Abstract
This study examined the similarity of epilimnetic bacterial community composition (BCC) across several within- and among-lake spatial scales, and the environmental factors giving rise to similar bacterial communities in different lakes were also explored. Samples were collected from 13 northern and southern Wisconsin lakes representing gradients in lake size, productivity, dissolved organic carbon and humic acid contents, and pH. Hypotheses regarding patchy distribution of bacterial communities in lakes were tested by comparing samples collected from nearby (tens of meters) and distant (hundreds of meters) sampling sites in the same lake. BCC was characterized by using a molecular fingerprinting technique, automated ribosomal intergenic spacer analysis (ARISA). Overall, samples collected at the 10-m, 100-m, and between-lake scales differed by 13, 17, and 75%, respectively. Variation at these last two scales was significant. The development of within-lake variation in BCC appeared to depend on the isolation of water by lake shoreline features such as bays or narrow constrictions. ARISA profiles from northern lakes had fewer peaks and were less similar to each other than were those of the southern lakes, suggesting that regional features do not necessarily lead to the development of similar bacterial communities. Lakes at similar positions on productivity and dissolved organic carbon concentration gradients had similar bacterial communities, and bacterial diversity was positively correlated with lake productivity and water temperature. Factorial studies taking into account these gradients, as well as regional spatial scales, should provide much insight into the nature of aquatic bacterial biogeography.
The purpose of this study was to describe changes in bacterial community composition (BCC) at several within- and among-lake spatial scales. In addition, this study sought to investigate whether lakes with similar chemical and physical properties harbor similar bacterial communities and to determine the features of lakes most important in structuring BCC. The answers to these questions are of significance for the design and implementation of studies attempting to describe BCC in freshwater systems.
Previous work has shown variation in BCC over different depths in lakes (1, 3, 12, 17, 33, 35, 36), but little attention has been paid to horizontal variation. Earlier work (16, 17, 29) suggested that bacterioplankton may be distributed in patches varying in size from <1 to >20 m, but these studies were based on plate counts of bacteria and thus contained no information about the composition of bacterial communities. In addition, organisms responding to the growth media used for plate counts may not be representative of natural communities (37), and thus, the results of these studies may not be characteristic of whole-community behavior. Culture-independent molecular methods of microbial ecology can shed light on the variation of BCC for organisms not easily cultured.
While little is known about horizontal variation of BCC within lakes, there is much evidence that BCC is extremely variable between lakes (4, 11, 18, 22, 23, 28, 34, 38). Different chemical and physical properties of these systems have been implicated as the primary causes of the observed differences in BCC. Among these are pH (24, 28), aluminum chemistry (28), humic acid content (22, 24), and lake trophic status (22, 38). In addition, there may be a regional component to variation in BCC (24) despite evidence that some bacterial clades have widespread distributions (10, 39). Determining which environmental factors correlate with changes in BCC requires data from a number of different lakes spread across several environmental gradients. In addition, examining lakes from different regions can help define the scale at which important community structuring forces act. Considerations of scale and knowledge of how biological system behavior changes across different scales of investigation are important for the design of informative studies in microbial ecology.
The present study investigated changes in BCC and its potential environmental correlates over several nested spatial scales. Specifically, this study sought to determine (i) how variation in BCC changes across different within- and between-lake spatial scales and (ii) what environmental conditions give rise to similar bacterial communities at larger (i.e., among-lake and region) spatial scales.
MATERIALS AND METHODS
Study design.
Data were collected from 13 lakes in northern and southern Wisconsin in July 2001 (Fig. 1A to C). The lakes were part of the North Temperate Lakes Long-Term Ecological Research study site (25). These lakes differed from each other in a number of chemical and physical parameters, including inorganic and organic nutrient content, pH, productivity, and size (Table 1). The lakes in southern Wisconsin were all part of the Yahara River chain of lakes (Fig. 1C). Little Rock Lake, in northern Wisconsin (Fig. 1B), was the site of a prior whole-lake manipulation (2), and it was still divided into two separate basins by a plastic curtain during the course of the present study.
FIG. 1.
Map and spatial details of the study design. (A) State map of Wisconsin showing the locations of the northern (Trout Lake) region and the southern (Madison Lakes) region. (B and C) Maps of the northern Trout Lake region (B) and the southern Madison Lakes region (C) showing lakes, stream connections, and study basins. The numbers within each study lake correspond to the basins described in Tables 1, 3, and 4. (D) Detail of the study design with Trout Lake as an example. The relationship of basin level and station level samples is shown. Numbers correspond to basins, and the points labeled A, B, and C correspond to the station level samples. Station names in bold and italics (e.g., 24B) indicate primary stations at which environmental samples were collected.
TABLE 1.
Study design and lake detailsa
| Lake name (abbreviation), no. of basins | Lat | Long | SA | z | P | N | DOC | Chl a | pH |
|---|---|---|---|---|---|---|---|---|---|
| Southern lakes | |||||||||
| Kegonsa (KE), 2 | 42.95 | 89.24 | 1,298.6 | 9.0 | 26.5 | 714 | 5.7 | 26.0 | 8.8 |
| Mendota (ME), 5 | 43.10 | 89.40 | 3,937.7 | 25.3 | 13.0 | 414 | 5.2 | 8.8 | 8.2 |
| Monona (MO), 4 | 43.05 | 89.37 | 132.4 | 22.5 | 15.2 | 567 | 5.4 | 11.9 | 8.2 |
| Waubesa (WA), 2 | 43.02 | 89.32 | 841.7 | 10.5 | 17.5 | 396 | 5.7 | 15.2 | 8.8 |
| Wingra (WI), 1 | 43.05 | 89.42 | 139.6 | 6.7 | 11.0 | 524 | 7 | 25.4 | 9.4 |
| Northern lakes | |||||||||
| Allequash (AL), 2 | 46.03 | 89.62 | 168.4 | 8.0 | 9.9 | 300 | 3.6 | 3.4 | 7.5 |
| Big Muskellunge (BM), 3 | 46.02 | 89.62 | 396.3 | 21.3 | 4.7 | 359 | 3.5 | 1.9 | 7.3 |
| Crystal bog (CB), 1 | 46.00 | 89.6 | 0.5 | 2.5 | 6.0 | 405 | 8.7 | 8.9 | 5.1 |
| Crystal Lake (CR), 1 | 46.00 | 89.62 | 36.7 | 20.4 | 1.0 | 196 | 2.3 | 1.9 | 6.0 |
| Little Rock (LR), 2 | 45.98 | 89.70 | 18.2 | 9.3 | 6.0 | 329 | 3.6 | 3.5 | 6.4 |
| Sparkling (SP), 2 | 46.00 | 89.70 | 64.0 | 20.0 | 2 | 264 | 3.4 | 1.6 | 7.3 |
| Trout bog (TB), 1 | 46.03 | 89.68 | 1.1 | 7.9 | 14 | 615 | 18.7 | 2.2 | 4.8 |
| Trout Lake (TR), 4 | 46.03 | 89.67 | 1,607.9 | 35.7 | 4.7 | 250 | 3 | 1.8 | 7.6 |
Lat is (N) latitude, Long is (W) longitude, SA is surface area (hectares), z is maximum depth (meters), P is total dissolved phosphorus (micrograms per liter), N is total dissolved nitrogen (micrograms per liter), DOC is in milligrams per liter, Chl a is epilimnetic chlorophyll a (micrograms per liter).
Ninety sampling stations were distributed throughout this set of lakes in order to accommodate a sampling strategy investigating variability on four different scales. From largest to smallest, these scales were designated as regional, lake, basin, and station levels.
The regional scale was used to investigate variability between northern and southern Wisconsin, and these two areas served as regional treatments. Eight lakes were located in Vilas County in northern Wisconsin, and five lakes were located in Dane County in southern Wisconsin (Table 1). Variation on the lake level scale was investigated by comparing samples collected from different lakes. Thus, there were 13 treatments at the lake level scale.
Basin and station level scales were used to investigate variability within lakes. There were 30 and 90 treatments for these two scales, respectively. To determine basin level variability, one to five basins were defined in each lake, depending on lake size and morphometry (Table 1). Because all five southern lakes were part of the Yahara River chain, a station was designated at the inflow and outflow of each lake (except for Lake Wingra, where only a single station was designated; Table 1).
Three stations were placed in each basin. These stations were arranged in the shape of an equilateral triangle centered on the deepest point of the basin and with sides approximately 30 to 40 m long. The distance between the centers of any two triangles (i.e., the distance between basin level samples) was at least 100 m. Thus, variability within lakes was determined on two scales, on the orders of magnitude of tens of meters (station level) and hundreds of meters (basin level). Figure 1 summarizes the design and scales of the spatial investigations.
Field sampling. (i) Bacterial community DNA.
All samples were collected in July 2001. Samples for bacterial community DNA analysis were collected from a column of water integrated from the surface of the lake down to either the bottom of the epilimnion (as defined by a temperature profile) or to the Secchi depth, whichever was shallower. Thus, only bacterial communities in the oxygenated portion of the photic zone were sampled. Water samples were prefiltered through a 10-μm nylon mesh (Spectrum), which excluded larger eukaryotes and filamentous and particle-associated bacteria. From 250 to 500 ml of water was then vacuum filtered through 0.2-μm-pore-size Nuclepore membrane filters (Supor-200; Gelman) to capture bacterial cells on the filters. Filters were placed into cryovials and frozen immediately in liquid nitrogen for transport back to the laboratory, where they were stored at −80°C until extracted with a FastPrep DNA purification kit (Bio 101, Inc.). Three replicate water samples were collected on filters at each station, resulting in a total of 270 filters.
(ii) Environmental data.
Each station was georeferenced by using a handheld global positioning system unit. In addition, data on the depth of the water column, the Secchi depth, and the depth of the epilimnion (based on a temperature profile) were collected at each station. The water temperatures at the surface, middle, and bottom of the epilimnion were recorded. All other environmental data were collected at a single, randomly determined station for each basin, under the assumption that the conditions were homogeneous on the station level scale. Dissolved oxygen concentrations were determined for the surface, middle, and bottom of the epilimnion by the Winkler titration method performed on site. Subsamples of water from the integrated water sample (see above) were filtered through 0.4-μm-pore-size polycarbonate membrane filters (Osmonics) for analysis of dissolved organic carbon (DOC), color, total dissolved nitrogen, total dissolved phosphorus, and concentrations of nitrate and ammonia. Up to 1 liter of water from the integrated samples was filtered through glass fiber filters (Whatman) in order to collect phytoplankton cells for analysis of chlorophyll a concentrations. The glass fiber filters and all other water samples were placed immediately on ice for transport back to the laboratory. At the laboratory, glass fiber filters were kept frozen and in the dark until analyzed. Water samples for total nitrogen and phosphorus were acidified with 1 ml of Optima HCl and then refrigerated. All other samples were refrigerated until analyzed.
Laboratory analyses. (i) ARISA.
Automated ribosomal intergenic spacer analysis (ARISA) is a method for constructing bacterial community fingerprints based on the length heterogeneity of the intergenic transcribed spacer region of bacterial rRNA operons (8). In the present study, ARISA profiles were assumed to be indicative of BCC, and variation between ARISA profiles generated from different samples was assumed to reflect variation in the respective bacterial communities. PCR for ARISA was carried out by the method of Fisher and Triplett (8) as modified by Yannarell et al. (38), except that 20 PCR cycles were used to minimize the PCR bias associated with substrate depletion and template reannealing (30, 32).
Alignment of ARISA profiles was performed with the help of Genotyper (version 2.1; PE Applied Biosystems). The peak heights and areas of all of the ARISA profiles collected from the same lake were normalized so that the total signal strength (in fluorescence units) was the same. To distinguish ARISA peaks from baseline noise, ARISA peaks were required to be between 390 and 1,000 bp in size, with normalized peak heights of at least 150 fluorescence units. To eliminate shoulder peaks, which are common PCR artifacts, peaks within 1.2 bp of higher peaks were eliminated from profiles. These criteria were strictly adhered to so that subjective case-by-case determinations of ARISA peak presence or absence did not influence the variation in ARISA profiles. Although peak height was used in this manner to separate ARISA peaks from baseline noise and other artifacts, it was recognized that peak heights were not indicative of relative abundances of bacterial species owing to PCR bias (30-32). Therefore, ARISA profiles were transformed into presence-absence arrays, with ARISA peaks defined by size in base pairs and scored as 1 if present and 0 if absent but observed elsewhere in the data set.
(ii) Environmental data.
DOC was analyzed by high-temperature combustion on a Shimadzu TOC-5000 analyzer using potassium hydrogen phthalate standards. Color was determined with a Kontron 930 spectrophotometer. Total dissolved nitrogen and total dissolved phosphorus were analyzed on a segmented flow colorimeter following potassium persulfate-sodium hydroxide digestion. Ammonium and nitrate-nitrite were analyzed on a segmented-flow colorimeter using a copper-cadmium column to oxidize nitrate to nitrite for colorimetric detection. Chlorophyll a was extracted from glass fiber filters with Optima methanol following maceration and homogenization. Chlorophyll a concentration was determined with a Kontron 930 spectrophotometer. Each of these analyses was conducted as outlined by the North Temperate Lakes Long-Term Ecological Research Site (25), and more detail about these protocols is available via the “online datasets” link at http://lter.limnology.wisc.edu/index.html.
Data analyses. (i) Bacterial community variability.
Nonparametric approaches to data analysis were used in this work to avoid problems arising from the nonnormal distribution of variables and from the low sample-to-variable ratio in the data sets (5, 7). The similarity of ARISA profiles derived from different bacterial communities was assessed by using Sorenson's index (26). Patterns in the sample-by-sample matrix of Sorenson's values (i.e., the similarity matrix) were explored by using nonmetric multidimensional scaling (MDS). This ordination technique has been shown to faithfully represent the relationships inherent in multivariate data sets, and it performs well regardless of nonlinearity in the relationships between variables (27). On MDS plots, the samples with the most similarity are represented on the plot with the points plotted closest together, and the samples with the least similarity are represented on the plot with the points located farthest apart (20). The degree to which the plot matched the underlying similarity matrix was assessed by using Kruskal stress formula 1 (21), with values of less than 0.1 representing good ordinations with little risk of misinterpretation (5). Because any given MDS calculation may fail to arrive at the best possible solution, 20 separate MDS calculations were performed for each plot by using random starting configurations, and the best solution was taken to be the one producing the lowest stress. Three-dimensional solutions were sought if two-dimensional solutions failed to produce an acceptably small stress.
MDS plots were also used to investigate the relationship between measured environmental variables and patterns in BCC. The loadings (i.e., correlation) of these variables on the MDS axes were determined by correlating MDS axis scores for each sample with the value of each environmental variable for that sample using the Pearson product-moment correlation coefficient (ρ).
(ii) Assessing patchiness at different scales.
In the present context, a patch is defined as a contiguous region of limited spatial extent in which BCC is distinct from that of other regions. Thus, samples collected from the same patch could be grouped for statistical comparison with samples from other patches in a fashion analogous to analysis of variance. The “scale” of patchiness would depend on the size of the patches, so patchiness on the station level scale could be inferred if samples collected from the same station were more similar to each other than to those collected from different stations. This would imply that patches were no bigger than the distance between stations (i.e., 30 to 40 m in the present case). These comparisons could be carried out for each scale of observation included in this study, with appropriate measures being taken to account for the fact that the spatial scales of observation were nested within one another.
For each level of investigation, each sample was assigned, a priori, to a group corresponding to the station, basin, lake, or region from which the sample was collected. To test the hypothesis that the within-group ARISA profile similarity was greater than among-group similarity, an analysis of similarity (ANOSIM) (5, 7) was conducted. ANOSIM is a nonparametric technique designed to allow formal statistical comparisons for multivariate data sets in a manner similar to analysis of variance. Briefly, this procedure uses the Sorenson similarity matrix to calculate R = (rB − rW)/[0.25 · n · (n − 1)]. rW is the average of all rank similarities for samples within the same group, rB is the average of all rank similarities for samples between different groups, and n is the total number of samples under consideration. Values of R near 1 indicate complete separation of sample groups, while values near 0 indicate no separation between groups (5). Having determined R, ANOSIM then randomly assigns samples to different groups to generate a null distribution for R, i.e., Monte Carlo tests (13), and to test whether within-group samples were more closely related to each other than would be expected at random.
Because the study design for the present work involved four levels of spatial investigation, with each level nested within one or more higher levels, the existence of patchiness at any given scale had consequences for the detection of patchiness on both higher and lower levels. For example, significant patchiness on the lake scale would render meaningless comparisons involving basins from different lakes. For higher levels of investigation, patchiness could lead to a problem equivalent to pseudoreplication (5, 15). For this analysis, patchiness was always assumed a priori to exist at the next higher level of investigation, and global ANOSIM was always conducted with treatment levels nested within the next higher level of aggregation, as described by Clarke (5) for nested ANOSIM. This method constrained Monte Carlo randomization of samples within the nested group so that, for example, samples could be assigned to a different basin within the same lake but would never be randomly assigned to a basin in another lake. To avoid problems of pseudoreplication, some replicate samples were aggregated as described by Clarke (5). For example, if significant basin level patchiness was found, then all samples from the same basin were aggregated into a single replicate for the lake scale analysis; if no patchiness was found at the basin level, then basin samples were not aggregated.
(iii) ARISA fragment sensitivity analysis.
The Sorenson similarity index, which applies a presence-absence transformation to data, can be disproportionately affected by the behavior of rare data elements, or ARISA fragments in this case (6). To investigate the influence of rare ARISA fragments on the ability to detect patchiness, a sensitivity analysis was conducted by using the data set collected for Lake Monona, one of the large southern lakes (Table 1). Nested ANOSIM (stations within basins) was repeated for this lake by using data sets from which rare ARISA fragments had been removed. An ARISA fragment was eliminated from a profile if its peak area constituted less than 0.1, 0.5, 1, 2, and 3% of the total signal; thus, five new data sets were generated corresponding to successive levels of sensitivity from 0.1 through 3%. The influence of these removals was assessed for (i) mean ARISA fragment richness of samples, (ii) mean similarity of replicate samples, and (iii) the results of station and basin level ANOSIM analyses.
RESULTS
ARISA profiles derived from replicate samples collected from the same sampling station were, on average, 86.9% ± 0.6% (standard error [SE]; n = 86) similar to each other. The different within-lake scales of aggregation had little effect on ARISA profile similarity. On average, ARISA profile similarity was 87.8% ± 0.7% (SE; n = 30) for samples collected in the same basin and 86.3% ± 1.6% (SE; n = 13) for samples collected from the same lake. Samples from lakes in the southern region were more similar to each other than were samples collected from lakes in the northern region, and southern lakes had higher average ARISA fragment richness than did northern lakes (Fig. 2).
FIG. 2.
Mean Sorenson similarity and ARISA fragment richness of ARISA profiles obtained from northern and southern lakes. Bars represent SE, with 10 (south) and 28 (north) comparisons for similarity and 5 (south) and 8 (north) lakes comparisons for richness.
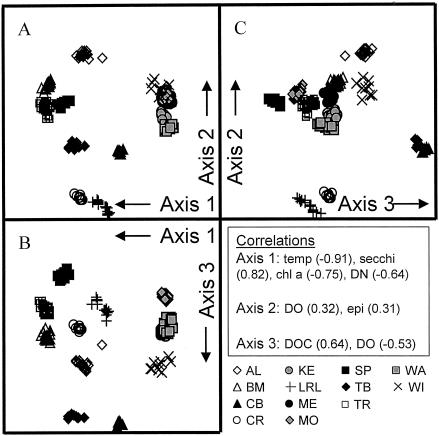
The greatest component of variation between bacterial communities was related to differences between lakes rather than within lakes. The average dissimilarity (i.e., dissimilarity = 100 − similarity) of samples collected from different stations within the same basin was 13.0% ± 1.8% (SE; n = 30 basins), and the average dissimilarity of samples collected from different basins within the same lake was 17.0% ± 3.5% (SE; n = 9 lakes). However, the average dissimilarity of samples collected from different lakes was 74.8% ± 9.3% (SE; n = 2 regions). Three-dimensional nonmetric MDS of all ARISA profiles was sufficient to separate ARISA profiles into groups depending on which lake they were collected from (Fig. 3). The geographic distance between the lakes was not reflected in the distance between these lake groups on the plot, and neither was the spatial configuration of the lake groups on the plot representative of the position of the lakes in geographic space (compare Fig. 1 and 3). The first MDS axis clearly separated profiles of the northern lakes (high scores) from those of the southern lakes (low scores), and the axes of this plot were highly correlated to several measured environmental variables (Fig. 3). Axis 1 was highly negatively correlated with temperature, reflecting the fact that the average epilimnetic temperature of the southern lakes was 5°C higher than that of the northern lakes during the sampling period (data not shown). Additionally, axis 1 was negatively correlated with the chlorophyll a concentration and dissolved nitrogen and positively correlated with Secchi depth. Axis 2, while representing much of the variability in BCC in different lakes, was not highly correlated to any of the variables measured in this study. Axis 3 was positively correlated with DOC and negatively correlated with dissolved oxygen.
FIG. 3.
Three-dimensional nonmetric MDS plot for ARISA profiles collected from study lakes. Stress = 0.09. The symbols are coded to represent the lakes from which profiles were obtained. Lake abbreviations are listed in Table 1. The arrows indicate the directions in which scores on the various axes increase, and Pearson product-moment correlation coefficients of the axes with various environmental variables are provided in the insert. A, axes 1 and 2; B, axes 1 and 3; C, axes 2 and 3. temp, temperature; chl a, chlorophyll a; DN, dissolved nitrogen; DO, dissolved oxygen; epi, epilimnion.
Separate ordinations of ARISA profiles from the northern and southern regions underscored the variation in community composition between lakes (Fig. 4). Within both regions, a large component of variation in BCC was related to the thermal characteristics of the lakes, as reflected by water temperature and the depth of the epilimnion (Fig. 4).
FIG. 4.
Two-dimensional nonmetric MDS plots for ARISA fragments collected from northern and southern lakes. The symbols are coded to represent the lakes from which profiles were obtained. Lake name abbreviations are listed in Table 1. Because plots were generated from separate ordinations, absolute distances on different plots cannot be considered equal. The arrows indicate the directions in which scores on the various axes increase, and Pearson product-moment correlation coefficients of the axes with various environmental variables are provided. (A) Profiles for northern lakes. Stress = 0.12. (B) Profiles for southern lakes. Stress = 0.13. epi, epilimnion; temp, temperature; DP, dissolved phosphorus; DN, dissolved nitrogen; chl a, chlorophyll a.
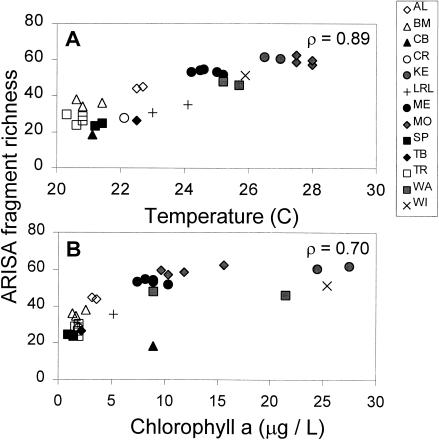
Across both regions, there were significant positive relationships between ARISA fragment richness and several environmental variables (Fig. 5). For each basin, the mean ARISA fragment richness was positively correlated with water temperature (Fig. 5A; ρ = 0.886, P ≪ 0.001), chlorophyll a (Fig. 5B; ρ = 0.698, P ≪ 0.001), and total dissolved nitrogen (data not shown; ρ = 0.661, P ≪ 0.001). Richness was also negatively correlated with Secchi depth (data not shown; ρ = −0.633, P ≪ 0.001).
FIG. 5.
Relationship between ARISA fragment richness and water temperature (A) or chlorophyll a (B). Data from each study basin are presented. ARISA fragment richness is the mean number of fragments seen at each primary station from which environmental samples were taken. ρ = Pearson product-moment correlation coefficient.
Patchiness at different scales.
Global ANOSIM tests (i.e., as opposed to pairwise tests) revealed significant evidence for patchiness at the basin and lake level scales, but no patchiness was detected at the station or regional level scales (Table 2). Individual pairwise contrasts between lakes all produced an R value of 1 (data not shown).
TABLE 2.
Global ANOSIM statistics for tests involving patchiness at four spatial scalesa
| Scale | Sample statistic R | No. of Monte Carlo permutations with scores ≥ Rb | P value |
|---|---|---|---|
| Stations (nested in basins) | −0.13 | 999 | 1.000 |
| Basins (nested in lakes) | 0.231 | 0 | 0.001 |
| Lakes (with basins nested) | 1.0 | 0 | 0.001 |
| Regions (with lakes nested) | −0.066 | 693 | 0.694 |
The magnitude of R represents the degree of separation between groups defined at the indicated scale (see Materials and Methods).
Out of 999.
Pairwise comparisons of basin level groupings (nested within lake level groups, but with replicate samples not aggregated by station) revealed that basin level patchiness in ARISA profiles depended on the lake, and there was detectable basin scale patchiness in the following lakes: Big Muskellunge, Little Rock, Mendota, Monona, and Trout (Table 3). The degree of basin level heterogeneity in these lakes was variable. Generally, differences between basins were small, as indicated by low R values (Table 3), and MDS plots of samples from these lakes reflected the incomplete separation of samples by basin (Fig. 6C and D). However, large differences between basins were seen in Little Rock Lake (basins 10 and 11) and in Trout Lake (basin 27 from each other basin; Table 3), and MDS plots clearly show this separation (Fig. 6A and B). In most cases, basins showing significant differences were separated to some degree from other basins by features of the lake shoreline morphometry (compare Fig. 1 and 6).
TABLE 3.
ANOSIM statistics for pairwise comparisons involving patchiness at the basin level scalea
| Lake, comparison | Sample statistic R | No. of Monte Carlo permutations with scores ≥ Rb | P value |
|---|---|---|---|
| Big Muskellunge Lake | |||
| Basin 3 vs basin 4 | 0.146 | 9 | 0.010 |
| Basin 4 vs basin 5 | 0.352 | 0 | 0.001 |
| Little Rock Lake, basin 10 vs basin 11 | 1.0 | 0 | 0.001 |
| Lake Mendota | |||
| Basin 12 vs basin 15 | 0.25 | 24 | 0.025 |
| Basin 14 vs basin 15 | 0.16 | 43 | 0.044 |
| Basin 15 vs basin 16 | 0.32 | 17 | 0.018 |
| Lake Monona | |||
| Basin 17 vs basin 19 | 0.196 | 26 | 0.027 |
| Basin 18 vs basin 19 | 0.247 | 11 | 0.012 |
| Trout Lake | |||
| Basin 24 vs basin 26 | 0.293 | 13 | 0.014 |
| Basin 24 vs basin 27 | 0.605 | 0 | 0.001 |
| Basin 25 vs basin 26 | 0.177 | 28 | 0.029 |
| Basin 25 vs basin 27 | 0.713 | 0 | 0.001 |
| Basin 26 vs basin 27 | 0.894 | 0 | 0.001 |
The magnitude of R represents the degree of separation between defined basin groups (see Materials and Methods). Only significant comparisons are shown.
Out of 999.
FIG. 6.
Nonmetric MDS plots for selected basin level analyses. For the spatial arrangement of the basins within these lakes, see Fig. 1. Depicted are the basin level plots for Little Rock Lake (A), Trout Lake (B), Lake Mendota (C), and Lake Monona (D). The numbers represent the basins from which the respective ARISA profiles were derived. Because plots were generated from separate ordinations, absolute distances on different plots cannot be considered equal. Stress values for plots are 0.01 (A), 0.17 (B), 0.13 (C), and 0.15 (D).
Sensitivity analysis.
Under the ARISA and Genotyper analysis conditions described in Materials and Methods, ARISA profiles from Lake Monona had an average richness of 58.22 ± 0.70 (SE; n = 36) fragments per profile. The ANOSIM for basin level heterogeneity was not affected until rarer fragments were removed whose signal strength (in terms of peak area) was less than 1% of the total signal, and this resulted in the loss of almost half of the ARISA fragments from profiles (Table 4). Removal of fragments whose signal strength was less than 2% of the total signal dramatically affected the results of the ANOSIM. However, under this criterion, less than 25% of ARISA fragments found in this lake were being included in the analysis (Table 4). Additionally, under this criterion the average similarity of profiles derived from the same basin was at its lowest (Table 4), and this was especially pronounced in basin 19 (where similarity dropped to 77.8%), the basin responsible for heterogeneity in the original analysis. Removal of peaks with a signal strength of less than 3% of the total signal, which left only 7.3 ± 0.2 (SE; n = 36) fragments per profile, yielded ANOSIM results similar to the original analysis (Table 4), and thus, there was detectable basin level heterogeneity in the distribution of these major community components in Lake Monona. ANOSIM results of station level analyses were not affected by any of these changes.
TABLE 4.
Results of sensitivity analysis with Lake Monona data set
| Category and comparison | Avg ARISA fragment richness (SE; n = 36)c | Avg similarity (%) of profiles from same basin (SE; n = 4) | ANOSIM statistic R |
|---|---|---|---|
| Peaks with < 0.1% of area removed | 57.78 (0.74) | 85.94 (1.23) | |
| Basin 17 vs basin 19 | 0.174a | ||
| Basin 18 vs basin 19 | 0.239b | ||
| Peaks with < 0.5% of area removed | 41.97 (0.43) | 88.57 (0.69) | |
| Basin 17 vs basin 19 | 0.156a | ||
| Basin 18 vs basin 19 | 0.275b | ||
| Peaks with < 1% of area removed | 30.75 (0.35) | 85.12 (0.73) | |
| Basin 17 vs basin 19 | 0.195a | ||
| Basin 18 vs basin 19 | 0.085 | ||
| Peaks with < 2% of area removed | 13.69 (0.28) | 82.35 (1.58) | |
| Basin 17 vs basin 19 | 0.008 | ||
| Basin 18 vs basin 19 | −0.046 | ||
| Peaks with < 3% of area removed | 7.31 (0.20) | 80.99 (0.75) | |
| Basin 17 vs basin 19 | 0.236b | ||
| Basin 18 vs basin 19 | 0.245b | ||
| Basin 19 vs basin 20 | 0.191a |
Difference significant at the α = 0.05 level.
Difference significant at the α = 0.01 level.
Richness is the number of fragments per profile.
DISCUSSION
Horizontal heterogeneity in lakes.
The horizontal heterogeneity in BCC seen in this study is similar to the variation in plate counts of bacteria noted by several previous studies (16, 17). Although there was evidence of significant horizontal heterogeneity in BCC at the scale of hundreds of meters (i.e., basins) in lakes, this finding was not consistent in all of the lakes investigated. In addition, the magnitude of the within-lake variation was small compared to the variability noted between lakes (Fig. 3 and 4). In all cases, the basins identified as significantly different were at least partially isolated from the other basins investigated, and this was especially true for the lakes showing the greatest degree of heterogeneity, Trout Lake and Little Rock Lake (Fig. 1 and 6). Basin 27 in Trout Lake is separated from the rest of the lake by a narrow constriction of water, and basin 26, which also displayed differences in BCC, is surrounded by islands. The most extreme instance of within-lake heterogeneity occurred in Little Rock Lake, where basins 10 and 11 were still separated by a plastic curtain from an earlier experiment in which basin 10 had been acidified through addition of sulfuric acid (2). Despite evidence that many features (including pH) of the two basins of Little Rock Lake have converged following the end of that lake's manipulation (9), residual effects of the acidification cannot be ruled out as potential causes of heterogeneity in BCC witnessed in this study. However, the observation of heterogeneity in other, nonmanipulated lakes, as well as the confinement of that heterogeneity to isolated basins in all of these lakes, strongly suggests that restricted water flow is a prerequisite for the development of different BCCs in different areas of a lake's epilimnion. It is likely that wind-induced circulation patterns serve to homogenize BCC across the lake's epilimnion, and if so, the heterogeneity noted here should be ephemeral in all but the most extreme cases. However, as the present study design used a single time point in each of these lakes, it is not possible to generalize about the stability of this patchiness.
The use of Sorenson's index can weight the results of a similarity analysis toward the rare elements (ARISA fragments in the present case [6]). In the present context of patchiness in BCC, this could have one of two potential effects. First, if these rare elements tend to be randomly or evenly (i.e., not patchily) distributed, then their inclusion would tend to contribute “noise” to the analysis and removal of these elements from the analysis would uncover patchiness in BCC that was previously hidden. On the other hand, if these rare elements are patchily distributed, then removing them from the analysis would confound the ability of ANOSIM to detect the pattern in the remaining community, particularly if only the rare elements have patchy distributions. The sensitivity analysis performed here for Lake Monona was more consistent with the latter scenario, as removing ARISA fragments whose signal strength constituted <1 and <2% of the total signal caused ANOSIM to fail to detect patchiness in BCC (Table 4). However, this does not indicate that only the rare ARISA fragments showed patchy distribution. The primary effect of removing the rarest ARISA fragments was to decrease the similarity of samples collected from the same basin rather than to increase the similarity of samples collected in different basins. This is analogous to increasing the variance of treatment groups in analysis of variance, and thus, the failure of ANOSIM to reject the null hypothesis in this case is likely due to decreased statistical power. In addition, ANOSIM performed with only the seven largest ARISA peaks in each profile gave results comparable to those done with the entire ARISA profiles. While PCR bias can distort the post-PCR ratio of templates (30, 31), making quantitative evaluation of ARISA profiles difficult, it is likely that these large ARISA peaks represented major components of the Lake Monona BCC. Thus, there is evidence that both the rare and the major components of this lake's bacterial communities showed a degree of patchiness in their distributions.
Palmer et al. (29), using dilution plate counts, concluded that samples collected at locations >20 m apart in Lake Washington would contain bacteria from different patches. In the present context, this would correspond to the station level scale, for which no evidence of patchiness was found (Table 2). It is likely that the dilution plating of Palmer et al. (29) uncovered patchiness in the population sizes of bacteria, and this patchiness would have been masked by the presence-absence transformation applied in the present study. It is also possible that the organisms responding to the growth media of Palmer et al. formed minor parts of the bacterial community as a whole, and they may not have been detected in the present work owing to the criteria used to distinguish ARISA fragments from noise. It should also be noted that in the present study the Sorenson distance between stations was similar to the Sorenson distance within stations (roughly 13% in both cases). This may indicate that variation in BCC exists at a finer spatial scale than was investigated here, and this variation is consistent across different sampling stations without giving rise to distinct patches of BCC. There is evidence that variability in BCC and bacterial abundance exists at a nanoscale (19). Such ultrafine-scale patchiness is almost certainly important to the biology of bacterioplankton, but since most sampling strategies would include many such nanoscale patches, it would have a minor influence in studies attempting to characterize BCC in lakes.
The detection of patchiness in these systems has implications for the design of studies examining BCC, but the importance of those implications changes dramatically over different scales of observation. Among-lake variation in BCC was by far the most significant source of variation witnessed in the present study, with samples obtained in the same lake having an average Sorenson dissimilarity of 13 to 17%, compared to an average dissimilarity of almost 75% for samples obtained from other lakes. Thus, within-lake variation could essentially be ignored in multilake studies, as long as samples from the lakes were obtained from areas with access to the free exchange of water. Even for investigations on the single-lake scale, within-lake patchiness may have little impact on the results. Most of the within-lake variation witnessed in the present study was associated with isolated parts of the lake, and so the existence of heterogeneity depends on where one looks for it. If water currents are responsible for homogenizing epilimnetic bacterial communities, then the heterogeneity observed in this study may be short-lived. However, more thorough fine-scale studies of horizontal heterogeneity in lakes are required before its impacts can be written off entirely, and such studies should include a temporal component to characterize the life spans and movement of community patches.
Among-lake variation.
The among-lake component was by far the largest component of variation in BCC observed in this study (Fig. 3 and 4). This result is consistent with previous work showing major differences in BCC among different lakes (11, 28, 38).
Despite the differences in BCC in different lakes, the ARISA profiles from lakes with similar characteristics clustered together on MDS plots, and the axes of these plots were often highly correlated with some of the environmental variables measured in this study. This suggests that similar bacterial communities form in lakes that are close together on certain important environmental gradients. The correlations with axis 1 of Fig. 3 suggest that this axis represents a productivity gradient, with the high-productivity, eutrophic southern lakes at one end and the lower-productivity, oligotrophic northern lakes at the other end. However, as all of the southern lakes in this study were eutrophic and all of the northern lakes were relatively unproductive, this inference must be approached with caution. A more factorial design, with productive and unproductive lakes from both regions of the state, is necessary to adequately separate out the influence of lake productivity from what may possibly be a correlated regional effect.
The correlations with axis 3 of Fig. 3 suggest that this axis represents a gradient related to the amount of available carbon substrates and the degree of net heterotrophy or net autotrophy in these lakes. Thus, it appears that lake productivity and the concentration of substrates available for metabolism represent two major factors structuring the composition of bacterial communities in lakes. In a study of Adirondack lakes, Methé and Zehr (28) found that bacterial assemblages were influenced by aluminum chemistry, pH, and DOC concentrations, and Lindström and Leskinen (24) confirmed that DOC and pH are important determinants of BCC in lakes. Other work has shown that bacterial assemblages in different lakes are influenced by food web interactions with phytoplankton, protoplankton, and zooplankton (22, 23, 34). Multilake studies investigating the effects of these forces on bacterial communities across productivity and DOC gradients should prove to be fruitful ground for microbial ecology.
Strikingly, ARISA fragment richness also appears to follow certain environmental gradients. ARISA profiles from the southern lakes were richer than those from the northern lakes (Fig. 2), and this presumably indicates that bacterial communities in southern lakes are more diverse. Reflecting this, ARISA profile richness was highly negatively correlated with axis 1 of Fig. 3 (ρ = −0.84) and highly correlated with water temperature (Fig. 5A). Additionally, ARISA profile richness was significantly correlated with factors relating to lake productivity, including chlorophyll a (Fig. 5B) and total dissolved nitrogen (data not shown). Horner-Devine et al. (14) investigated bacterial productivity-diversity relationships in experimental ponds and showed that, while there was no overall relationship between productivity and diversity, there were significant relationships when certain bacterial divisions were considered separately. Thus, there is evidence that the diversity of some bacterial clades may vary along a productivity gradient. These results, along with those of the present study, suggest that this is yet another promising area of investigation.
Regional-scale patterns.
Although there was no evidence of patchiness on the regional scale (Table 2), there were notable differences between northern and southern lakes. As already noted, southern lakes appeared to have more diverse bacterial communities than northern lakes. There was more variation in the bacterial communities of the northern lakes (Fig. 2), and this fact is likely responsible for the low R value in the regional ANOSIM analysis, as there was no distinct northern bacterial assemblage. In a survey of Scandinavian lakes, Lindström and Leskinen (24) also found that bacterial communities from lakes in some regions were very similar to each other, while in other regions BCC varied greatly from lake to lake. It may be a general feature of the biogeography of bacterioplankton that some regions harbor much more diversity than others. However, as in the present study, the study of Lindström and Leskinen (24) may have conflated regional effects on BCC with features of the lakes themselves (e.g., humic acid content). This underscores the need for factorial experiments undertaken at the regional scale.
During the study period, the southern lakes were warmer than the northern lakes by about 5°C, which reflects the difference in climate between these two regions. Southern Wisconsin lakes warm up earlier in the year and attain higher maximum temperatures than northern Wisconsin lakes. Another potential confounding factor in the present study is the fact that all of the southern lakes were linked together into a single flowage by the Yahara River. This overland connection may have contributed to the similarity in BCC in these lakes, and samples collected from these lakes may not necessarily be considered completely independent in terms of a regional analysis. However, three of the northern lakes, Trout Lake, Allequash Lake, and Big Muskellunge Lake, also had overland connections (Fig. 1), and BCC in the middle lake (Allequash) was quite distinct from that of the other two (Fig. 3). There was also no evidence that overland connections improved the similarity coefficients comparing BCC in a series of lakes in northeastern Indiana (18). Thus, it is more likely that the similarity in BCC noted in the southern lakes in the present study was due to the similar natures of these lakes (Table 1) and not to the overland connection. Nevertheless, the existence of several potential confounding factors means that any conclusions about regional differences between northern and southern lakes should be viewed with caution until more stringent tests have been applied.
Despite the lack of a significant regional ANOSIM result, and despite the caution with which conclusions on the regional scale must be drawn, the separation of northern and southern lakes on axis 1 of Fig. 3 suggests that bacterial communities may be subject to different structuring forces in the two regions. Separate MDS and environmental analysis of northern and southern regions (Fig. 4) revealed that thermal characteristics of the lakes, such as water temperature and the depth of the epilimnion, could influence the epilimnetic BCC within both regions. Because the present study used a single time point in each lake, and because all samples were collected in the same season (midsummer), it is unlikely that temperature, in any given region, reflects the impact of seasonal fluctuations, as has been demonstrated by previous authors (34, 38). In the present case, water temperature may be related to the size of the lake, with larger lakes showing cooler temperatures owing to the greater thermal inertia of larger volumes of water. However, it is also possible that water temperature has a direct selective effect on BCC in lakes, and this hypothesis bears further investigation. As has already been noted, temperature, along with productivity-related variables, was related to ARISA fragment richness across both regions (Fig. 5).
BCC in northern Wisconsin lakes also appears to be influenced by DOC, nitrogen concentration, and primary productivity (Fig. 4A), and this lends further support to the conclusion that lake productivity and substrate availability are important structuring gradients in lakes. Southern lakes appear to be influenced by phosphorus and nitrogen compounds (Fig. 4B), but the correlations are weak. The lack of evidence for strong structuring forces in southern lakes is most likely due to the similarity of these lakes (Table 1).
Conclusions.
This study has demonstrated that midsummer epilimnetic bacterial communities show variation at both within- and among-lake scales. Despite the evidence for patchiness in BCC in some of these lakes, by far the greatest portion of the variation was in the among-lake component. Heterogeneity in lakes may be ephemeral, and it appears to be largely the product of reduced water exchange between isolated portions of the lake. Thus, investigations of BCC involving many different lakes may not need to be overly concerned about within-lake horizontal heterogeneity unless there is reason to suspect that free exchanges of water are hampered in different parts of the lake. Among-lake variation in BCC appears to be structured by strong gradients of lake productivity and DOC availability, although regionalization of bacteria may play some role. There is a need for large-scale studies of BCC involving the simultaneous evaluation of regionalization, lake trophic status, and DOC availability.
Acknowledgments
This work was funded by NSF grants DEB 9977903, DEB 9632853, and DEB 0217533, awarded to the Center for Limnology at the University of Wisconsin—Madison.
We thank G. Lauster, A. Kent, T. Kratz, and J. Thoyre for valuable assistance with data collection and with preparation of the manuscript. We also thank J. Chipman for graciously producing the images for Fig. 1.
REFERENCES
- 1.Bosshard, P. P., R. Stettler, and R. Bachofen. 2000. Seasonal and spatial community dynamics in the meromictic Lake Cadagno. Arch. Microbiol. 174:168-174. [DOI] [PubMed] [Google Scholar]
- 2.Brezonik, P. L., L. A. Baker, J. R. Eaton, T. M. Frost, P. Garrison, T. K. Kratz, J. J. Magnuson, W. J. Rose, B. K. Shephard, W. A. Swenson, C. J. Watras, and K. E. Webster. 1986. Experimental acidification of Little-Rock Lake, Wisconsin. Water Air Soil Pollut. 31:115-121. [Google Scholar]
- 3.Casamayor, E. O., G. Muyzer, and C. Pedros-Alio. 2001. Composition and temporal dynamics of planktonic archaeal assemblages from anaerobic sulfurous environments studied by 16S rDNA denaturing gradient gel electrophoresis and sequencing. Aquat. Microb. Ecol. 25:237-246. [Google Scholar]
- 4.Casamayor, E. O., H. Schafer, L. Baneras, C. Pedros-Alio, and G. Muyzer. 2000. Identification of and spatio-temporal differences between microbial assemblages from two neighboring sulfurous lakes: comparison by microscopy and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 66:499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke, K. R. 1993. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18:117-143. [Google Scholar]
- 6.Clarke, K. R., and R. H. Green. 1988. Statistical design and analysis for a 'biological effects' study. Mar. Ecol. Prog. Ser. 46:213-226. [Google Scholar]
- 7.Field, J. G., K. R. Clarke, and R. M. Warwick. 1982. A practical strategy for analysing multispecies distribution patterns. Mar. Ecol. Prog. Ser. 8:37-52. [Google Scholar]
- 8.Fisher, M. M., and E. W. Triplett. 1999. Automated approach for ribosomal intergenic spacer analysis of microbial diversity and its application to freshwater bacterial communities. Appl. Environ. Microbiol. 65:4630-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost, T. M., P. K. Montz, and T. K. Kratz. 1998. Zooplankton community responses during recovery from acidification in Little Rock Lake, Wisconsin. Restor. Ecol. 6:336-342. [Google Scholar]
- 10.Glöckner, F. O., E. Zaichikov, N. Belkova, L. Denissova, J. Pernthaler, A. Pernthaler, and R. Amann. 2000. Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of actinobacteria. Appl. Environ. Microbiol. 66:5053-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiorns, W. D., B. A. Methé, S. A. NierzwickiBauer, and J. P. Zehr. 1997. Bacterial diversity in Adirondack Mountain lakes as revealed by 16S rRNA gene sequences. Appl. Environ. Microbiol. 63:2957-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Höfle, M. G., and I. Brettar. 1995. Taxonomic diversity and metabolic-activity of microbial communities in the water column of the central Baltic Sea. Limnol. Oceanogr. 40:868-874. [Google Scholar]
- 13.Hope, A. C. A. 1968. A simplified Monte Carlo significance test procedure. J. R. Stat. Soc. Ser. B 30:582-598. [Google Scholar]
- 14.Horner-Devine, M. C., M. A. Leibold, V. H. Smith, and B. J. M. Bohannan. 2003. Bacterial diversity patterns along a gradient of primary productivity. Ecol. Lett. 6:613-622. [Google Scholar]
- 15.Hurlbert, S. H. 1984. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 54:187-211. [Google Scholar]
- 16.Jones, J. G. 1977. The effect of environmental factors in estimated viable and total populations of planktonic bacteria in lakes and experimental enclosures. Freshwater Biol. 7:67-91. [Google Scholar]
- 17.Jones, J. G., and B. M. Simon. 1980. Variability in microbiological data from a stratified eutrophic lake. J. Appl. Bacteriol. 49:127-135. [Google Scholar]
- 18.Konopka, A., T. Bercot, and C. Nakatsu. 1999. Bacterioplankton community diversity in a series of thermally stratified lakes. Microb. Ecol. 38:126-135. [DOI] [PubMed] [Google Scholar]
- 19.Krembs, C., A. R. Juhl, R. A. Long, and F. Azam. 1998. Nanoscale patchiness of bacteria in lake water studied with the spatial information preservation method. Limnol. Oceanogr. 43:307-314. [Google Scholar]
- 20.Kruskal, J. B. 1964. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1-27. [Google Scholar]
- 21.Kruskal, J. B., and M. Wish. 1978. Multidimensional scaling. Sage Publications, Beverley Hills, Calif.
- 22.Lindström, E. S. 2000. Bacterioplankton community composition in five lakes differing in trophic status and humic content. Microb. Ecol. 40:104-113. [DOI] [PubMed] [Google Scholar]
- 23.Lindström, E. S. 2001. Investigating influential factors on bacterioplankton community composition: results from a field study of five mesotrophic lakes. Microb. Ecol. 42:598-605. [DOI] [PubMed] [Google Scholar]
- 24.Lindström, E. S., and E. Leskinen. 2002. Do neighboring lakes share common taxa of bacterioplankton? Comparison of the 16S rDNA fingerprints and sequences from three geographic regions. Microb. Ecol. 44:1-9. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson, J. J., T. K. Kratz, T. F. Allen, D. E. Armstrong, B. J. Benson, C. J. Bowser, D. W. Bolgrien, S. R. Carpenter, T. F. Frost, S. T. Gower, T. M. Lillesand, J. A. Pike, and M. G. Turner. 1997. Regionalization of long-term ecological research (LTER) on north temperate lakes. Verh. Int. Verein Limnol. 26:522-528. [Google Scholar]
- 26.Magurran, A. E. 1988. Ecological diversity and its measurement. Princeton University Press, Princeton, N.J.
- 27.McCune, B., and J. B. Grace. 2002. Analysis of ecological communities. MjM Software Design, Gleneden Beach, Oreg.
- 28.Methé, B. A., and J. P. Zehr. 1999. Diversity of bacterial communities in Adirondack lakes: do species assemblages reflect lake water chemistry? Hydrobiologia 401:77-96. [Google Scholar]
- 29.Palmer, F. E., J. R. Methot, and J. T. Staley. 1976. Patchiness in the distribution of planktonic heterotrophic bacteria in lakes. Appl. Environ. Microbiol. 31:1003-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polz, M. F., and C. M. Cavanaugh. 1998. Bias in template-to-product ratios in multitemplate PCR. App. Environ. Microbiol. 64:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki, M., M. S. Rappe, and S. J. Giovannoni. 1998. Kinetic bias in estimates of coastal picoplankton community structure obtained by measurements of small-subunit rRNA gene PCR amplicon length heterogeneity. Appl. Environ. Microbiol. 64:4522-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki, M. T., and S. J. Giovannoni. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbach, E., K. L. Vergin, L. Young, A. Morse, G. L. Larson, and S. J. Giovannoni. 2001. Unusual bacterioplankton community structure in ultra-oligotrophic Crater Lake. Limnol. Oceanogr. 46:557-572. [Google Scholar]
- 34.Van der Gucht, K., K. Sabbe, L. De Meester, N. Vloemans, G. Zwart, M. Gillis, and W. Vyverman. 2001. Contrasting bacterioplankton community composition and seasonal dynamics in two neighbouring hypertrophic freshwater lakes. Environ. Microbiol. 3:680-690. [DOI] [PubMed] [Google Scholar]
- 35.Vila, X., and C. A. Abella. 1999. Spectroradiometric identification of phototrophic microorganisms in planktonic aquatic environments. Aquat. Microb. Ecol. 20:225-230. [Google Scholar]
- 36.Vila, X., C. A. Abella, J. B. Figueras, and J. P. Hurley. 1998. Vertical models of phototrophic bacterial distribution in the metalimnetic microbial communities of several freshwater North-American kettle lakes. FEMS Microbiol. Ecol. 25:287-299. [Google Scholar]
- 37.von Wintzingerode, F., U. B. Gobel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]
- 38.Yannarell, A. C., A. D. Kent, G. L. Lauster, T. K. Kratz, and E. W. Triplett. Temporal patterns in bacterial communities in three temperate lakes of different trophic status. Microb. Ecol., in press. [DOI] [PubMed]
- 39.Zwart, G., W. D. Hiorns, B. A. Methé, M. P. Van Agterveld, R. Huismans, S. C. Nold, J. P. Zehr, and H. J. Laanbroek. 1998. Nearly identical 16S rRNA sequences recovered from lakes in North America and Europe indicate the existence of clades of globally distributed freshwater bacteria. Syst. Appl. Microbiol. 21:546-556. [DOI] [PubMed] [Google Scholar]