jones AM, Pivik RT. Abnormal visual–vestibular interactions in psychosis. Biol Psychiatry. 1983;18:45–61. [PubMed] [Google Scholar]
- Karoumi B, Ventre-Dominey J, Dalery J. Predictive saccade behavior is enhanced in schizophrenia. Cognition. 1998;68:B81–B91. doi: 10.1016/s0010-0277(98)00052-3. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Hochrein A, Uwer R, Bondy B. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am J Psychiatry. 2003;160:696–702. doi: 10.1176/appi.ajp.160.4.696. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Kortenkamp S, Iacono WG, Grove WM. Antisaccade performance in patients with schizophrenia and affective disorder. J Abnorm Psychol. 1997;106:468–472. doi: 10.1037//0021-843x.106.3.468. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Taylor J, Iacono WG, Hammer MA. Heritability of different measures of smooth pursuit eye tracking dysfunction: a study of normal twins. Psychophysiology. 2000;37:724–730. [PubMed] [Google Scholar]
- Keating EG. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp Brain Res. 1991;86:311–323. doi: 10.1007/BF00228954. [DOI] [PubMed] [Google Scholar]
- Keating EG. Lesions of the frontal eye field impair pursuit eye movements, but preserve the predictions driving them. Behav Brain Res. 1993;53:91–104. doi: 10.1016/s0166-4328(05)80268-2. [DOI] [PubMed] [Google Scholar]
- Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Res. 2006;146:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Keefe R, Silverman J, Mohs R, Siever L, Harvey P, Friedman L, Roitman S, DuPre R, Smith C, Schmeider J, Davis K. Eye tracking, attention, and schizotypal symptoms in nonpsy-chotic relatives of patients with schizophrenia. Arch Gen Psychiatry. 1997;54:169–176. doi: 10.1001/archpsyc.1997.01830140081014. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18:537–542. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol. 1989;62:31–47. doi: 10.1152/jn.1989.62.1.31. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. Temporal properties of visual motion signals for the initiation of smooth-pursuit eye-movements in monkeys. J Neurophysiol. 1994;72:150–162. doi: 10.1152/jn.1994.72.1.150. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Gut-Fayand A, Amado I, Daban C, Bourdel M, Poirier MF, Berthoz A. Impairment of predictive saccades in schizophrenia. Neuroreport. 2001;12:465–469. doi: 10.1097/00001756-200103050-00009. [DOI] [PubMed] [Google Scholar]
- Kumra S, Sporn A, Hommer DW, Nicolson R, Thaker G, Israel E, Lenane M, Bedwell J, Jacobsen LK, Gochman P, Rapoport JL. Smooth pursuit eye-tracking impairment in childhood-onset psychotic disorders. Am J Psychiatry. 2001;158:1291–1298. doi: 10.1176/appi.ajp.158.8.1291. [DOI] [PubMed] [Google Scholar]
- Lander E. Splitting schizophrenia. Nature. 1988;336:105–106. doi: 10.1038/336105a0. [DOI] [PubMed] [Google Scholar]
- Larrison AL, Ferrante CF, Briand KA, Sereno AB. Schizotypal traits, attention and eye movements. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:357–372. doi: 10.1016/s0278-5846(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Larrison AL, Briand KA, Sereno AB. Nicotine improves antisaccade task performance without affecting prosaccades. Hum Psychopharmacol. 2004;19:409–419. doi: 10.1002/hup.604. [DOI] [PubMed] [Google Scholar]
- Larrison-Faucher A, Briand KA, Sereno AB. Delayed onset of inhibition of return in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:505–512. doi: 10.1016/s0278-5846(01)00298-6. [DOI] [PubMed] [Google Scholar]
- Latham C, Holzman PS, Manschrek TC, Tole J. Optokinetic nystagmus and pursuit eye movements in schizophrenia. Arch Gen Psychiatry. 1981;38:997–1003. doi: 10.1001/archpsyc.1981.01780340049006. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. Oxford University Press; New York: 2006. [Google Scholar]
- Lekwuwa GU, Barnes GR. Cerebral control of eye movements: the relationship between cerebral lesions sites and smooth pursuit deficits. Brain. 1996;119:473–490. doi: 10.1093/brain/119.2.473. [DOI] [PubMed] [Google Scholar]
- Lencer R, Trillenberg P, Trillenberg-Krecker K, Junghanns K, Kordon A, Broocks A, Hohagen F, Heide W, Arolt V. Smooth pursuit deficits in schizophrenia, affective disorder and obsessive-compulsive disorder. Psychol Med. 2004;34:451–460. doi: 10.1017/s0033291703001314. [DOI] [PubMed] [Google Scholar]
- Lencer R, Nagel M, Sprenger A, Heide W, Binkofski F. Reduced neuronal activity in the V5 complex underlies smooth-pursuit deficit in schizophrenia: evidence from an fMRI study. Neuroimage. 2005;24:1256–1259. doi: 10.1016/j.neuroimage.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Lencer R, Sprenger A, Harris MSH, Reilly JL, Keshavan MS, Sweeney JA. Effects of second-generation antipsychotic medication on smooth pursuit performance in antipsychotic-naive schizophrenia. Arch Gen Psychiatry. 2008;65:1146–1154. doi: 10.1001/archpsyc.65.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF, O’Driscoll GA. Smooth pursuit eye movement and schizotypy in the community. J Abnorm Psychol. 2006;115:779–786. doi: 10.1037/0021-843X.115.4.779. [DOI] [PubMed] [Google Scholar]
- LeVasseur AL, Flanagan JR, Riopelle RJ, Munoz DP. Control of volitional and reflexive saccades in Tourette’s syndrome. Brain. 2001;124:2045–2058. doi: 10.1093/brain/124.10.2045. [DOI] [PubMed] [Google Scholar]
- Levin S. Frontal lobe dysfunction in schizophrenia. I. Eye movement impairments. J Psychiatr Res. 1984;18:27–55. doi: 10.1016/0022-3956(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Levin S, Jones A, Stark L, Merrin EL, Holzman PS. Saccadic eye movements of schizophrenic patients measured by reflected light technique. Biol Psychiatry. 1982;17:1277–1287. [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Proctor LR. Vestibular responses in schizophrenia. Arch Gen Psychiatry. 1978;35:972–981. doi: 10.1001/archpsyc.1978.01770320066005. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Proctor LR. Vestibular dysfunction and psychopathology. Schizophr Bull. 1983;9:383–483. doi: 10.1093/schbul/9.3.383. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull. 1993;19:461–536. doi: 10.1093/schbul/19.3.461. [DOI] [PubMed] [Google Scholar]
- Levy DL, Lajonchere CM, Dorogusker B, Min D, Lee S, Tartaglini A, Lieberman JA, Mendell NR. Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophr Res. 2000;42:171–185. doi: 10.1016/s0920-9964(99)00122-x. [DOI] [PubMed] [Google Scholar]
- Levy DL, O’Driscoll G, Cook SR, Matthysse S, Holzman PS, Mendell NR. Antisaccade performance in biological relatives of schizophrenia patients: a meta-analysis. Schizophr Res. 2004;71:113–125. doi: 10.1016/j.schres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Li CR. Impaired detection of visual motion in schizophrenic patients. Prog Neuropsycho-pharmacol Biol Psychiatry. 2002;26:929–934. doi: 10.1016/s0278-5846(02)00207-5. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Levin S, Holzman PS. Horizontal and vertical pursuit eye movements, the oculocephalic reflex and the functional psychoses. Psychiatr Res. 1980;3:193–203. doi: 10.1016/0165-1781(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth-pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tyschen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance. J Neurosci. 2008;28:7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruff P, Danckert J, Pantelis C, Currie J. Saccadic and attentional abnormalities in patients with schizophrenia. Psychol Med. 1998;28:1091–1100. doi: 10.1017/s0033291798007132. [DOI] [PubMed] [Google Scholar]
- Matthysse S, Holzman PS. Genetic latent structure models: implications for research on schizophrenia. Psychol Med. 1987;17:271–274. doi: 10.1017/s003329170002479x. [DOI] [PubMed] [Google Scholar]
- Matthysse S, Parnas J. Extending the phenotype of schizophrenia: implications for linkage analysis. J Psychiatr Res. 1992;26:329–344. doi: 10.1016/0022-3956(92)90039-q. [DOI] [PubMed] [Google Scholar]
- Matthysse S, Holzman PS, Gusella J, Levy DL, Harte C, Jorgensen A, Moller L, Parnas J. Linkage of eye movement dysfunction to chromosome 6p in schizophrenia: A confirmation. Am J Hum Genet (Neuropsychiatric Genetics) 2004;128B:30–36. doi: 10.1002/ajmg.b.30030. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. Oculomotor delayed response task performance among schizophrenia patients. Neuropsychobiology. 1996;34:67–71. doi: 10.1159/000119294. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res. 1997;115:333–344. doi: 10.1007/pl00005702. [DOI] [PubMed] [Google Scholar]
- McKee SP. A local mechanism for differential velocity detection. Vision Res. 1981;21:491–500. doi: 10.1016/0042-6989(81)90095-x. [DOI] [PubMed] [Google Scholar]
- Morrow MJ, Sharpe JA. Deficits of smooth-pursuit eye movement after unilateral frontal lobe lesions. Ann Neurol. 1995;37:443–451. doi: 10.1002/ana.410370406. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Lasker AG, Cutting LE, Denckla MB, Zee DS. Oculomotor abnormalities in attention deficit hyperactivity disorder: a preliminary study. Neurology. 2001;57:423–430. doi: 10.1212/wnl.57.3.423. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Eggert T, Straube A. Internally and externally guided voluntary saccades in unmedicated and medicated schizophrenics. II. Saccadic latency, gain, and fixation suppression errors. Eur Arch Psychiatry Clin Neurosci. 1999;249:7–14. doi: 10.1007/s004060050059. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Armstrong IT, Hampton KA, Moore KD. Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J Neurophysiol. 2003;90:503–514. doi: 10.1152/jn.00192.2003. [DOI] [PubMed] [Google Scholar]
- Nagel M, Sprenger A, Nitschke M, Zapf S, Heide W, Binkofksi F, Lencer R. Different extraretinal neuronal mechanisms of smooth pursuit eye movements in schizophrenia: an fMRI study. Neuroimage. 2007;34:300–309. doi: 10.1016/j.neuroimage.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Tyler CW. Psychophysical isolation of movement sensitivity by removal of familiar position cues. Vision Res. 1981;21:427–433. doi: 10.1016/0042-6989(81)90089-4. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- O’Driscoll G, Callahan BL. Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain Cogn. 2008;68:359–370. doi: 10.1016/j.bandc.2008.08.023. [DOI] [PubMed] [Google Scholar]
- O’Driscoll G, Lenzenweger MF, Holzman PS. Antisaccades and smooth pursuit eye movements and schizotypy. Arch Gen Psychiatry. 1998;55:837–843. doi: 10.1001/archpsyc.55.9.837. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Benkelfat C, Florencio PS, Wolff AL, Joober R, Lal S, Evans AC. Neural correlates of eye tracking deficits in first-degree relatives of schizophrenic patients: a positron emission tomography study. Arch Gen Psychiatry. 1999;56:1127–1134. doi: 10.1001/archpsyc.56.12.1127. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Depatie L, Holahan ALV, Savion-Lemieux T, Barr RG, Jolicoeur C, Douglas VI. Executive functions and methylphenidate response in subtypes of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1452–1460. doi: 10.1016/j.biopsych.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Missal M, Lefèvre P. A dynamic representation of target motion drives predictive smooth pursuit during target blanking. J Vis. 2008;8(6):1–13. doi: 10.1167/8.15.6. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Grecu LM, Gangemi PF, Massi S, Parigi A, Arnetoli G, Quercioli L, Zaccara G. Smooth-pursuit eye movement and saccadic intrusions in obsessive-compulsive disorder. Biol Psychiatry. 1996;40:1164–1172. doi: 10.1016/s0006-3223(95)00607-9. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Quercioli L, Zaccara G, Ramacciotti AB, Arnetoli G. Eye movement abnormalities in anorexia nervosa. Psychiatry Res. 1998;78:59–70. doi: 10.1016/s0165-1781(97)00139-x. [DOI] [PubMed] [Google Scholar]
- Pantle A. Temporal frequency response characteristic of motion channels measured with three different psychophysical techniques. Percept Psychophys. 1978;24:285–294. doi: 10.3758/bf03206100. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS, Lenzenweger MF. Individual differences in spatial working memory in relation to schizotypy. J Abnorm Psychol. 1995;104:355–363. doi: 10.1037//0021-843x.104.2.355. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Merrigan WH. Motion perception following lesions of the superior temporal sulcus in the monkey. Cereb Cortex. 1994;4:247–259. doi: 10.1093/cercor/4.3.247. [DOI] [PubMed] [Google Scholar]
- Plant GT, Nakayama K. The characteristics of residual motion perception in the hemifield contralateral to lateral occipital lesions in humans. Brain. 1993;116:1337–1353. doi: 10.1093/brain/116.6.1337. [DOI] [PubMed] [Google Scholar]
- Radant AD, Claypoole K, Wingerson DK, Cowley DS, Roy-Byrne P. Relationship between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biol Psychiatry. 1997;42:797–805. doi: 10.1016/s0006-3223(96)00464-7. [DOI] [PubMed] [Google Scholar]
- Radant A, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, Freedman R, Green MF, Greenwood TA, Gur RE, Light GA, Meichle SP, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang MT, Turetsky BI, Tsuang DW. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophr Res. 2007;89:320–329. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Ram-Tsur R, Faust M, Caspi A, Gordon CR, Zivotofsky AZ. Evidence for oculomotor deficits in developmental dyslexia: application of the double-step paradigm. Invest Ophthalmol Vis Sci. 2006;47:4401–4409. doi: 10.1167/iovs.05-1657. [DOI] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C, Russell GFM. Action of a barbiturate drug (amylobarbitone sodium) on the vestibulo-ocular reflex. Brain. 1961;84:329–335. doi: 10.1093/brain/84.2.329. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal lobe lesions in humans. Exp Brain Res. 1994;102:110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Dick EL, O’Hearn KM, Sweeney JA. Response inhibition deficits in obsessive compulsive disorder: an indicator of dysfunction of frontostriatal circuits. J Psychiatry Neurosci. 1997;22:29–38. [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Hommer D, Breiger D, Varley C, Radant AD. Eye movement task related to frontal lobe functioning in children with attention deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1994;33:869–874. doi: 10.1097/00004583-199407000-00013. [DOI] [PubMed] [Google Scholar]
- Ross DE, Thaker GK, Holcomb HH, Cascella NG, Medoff DR, Tamminga CA. Abnormal smooth pursuit eye movements in schizophrenic patients are associated with cerebral glucose metabolism in oculomotor regions. Psychiatry Res. 1995;58:53–67. doi: 10.1016/0165-1781(95)02724-b. [DOI] [PubMed] [Google Scholar]
- Ross DE, Thaker GK, Buchanan RW, Lahti AC, Medoff D, Bartko JJ, Moran M, Thaker GK. Association of abnormal smooth pursuit eye movements with the deficit syndrome in schizophrenic patients. Am J Psychiatry. 1996;153:1158–1165. doi: 10.1176/ajp.153.9.1158. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Radant A. Amplitude criteria and anticipatory saccades during smooth pursuit eye movements in schizophrenia. Psychophysiology. 1999;36:464–468. [PubMed] [Google Scholar]
- Ross RG, Olincy A, Harris JG, Sullivan B, Radant A. Smooth pursuit eye movements in schizophrenia and attentional dysfunction: adults with schizophrenia, ADHD, and a normal comparison group. Biol Psychiatry. 2000;48:197–203. doi: 10.1016/s0006-3223(00)00825-8. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Hauser J. Dopamine D3 receptor (DRD3) gene polymorphism is associated with the intensity of eye movement disturbances in schizophrenic patients and healthy subjects. Mol Psychiatry. 2001;6:718–724. doi: 10.1038/sj.mp.4000927. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Hauser J. Eye movement disturbances in schizophrenia and a polymorphism of catechol-O-methyltransferase gene. Psychiatry Res. 2002;113:49–57. doi: 10.1016/s0165-1781(02)00245-7. [DOI] [PubMed] [Google Scholar]
- Sailer U, Eggert T, Strassnig M, Reidel M, Straube A. Predictive eye and hand movements are differentially affected by schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257:413–422. doi: 10.1007/s00406-007-0749-8. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Murasugi CM, Britten KH, Newsome WT. Microstimulation in visual area MT: effects on direction discrimination performance. J Neurosci. 1992;12:2331–2355. doi: 10.1523/JNEUROSCI.12-06-02331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalen L, Pyykko I, Korttila K, Magnusson M, Enbom H. Effects of intravenously given barbiturate and diazepam on eye motor performance in man. Adv Otolaryngol. 1988;42:260–264. doi: 10.1159/000416119. [DOI] [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Selectivity of macaque ventral intraparietal area (area VIP) for smooth pursuit eye movements. J Physiology. 2003;551:551–556. doi: 10.1113/jphysiol.2003.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker R, Cohen R, Berg P, Hubman W, Mohr F, Watzl H, Werther P. Smooth-pursuit eye movement dysfunction in schizophrenia: the role of attention and general psychomotor dysfunctions. Eur Arch Psychiatry Clin Neurosci. 1994;244:153–160. doi: 10.1007/BF02191891. [DOI] [PubMed] [Google Scholar]
- Schwartz BD, McGinn T, Winstead DK. Disordered spatiotemporal processing in schizophrenics. Biol Psychiatry. 1987;22:688–698. doi: 10.1016/0006-3223(87)90200-9. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry. 1995;37:394–401. doi: 10.1016/0006-3223(94)00127-O. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Babin SL, Hood AJ, Jeter CB. Executive functions: eye movements and neuro-psychiatric disorders. In: Squire LR, editor. Encyclopedia of neuroscience. Vol. 4. 2009. pp. 117–122. [Google Scholar]
- Sharpe JA, Morrow MJ. Cerebral hemispheric smooth pursuit disorders. Acta Neurol Belg. 1991;91:81–96. [PubMed] [Google Scholar]
- Sherr JD, Myers CS, Avila MT, Elliott A, Blaxton TA, Thaker GK. The effects of nicotine on specific eye tracking measures in schizophrenia. Biol Psychiatry. 2002;52:721–728. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- Shi DX, Friedman PR, Bruce CJ. Deficits in smooth pursuit eye movements after muscimol inactivation within the primate’s frontal eye field. Neurophysiology. 1998;80:458–464. doi: 10.1152/jn.1998.80.1.458. [DOI] [PubMed] [Google Scholar]
- Skottun BC, Skoyles JR. Contrast sensitivity and magnocellular function in schizophrenia. Vision Res. 2007;47:2923–2933. doi: 10.1016/j.visres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Slaghius WL. Contrast sensitivity for stationary and drifting frequency gratings in positive-and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Bowling AC, French RV. Smooth pursuit eye movement and direction motion contrast sensitivity in schizophrenia. Exp Brain Res. 2005;166:89–101. doi: 10.1007/s00221-005-2347-1. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Hawkes A, Holthouse T, Bruno R. Eye movement and visual motion perception in schizophrenia I: apparent motion evoked smooth pursuit eye movement reveals a hidden dysfunction in smooth pursuit eye movement in schizophrenia. Exp Brain Res. 2007a;182:399–413. doi: 10.1007/s00221-007-1000-6. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Holthouse T, Hawkes A, Bruno R. Eye movement and visual motion perception in schizophrenia II: global coherent motion as a function of target velocity and stimulus density. Exp Brain Res. 2007b;182:415–426. doi: 10.1007/s00221-007-1003-3. [DOI] [PubMed] [Google Scholar]
- Smyrnis N, Kattoulas E, Evdokomidis I, Stefanis NC, Avramopoulos D, Pantes G, Theleritis C, Stefanis CN. Active fixation performance in 940 young men: effects of IQ, schizotypy, anxiety and depression. Exp Brain Res. 2004;156:1–10. doi: 10.1007/s00221-003-1759-z. [DOI] [PubMed] [Google Scholar]
- Spengler D, Trillenberg P, Sprenger A, Nagel M, Kordon A, Junhanns K, Heide W, Arolt V, Hohagen F, Lencer R. Evidence from increased anticipation of predictive saccades for a dysfunction of front-striatal circuits in obsessive compulsive disorder. Psychiatry Res. 2006;143:77–88. doi: 10.1016/j.psychres.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Stevens J. Letter in response to eye tracking patterns in schizophrenia. Science. 1974;184:1201–1202. [PubMed] [Google Scholar]
- Stuve TA, Friedman L, Jesberger JA, Gilmore GC, Strauss ME, Meltzer HY. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol Med. 1997;27:143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, Carl JR. Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biol Psychiatry. 1998a;44:698–708. doi: 10.1016/s0006-3223(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Strojwas MH, Mann JJ, Thase ME. Prefrontal and cerebellar abnormalities in major depression: evidence from oculomotor studies. Biol Psychiatry. 1998b;43:584–594. doi: 10.1016/s0006-3223(97)00485-x. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry. 1999;46:671–680. doi: 10.1016/s0006-3223(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Levy DL, Harris MSH. Eye movement research with clinical populations. In: Hyona J, Munoz DP, Heide W, Radach R, editors. Progress in brain research. Brain’s eyes: neurobiological and clinical aspects of oculomotor research. Vol. 140. Elsevier Science; Amsterdam: 2002. pp. 507–522. [DOI] [PubMed] [Google Scholar]
- Tanabe JL, Tregellas JR, Martin LF, Freedman R. Effects of nicotine on hippocampal and cingulate activity during smooth pursuit eye movement in schizophrenia. Biol Psychiatry. 2006;59:754–761. doi: 10.1016/j.biopsych.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol. 1998;80:28–47. doi: 10.1152/jn.1998.80.1.28. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Regulation of the gain of visually guided smooth-pursuit eye movements by frontal cortex. Nature. 2001;409:191–194. doi: 10.1038/35051582. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. II. Relation to vector averaging pursuit. J Neurophysiol. 2002a;87:2700–2714. doi: 10.1152/jn.2002.87.6.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. I. Basic response properties to retinal image motion and position. J Neurophsyiol. 2002b;87:2684–2699. doi: 10.1152/jn.2002.87.6.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Enhancement of multiple components of pursuit eye movement by microstimulation in the arcuate frontal pursuit area in monkeys. J Neurophysiol. 2002c;87:802–818. doi: 10.1152/jn.00409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Nguyen JA, Tamminga CA. Increased saccadic distractibility in tardive dyskinesia: functional evidence for subcortical GABA dysfunction. Biol Psychiatry. 1989;25:49–59. doi: 10.1016/0006-3223(89)90146-7. [DOI] [PubMed] [Google Scholar]
- Thaker G, Cassady S, Adami H, Moran M, Ross D. Eye movements in spectrum personality disorders: comparison of community subjects and relatives of schizophrenic patients. Am J Psychiatry. 1996a;153:362–368. doi: 10.1176/ajp.153.3.362. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Buchanan RW, Moran M, Lahti AC, Kim CE. Does pursuit abnormality in schizophrenia represent a deficit in the predictive mechanism? Psychiatry Res. 1996b;59:221–237. doi: 10.1016/0165-1781(95)02759-9. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, Lahti AC. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55:830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Buchanan RW, Adami HM, Medoff DR. Smooth pursuit eye movements to extraretinal motion signals: deficits in patients with schizophrenia. Psychiatry Res. 1999;88:209–219. doi: 10.1016/s0165-1781(99)00084-0. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Avila MT, Hong LE, Medoff DR, Ross DE, Adami HM. A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology. 2003;40:277–284. doi: 10.1111/1469-8986.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK, Wonodi I, Avila MT, Hong LE, Stine OC. Catechol O-methyltransferase polymorphism and eye tracking in schizophrenia: a preliminary report. Am J Psychiatry. 2004;161:2320–2322. doi: 10.1176/appi.ajp.161.12.2320. [DOI] [PubMed] [Google Scholar]
- Thier P, Erickson RG. Responses of visual tracking neurons from cortical area MST-l to visual, eye and head motion. Eur J Neurosci. 1992;4:539–553. doi: 10.1111/j.1460-9568.1992.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Thier P, Ilg UJ. The neural basis of smooth-pursuit eye movements. Curr Opin Neurobiol. 2005;15:645–652. doi: 10.1016/j.conb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Thurston SE, Leigh RJ, Crawford TJ, Thompson A, Kennard C. Two distinct deficits of visual tracking caused by unilateral lesions of cerebral cortex in humans. Ann Neurol. 1988;23:266–273. doi: 10.1002/ana.410230309. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry. 2004;161:315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- Trillenberg P, Heide W, Junghanns B, Blankenburg M, Arolt V, Kompf D. Target anticipation and impairment of smooth pursuit eye movements in schizophrenia. Exp Brain Res. 1998;120:316–324. doi: 10.1007/s002210050405. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Maunsell JHR. Hierarchical organization and the functional streams in the visual system. Trends Neurosci. 1983;6:370–375. [Google Scholar]
- Vandenberg AV. Human smooth pursuit during transient perturbations of predictable and unpredictable target movement. Exp Brain Res. 1988;72:95–108. doi: 10.1007/BF00248504. [DOI] [PubMed] [Google Scholar]
- Warren S, Ross RG. Deficient cancellation of the vestibular ocular reflex in schizophrenia. Schizophr Res. 1998;34:187–193. doi: 10.1016/s0920-9964(98)00101-7. [DOI] [PubMed] [Google Scholar]
- Wertheim AH, Van Gelder P, Peselow LE, Cohen N. High thresholds for movement perception in schizophrenia may indicate abnormal extraneous noise levels of central vestibular activity. Biol Psychiatry. 1985;20:1197–1210. doi: 10.1016/0006-3223(85)90178-7. [DOI] [PubMed] [Google Scholar]
- White HR. Ocular pursuit in normal and psychopathological subjects. J Exp Psychol. 1938;22:17–31. [Google Scholar]
- Winograd-Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB. Oculo-motor differences between melancholic and non-melancholic depression. J Affect Disord. 2006;93:193–203. doi: 10.1016/j.jad.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Komatsu H, Yamasaki DSG, Dursteler MR. Cortical visual processing for oculomotor control. In: Cohen B, Bodis-Wollner I, editors. Vision and brain. Raven Press; New York: 1990. pp. 211–231. [Google Scholar]
- Xiao Q, Barborica A, Ferrera VP. Modulation of visual responses in macaque frontal eye field during covert tracking of invisible targets. Cereb Cortex. 2003;17:918–928. doi: 10.1093/cercor/bhl002. [DOI] [PubMed] [Google Scholar]
- Yee R, Baloh R, Marder S, Levy DL, Sakala S, Honrubia V. Eye movements in schizophrenia. Invest Ophthalmol Vis Sci. 1987;28:366–374. [PubMed] [Google Scholar]
- Zeki SM. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol. 1974;236:549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]