Abstract
As the nervous system ages, a variety of changes occur in metabolism supporting glial and neuronal function, resulting in greater susceptibility to disease conditions. Changes with aging in the metabolic unit (i.e., neurons, glial cells and blood vessels) have been reported to include alterations of vascular reactivity, impaired transport of critical substrates underlying metabolism, enhanced reactive oxygen species production and alterations in calcium signaling. Some diseases are focused on the elderly, particularly cerebral ischemia, cognitive limitations, iatrogenic hypoglycemia, malignant brain tumors (i.e., glioblastoma), and Alzheimer’s disease, partly due to metabolic alterations with aging. These metabolic changes with aging are discussed in light of primary theories of aging of the brain, which include mitochondrial, calcium dysfunction and enhanced oxidative damage. Here we focus on metabolic changes with aging which can influence the susceptibility of the brain to ischemia and cognitive function. Lastly, we describe treatment possibilities for these abnormal responses to aging, particularly the topic of caloric/dietary restriction, and possible mechanisms underlying this treatment direction.
Keywords: Aging, Mitochondrial dysfunction, ROS, NADH, Ischemia, LTP, Caloric restriction
The brain shows unique age-related changes in function and metabolism, particularly enhanced vulnerability to a number of insults and diseases [1–3]. This may be due to decreased production of new cells [4–6] or increased cellular damage associated with aging. A variety of diseases show a higher incidence during advanced age, including alterations in memory and cognitive function [7], as noted particularly in Alzheimer’s disease, Parkinson’s disease [8, 9], malignant intrinsic brain tumors (i.e., glioblastomas [10]; as well as ischemia and stroke.
Even in aged healthy individuals the brain’s innate ability to respond to metabolic challenges can be altered, resulting in enhanced cellular injury, fatigue, and altered cognitive function in response to energy substrate deprivation as well as increased metabolic demand [11–13]. Moreover, the loss of plasticity of neuronal stem cells with aging in response to milder partial deafferentation [6] can also contribute to the cognitive impairment with aging. Progressive deterioration of cellular homeostatic reserves and alterations in calcium-dependent signaling mechanisms have been proposed as some of the key events associated with brain aging [14–18]. Other processes associated with cellular aging include increased reactive oxygen species (ROS) production and mitochondrial dysfunction [19, 20].
There are two main approaches to describing underlying theories of aging [17]. The first is that there are programmed changes during aging which are genetically determined but which may be considerably delayed in onset. Such programmed changes may be included under the concept of involution or programmed senescence, similar to the concept of progressive telomere shortening with each cell division. The second general approach includes alterations of cells resulting from damage or errors which accumulate over time (i.e., wear and tear changes). Such changes occur in joints, for example, due to wear rates occurring faster than repair rates. In the nervous system, it is likely that enhanced susceptibility to metabolic stress may be due to a combination of factors derived from both genetically programmed alterations in metabolism as well as accumulated damage from a variety of sources, such as reactive oxygen species and secondary target damage, nuclear and mitochondrial DNA mutations (from causes such as radiation damage, faulty repair, etc), and differences in Ca2+ buffering and signaling.
This review has four main aspects. We will first describe a number of general aging-related metabolic changes which can lead to impaired metabolism, as suggested by theories of aging. Next, we will discuss two specific areas of impairment commonly noted in aging: enhanced vulnerability to ischemic injury (i.e., stroke) and alterations in cognitive function both at the cellular and systems level. Lastly, we will discuss mechanisms of treatments which have been studied, to attempt to reverse age-related alterations, particularly the topic of caloric restriction. Our conclusion is that genetically programmed changes in brain function and repair, in combination with acquired aging damage can lead to impaired responses to metabolic stressors, and that some of these changes can be treated or possibly even prevented.
Aging and metabolic impairments
Aging Related Decline in Cellular Energy Metabolism
Analysis of the aging brain has generally shown signs of overall reduced energy metabolism. For example, positron emission tomography (PET) imaging of radio-labeled glucose uptake in the brains of normal human participants indicated widespread decreases in glucose utilization with advancing age [21]. Similarly, several in vivo studies have reported a progressive decline of oxygen utilization with age [22]. These changes have been often attributed to widespread neuronal loss or a decrease of substrate supply, poor nutrition, or an impaired neurovascular response to increased metabolic demand with brain activation, rather than intrinsic changes within the brain itself in bioenergetics.
Energy ATP stores and resting blood and brain extracellular glucose are not significantly different between aged and young adult animals [11, 23]. During training or stress energy stores are rapidly utilized to replace the neurotransmitter pool and to restore the membrane potential. However, with such metabolic challenges aging individuals appear to have a decreased ability to cope with such a rapid increase in energy demand, resulting in increased fatigability, decreased performance and dysfunction that can lead to long term damage. For example, several studies have demonstrated that blood levels of glucose increase with training or stress in young individuals in response to epinephrine release, but do not similarly elevate in aging individuals, decreasing their ability to cope with an increased energy demand [24, 25]. In addition, during training the level of glucose in the ECF was shown to be decreased with age. Aged rats demonstrate a substantially larger decrease in hippocampal extracellular glucose levels during training and the depletion of glucose within the extracellular space was of longer duration.
In aging subjects, failing to provide sufficient glucose to the brain during training results in performance deficits on a series of tasks. Increased glucose availability in selective brain areas during cognitive task can positively modulate performance (especially in aged animals during high performance cognitive task). For example, microinjection of glucose into the medial septum, hippocampus, striatum and amygdala, can enhance memory processing [24]. These observations indicate that an aging individual may be at greater risk of exposure to relative substrate deprivation, especially during prolonged high demand cognitive tasks or training. Susceptibility to varying levels of low glucose has not been analyzed in aged brain tissue, particularly whether there may exist irreversible damage at shorter intervals compared to young brain tissue, as is true with ischemia [26–28]. Analyzing metabolism may lead to long-term preventative treatments, which could be used to improve metabolic buffering in the CNS [29].
Mitochondrial Dysfunction with Aging
In addition to changes in the delivery of metabolic substrate to the brain, alterations of mitochondrial function in vulnerable brain areas are likely another cause of decreased brain metabolism (oxygen uptake) in aging individuals [1]. Mitochondrial dysfunction is considered part of the aging process and has been reported to occur during common neurodegenerative disorders such as Parkinson’s and Alzheimer’s [8, 30, 31]. Electron microscopy studies have described a significant increase of damaged mitochondria in several brain regions including the hippocampus of aging rats, which includes sign of edema, damaged cristae and disrupted membranes, lysosome-like structures and lypofuscin deposition [32]. In addition, alteration of the components of the electron transport chain can occur with aging as a result of oxidative stress-related damage or accumulation of mitochondrial DNA (mtDNA) mutations in genes that encode mitochondrial proteins resulting in defective electron transport activity, and compromised energy metabolism. In table 1 we have summarized most of the age-related mitochondrial alterations and their potential contribution to the increased vulnerability to metabolic stress during aging.
Table 1:
Age-related mitochondrial alteration contributing to increased vulnerability to metabolic stress during aging
| Age-related mitochondrial alteration | Possible pathological implications | References |
|---|---|---|
| Decreased complex I activity (whole brain and non-synaptic mitochondria) | Enhanced production of ROS. Alteration of oxidative metabolism, inability to meet metabolic demand. Parkinson’s disease. Decreased memory and exploratory capacity | [19, 33, 35–37] |
| Decreased complex IV activity non synaptic mitochondria and synaptic mitochondria | Impaired ability to respond to increased ATP demand. Reduced ATP production. Cognitive deficits. Alzheimer disease | [33, 35, 36] |
| Decrease in ADP stimulated respiration (state 3) | Decrease in spare respiratory capacity, decline of physiological function, inability to accelerate the rate of oxidative metabolism during increased metabolic demand, metabolic crisis, ischemia-reperfusion injury. | [33, 40, 43] |
| Increased mutations of the mtDNA | Respiratory chain deficiency, increased apoptosis. Parkinson’s disease. | [44, 45, 47]. |
| Increased ROS generation at complex I | Mitochondria ultrastructural damage, inhibition of complex I activity, Parkinson’s disease, and Alzheimer disease, exacerbation of ischemia-induced neuronal damage. | [37, 52, 195] |
| Increased oxidative stress lipid peroxidation, protein damage, and mtDNA oxidative lesions | Damage to mitochondrial structures, inhibition of complex I activity, mtDNA mutations | [19, 33, 35–37] |
| Mitochondria depolarization (Δψm) | Increased oxidation of NADH and NADPH, inhibition of glutathione reduction and increased ROS generation. | [14, 34, 195] |
| Mitochondrial swelling and increased mitochondria fragility | Mitochondrial damage and cell death | [14, 32] |
Age-Induced Alteration of Mitochondrial Electron Transfer Activity and Respiration
Mitochondria isolated from the brain of older animals showed overall signs of mitochondrial dysfunction, such as a decrease in the electron transfer activity, rate of oxygen consumption and membrane potential (Table 1). However, changes are specific for selected brain areas and may vary between non synaptic and synaptic mitochondria [19, 33, 34]. Studies focused on measuring the specific activity of the electron transport chain complexes have shown consistently a decline in the electron transfer in complex I and IV in mitochondria isolated from the whole brain of >12 months rats [35–37] and in synaptosome free mitochondria in aged mice [38]. In contrast, the activity of complex II/III was mostly unaffected in these preparations. The decrease in the activity of complex I, noted in 12-month old rats, becomes more severe in 22-month old animals, and was greater in brain areas such as the hippocampus, compared to the prefrontal cortex or to the whole brain. Complex I activity decreased by 73% and 30% in the hippocampus and in the cortex respectively in mitochondria isolated from 22-month old rats versus 35% in the whole brain [33] Similarly, complex IV was affected by aging (cytochrome oxidase); its activity was decreased by 54% in the hippocampus and by 36% in the prefrontal cortex of 22-month old rats [33]. Interestingly, only few investigators have reported a decrease in the electron transfer activity at the level of complex II/III of the electron transport chain [34, 38]. Interestingly, a decrease in the electron transfer activity at the level of complex II/III of the electron transport chain has seldom been reported [34, 38]. For example, Kilbride et al. (2008) have described that in synaptic mitochondria the activity of complex II/III, and IV was decreased in middle age rats [34].
Alterations in the electron transfer activity may result in a decreased rate of proton extrusion with a consequent decrease of the efficiency of oxidative phosphorylation or mitochondrial respiration. Compared with younger rats, only the rate of ADP stimulated respiration (state 3) was decreased in brain mitochondria isolated from aging rats, while the rate of respiration during resting condition (state 4) was not altered [33, 39]. Consistent with a deficit in complex I activity, the rate of respiration in state 3 was decreased in synaptic and non-synaptic mitochondria when measured in the presence of glutamate and malate as a substrate both in old (∼12 months) as well as in senescent rats (>24 months old) [19, 37, 40]. In contrast, the respiratory rates with succinate present as a substrate were less affected. The percentage decrease in respiration (state 3- complex I driven) was higher in the cortex and in the hippocampus compared with the whole brain, suggesting that mitochondria in these brain areas (especially the hippocampus) are more affected by age [33]. The age-dependent decrease in oxygen utilization was more dramatic in synaptic mitochondria of 28 months old rats, showing an 83% decrease of the rate of state 3 malate + glutamate driven respiration compared to a 33% decrease in non-synaptic mitochondria [40].
Over-expression of electron transport chain proteins in part can compensate for age-induced alterations of the electron transport chain activity [41] so that mitochondria can maintain a normal rate of respiration and adequate ATP supply during resting conditions. However, from these findings investigators have concluded that dysfunctional mitochondria may lack the ability to respond to a sudden increase in metabolic demand or may be more vulnerable to pathological metabolic stress [19, 42] such as ischemia-reperfusion or hypoglycemia.
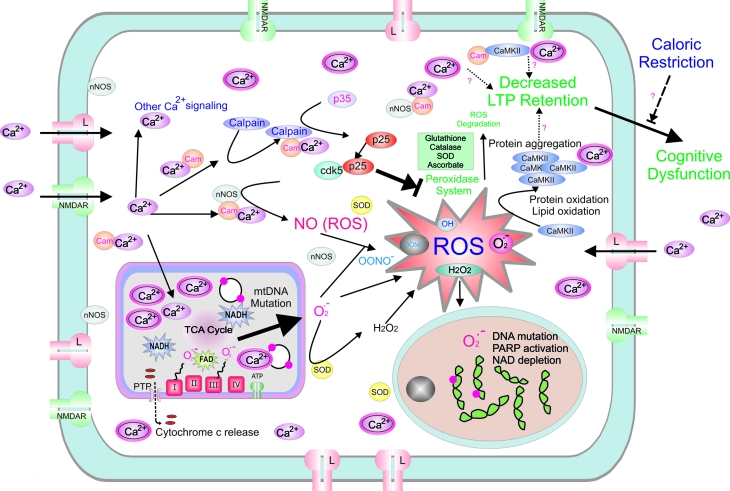
Electron transport chain dysfunction can occur as a result of direct damage by oxygen free radicals and reactive nitrogen species to the structures constituting the respiratory complexes [43]. In addition, mutations of the mtDNA can result in altered protein synthesis of the electron transport chain components. Based on the oxidative-stress theory, aging cells with defective electron transfer activity have higher rates of ROS production which can possibly lead to further mtDNA damage (Fig.2).
Figure 2.
The figure summarizes major changes that have been reported during aging processes involving neurons. Schematic drawing illustrates increased L-type calcium channel expression, increased Ca2+ entry, calpain activation, unregulated cdk5 activity and accumulation of oxidative damage including protein oxidation and aggregation leading to decreased LTP retention, higher levels of somatic mtDNA and nuclear DNA mutations, and eventually cognitive function. NMDAR; N-methyl-D-aspartate receptor, CaMKII; Ca2+/calmodulin-dependent protein kinase II, cam; calmodulin, Cdk5; cyclin-dependent kinase, ROS; reactive oxygen species, ATP; Adenosine-5′-triphosphate, mtDNA; Mitochondrial DNA, LTP; long term potentiation, NAD; Nicotinamide adenine dinucleotide, nNOS – neuronal nitric oxide synthetase, TCA cycle; tricarboxylic acid cycle, Arg; arginine, HSD; hypoxic synaptic depression, PARP; Poly (ADP-ribose) polymerase, SOD; superoxide dismutase, PTP; permeability transition pore.
Somatic mtDNA Mutations
Somatic mutations of mtDNA can also play a role in the decline of mitochondrial oxidative metabolism in the brain during aging. Because the mtDNA contains 37 genes that encode for 13 proteins, all of which are subunits of the respiratory chain complexes, mutations in genes that encode for these proteins can compromise mitochondrial function. Abnormalities of mtDNA have been shown to accumulate with age. Both single-point mutations and, more frequently, deletions of mtDNA have been reported in the brain as well as in other tissues of aging individuals (for review see: [44, 45]). The levels of deleted mtDNA vary dramatically between tissues; in the brain the most affected regions are in the cerebral cortex, putamen and substantia nigra. Because the mutated mtDNA can coexist with normal DNA strands (heteroplasmic mutation), low levels of mutation are considered as having little impact on the oxidative phosphorylation. For example, it has been reported that respiration-chain deficiency occurs only when the level of mutation reaches a certain threshold of about 60% mutation of the total mtDNA [46]. However, mtDNA mutations can undergo clonal expansion leading to a significant increase of mutated mtDNA in each individual cell.
The early analysis of mtDNA isolated from brain homogenates reported a low frequency of mutation in the brain of aging mice [44]. With the utilization of novel approaches that can detect and quantify mtDNA deletion in single cells, investigators recently demonstrated that neurons in certain brain areas can accumulate high levels of deletions that can eventually compromise cellular function. For example, Bender and colleagues [47], using real-time PCR analysis of mtDNA in single neurons, were able to detect an age-dependent accumulation of mtDNA deletions in the substantia nigra of both Parkinson’s disease patients and age-matched individuals. Further, it was demonstrated that the levels of deletions were higher in cytochrome c oxidase (COX)-deficient neurons than in neurons with normal COX activity [47, 48] strongly suggesting that accumulation of mtDNA deletions with age may be responsible for the development of respiratory chain deficiency. In addition, expansion of mtDNA mutations can eventually be responsible for cell loss, probably by apoptosis. For example in aging individuals, it has been reported that cells in the substantia nigra die at the rate of 5% per 10 years [49].
There is experimental support for the hypothesis that mutation of the mtDNA with age occurs as results of accumulation of mutagenic oxidative lesions like 8-oxodeoxyguanine in the mtDNA. Interestingly, a recent study reported that increased oxidative damage to the mtDNA in transgenic mice lacking a specific DNA glycosylase repair system (8-oxoguanine glycosylase, also known as OGG1) did not correlate with mitochondrial respiratory chain dysfunction, suggesting that additional mechanisms underlying mtDNA oxidative damage may be responsible for age-related mtDNA deletions linked to mitochondrial dysfunction [50]. Based on these results as well as data obtained from studies in the “mtDNA mutator mice” Larsson has recently proposed [44] that most mtDNA mutations may be produced by replication error rather than oxidative damage.
Oxidative Stress in Aging
Reactive oxygen species (ROS) are continuously produced in brain cells as a consequence of aerobic metabolism. Investigators have recently estimated that under physiological conditions ∼0.2 % of oxygen consumption results in superoxide anion radical formation (O2• −) [51] due to a small number of electrons that “leak” early to molecular oxygen at various sites in the electron transport chain [52]. This reaction starts a chain of events that eventually results in the production of more reactive oxygen species such as of hydrogen peroxide (H2O2), hydroxyl radical (•OH) and peroxynitrate (ONOO−) [53]. This last one is generated by the reaction of O2• − with nitric oxide (NO). The brain has adapted to use ROS at low concentration as specific signaling molecules that are essential to maintain neuronal homeostasis. For example, NO, O2 and H2O2 can modulate various aspects of synaptic function and metabolism, as well as affect vascular permeability to substrates. However, if the production of ROS is higher and not adequately contained by the antioxidant system, it can cause oxidative damage to both cellular and mitochondrial macromolecules (Fig.2). To prevent oxidative damage, the cell has numerous scavenger mechanisms that are present both outside and inside the cells, in particular in the mitochondria, such as the glutathione redox system, superoxide dismutase enzymes family and catalase.
The hypothesis that oxidative damage is responsible for the aging process as well as age-related disease is based on reports from numerous studies showing a correlation between increasing age and the accumulation of oxidation products of phospholipids, proteins, and DNA. The content of oxidation product TBARS (Thiobarbituric acid reactive substances) and protein carbonyl indicate lipid peroxidation and protein damage respectively, and both were consistently increased in tissue homogenates of the liver and the brain of aged animals[19].
Oxidative damage by ROS to certain components of mitochondrial structures such as membrane phospholipids may result in the alteration mitochondrial enzyme function. For example, oxidative damage to cardiolipin can alter directly complex I activity; these phospholipids are localized in the inner mitochondrial membrane and regulate several mitochondrial bioenergetics processes, including the inner membrane super molecular assembly and electron transport [37]. Supplementation of brain mitochondria with cardiolipin, to correct the age-induced decline of cardiolipin content due to oxidative damage, restored the activity of complex I in 24 month old rats to control level [37].
Significant age-related increase of oxidative damage to the DNA, estimated by measuring 8-oxo-7,8-dihydro-2′-deoxyguanosine (oxo8dG), has been observed in numerous tissues, including the liver, muscle, kidney, heart, and brain. In the brain the levels of oxo8dG were particularly elevated because of the high metabolic activity [54]. Although free radicals can be produced at various levels in the cell, as discussed earlier, the mitochondrial respiratory chain is considered the main source of free radical generation and at the same time the target for ROS induced damage. Mitochondria can produce ROS at complexes I and III. However, in brain mitochondria isolated from aged rodents increased production of superoxide radicals and hydrogen peroxide were observed predominantly in mitochondria respiring on glutamate and malate (complex I specific substrates) and supplemented with rotenone + NADH. These data support the hypothesis that complex I dysfunction may be responsible for age-dependent enhanced production of ROS [37, 40, 43]. Free radical generation at complex I has been implicated in aged-induced oxidative damage to mitochondrial structures and DNA leading to the decline of mitochondrial function. Analysis of DNA in human brain tissue showed that the amount of oxo8dG is increased with age in both nuclear and mitochondrial DNA isolated from the cortex and the cerebellum of individuals from 47 to 97 years of age, and in the mtDNA this increase was 10-fold higher [55]. However, not all investigators have confirmed these findings. Sanz and collaborators [56] have reported that levels of oxo8dG in brain mitochondria were similar to younger animals. Nevertheless, caloric restriction leads to a decrease of the levels of DNA oxidation in both nuclear and mitochondrial DNA (see further discussion below).
Additional evidence in support of the oxidative stress theory of aging includes findings that ROS production is lower in cells of long-lived than short-lived species, and that therapeutic interventions can reduce the accumulation of oxidative damage, prolonging the life span of certain animal species. However, because therapeutic interventions, such as caloric restriction, do not exclusively target free radical generation but can affect various physiological processes, several investigators have questioned these results [57]. To verify directly the role of ROS on aging cells and mitochondria investigators have recently studied transgenic mice that either under underexpress or overexpress a wide variety of genes encoding for antioxidant enzymes. Interestingly, many of these genetic manipulations did not always affect the life span of the animals.
For example, mice deficient in manganese superoxide dismutase (Mn-SOD) and glutathione peroxidase-1 (GPx1) showed increased oxidative damage and age associated pathology, but not reduction in longevity [58]. A significant decrease in life span and demonstration of phenotypes of accelerated aging, including hearing loss, macular degeneration, and muscular atrophy, is found in SOD1−/− knockout mice (where the gene encoding for copper and zinc superoxide dismutase (CuZn-SOD) has been deleted). However, these mice have also a high incidence of hepatocellular carcinoma suggesting that a novel pathology due to the genetic manipulation may in this case contribute to the reduced life span [57].
Conversely, the overexpression of antioxidant enzymes such us Mn-SOD, CuZn-SOD, glutathione peroxidase, and catalase all resulted in increased resistance to oxidative stress and reduction of accumulation of oxidative damage with age [59], but increased life span was observed only in few selected cases [60]. Thus, increased ROS buffering may not in itself promote a longer lifespan, limiting the power of the simple oxidative stress hypothesis of aging.
Alterations in Calcium Buffering and Signaling
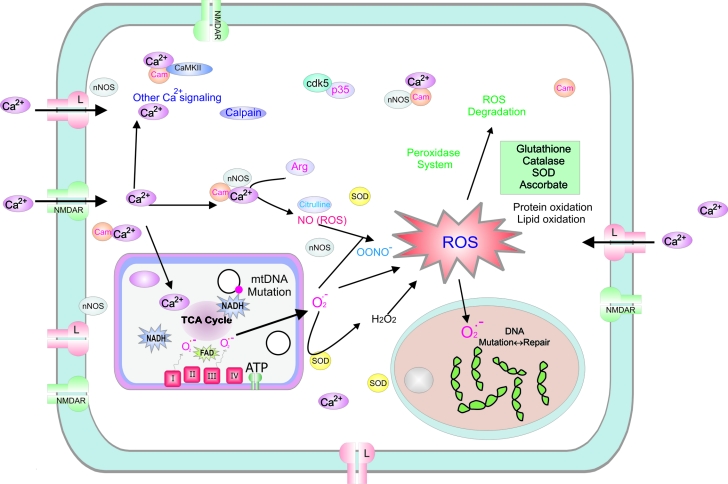
Calcium influx into cells is a critical mediator of cellular metabolism, since calcium regulates many metabolic enzymes as well as mitochondrial function. Calcium influx occurs via voltage-gated calcium channels as well as N-methyl D-aspartate receptors (NMDA) glutamate channels, and is likely as important as ADP for enhancing rates of metabolic enzymes, particularly in the tricarboxylic acid cycle (TCA). However, calcium itself is highly toxic to cells at higher levels, and intracellular levels (including within mitochondria) must be maintained very low to keep signaling optimal [18, 61–63]. A primary intracellular calcium buffer in neurons is the endoplasmic reticulum (ER), which has specific receptors and mechanisms for both calcium uptake and calcium release, depending on cellular activity and calcium levels. One long-held concept is that calcium levels may slowly increase during aging, thus affecting critical calcium signaling throughout cells, including altering Ca2+-stimulated currents (such as Ca2+-mediated after hyperpolarization) and affecting cellular activity [64]. In addition, ER may decrease its calcium buffering capacity in aged neurons in particular, potentially shifting the burden of intracellular calcium buffering onto mitochondria; however, this increased calcium uptake into mitochondria can significantly affect the signaling role which calcium assumes for rate of metabolism, by changing TCA cycle enzyme activity. Age-dependent alteration of intracellular Ca2+ buffering may be more dramatic in selected brain areas. For example, in the hippocampus of old animals the expression of calcium binding proteins Calbindin-28 and Calretinin is down-regulated and mitochondria have reduced Ca2+ buffer capacity. This will determine a prolonged Ca2+ transient and a greater risk for Ca2+ dependent neurotoxicity and cell death after ischemia [65]. Thus, calcium transients, normally useful for intracellular signaling (Fig. 1), may become distorted and amplified, potentially resulting in dysregulation of ubiquitous calcium effects on enzymes and metabolic processes [66] (Fig.2).
Figue 1.
Schematic diagram of a typical neuron shows Ca2+ dependent and reactive oxygen species (ROS) signaling that modulate energy production in young adult (i.e., normal). Cellular response following neuronal stimulation includes an increase in intracellular Ca2+ influx (via NMDA receptors and L-type calcium channels in particular) and increased metabolic activity in the mitochondria. Oxygen turnover in the electron transport chain can generate superoxide anion at complex I and III, which then leads to a variety of ROS, which are subsequently normally degraded by glutathione, catalase, superoxide dismutase [194] and ascorbate. ROS can lead to protein oxidation, lipid oxidation and somatic DNA mutations at both the mitochondrial (i.e., mtDNA) and nuclear levels. NMDAR; N-methyl-D-aspartate receptor, CaMKII; Ca2+/calmodulin-dependent protein kinase II, cam; calmodulin, Cdk5; cyclin-dependent kinase, ROS; reactive oxygen species, ATP; Adenosine-5′-triphosphate, mtDNA; Mitochondrial DNA, LTP; long term potentiation, NAD; Nicotinamide adenine dinucleotide, nNOS – neuronal nitric oxide synthetase, TCA cycle; tricarboxylic acid cycle, Arg; arginine, SOD; superoxide dismutase.
Perturbed energy homeostasis and metabolic stress due to hypoxia/ischemia in aging
Increased Susceptibility to Ischemia with Aging
The aging brain is more susceptible to hypoxia in addition to hypoglycemic insults, with increased risk of developing short-term as well long-term consequences [31, 67, 68] (Table 2). Neuronal damage following cerebral ischemia results in cell damage and cognitive impairment, which are more dramatic in the aging population. Deprivation of energy supply (oxygen and glucose) to the brain due to a reduction of cerebral blood flow during cerebral ischemia activates a cascade of events that eventually results in cell death, especially in vulnerable brain areas such as the hippocampus, caudate nucleus (striatum), cerebral cortex and cerebellum. Hippocampal function is crucial for acquisition of new information and damage to the hippocampus can result in impaired learning ability. Age represents a risk factor for post-stroke dementia, which is correlated with an elevated risk of death and stroke recurrence [69].
Table 2:
Cellular events contributing to increased neuronal vulnerability to hypoxia/ischemia with aging
| Events | Young adult | Aging |
|---|---|---|
| Regulation of the cerebrovascular system | Vasodilatation, in response to increased energy demand | Enhanced vasoconstriction and impaired vasodilatation. Narrowing of the intracranial arteries or small vessel occlusion due to severe atherosclerosis [70, 71] |
| Glycolysis | Can utilize available glucose during hypoxia | Failure to up-regulate glycolysis during hypoxia [27] |
| Cellular respiration | Increased O2 uptake in response to K+ -induced stimulation | Decreased ability to increase O2 consumption after K+ -induced stimulation [75] |
| Latency to spreading depression | HSD ∼5 min after the onset of hypoxia | HSD ∼4 min after the onset of hypoxia [73] |
| PCr and ATP recovery | Rapid recovery after 5 min of ischemia (20 min) | Slower recovery (60 min) [81] |
| pH recovery | Rapid recovery after 5 min of ischemia (20 min) | Slower recovery (50 min) [81] |
| Ionic homeostasis | Complete recovery of extracellular [K+ ] after hypoxia during reoxygenation | Incomplete recovery extracellular [K+] after hypoxia during reoxygenation [73] |
| ATP-ase activity | Substantial upregulation (800%) of Na+/K+ ATP-ase activity 1hr after an ischemic insult of 15 min | Limited upregulation (300%) of Na+/K+ ATP-ase activity 1hr after an ischemic insult of 15 min [91] |
| Hyperoxidation | No hyperoxidation after 2.5 min of hypoxia post HSD | Hyperoxidation after 2.5 min of hypoxia post HSD [26] |
Cerebrovascular Changes in Substrate Supply
Changes in the cerebrovascular system associated with aging may underlie the increased incidence of ischemic stroke. Structural alteration of the vasculature such as narrowing of the intracranial arteries or small vessel occlusion due to severe atherosclerosis may increase the susceptibility to substrate deprivation. In addition, enhanced vasoconstriction and impaired vasodilatation, in response to increased energy demand, result in a decrease of cerebral blood flow, reduced CMRO2 and CGU even in healthy aging individuals [70]. After an ischemic event, these alterations of vascular reactivity may limit the energy supply during reperfusion at the time at which tissue energy demand is extremely elevated, leading to more extensive damage [71]. Interestingly, investigators have found that in animals exposed to brain ischemia the rate of lethality increased with age [39, 72]; moreover, in brain slices isolated from aged animals, the incidence of cell death and synaptic failure after exposure to hypoxic/ischemic insult is higher compared to slices isolated from young adult animals [26, 73]. These observations indicate that, in addition to systemic changes, metabolic alterations [74] at the cellular level of both neurons and astrocytes may also contribute to the increased susceptibility of aged individuals to ischemiareperfusion injury.
Cellular Events Underlying Ischemia
The cascade of cellular events occurring during ischemia includes a dramatic decrease of neuronal ATP, membrane depolarization, glutamate release and increase in intracellular Ca2+, Na+ and Cl−. The ion gradients collapse and within a few minutes all neurons depolarize due to the failure of the Na+/ K+ ATPase and other transport systems dependent on ATP [75]. These events can be reversible following the ischemic episode during reperfusion, or they can trigger neuronal death. Some of the metabolic changes, such as the increase in intracellular Ca2+, are critical in determining irreversible damage to the neurons after ischemia. The large increase in cytosolic Ca2+ causes the generation of free radicals and the activation of intracellular proteases and kinases that initiate cell death mechanisms ([76] for review). High intracellular Ca2+ can also trigger mitochondrial injury. During ischemia and reperfusion, excessive mitochondrial Ca2+ sequestration compromises mitochondrial function, resulting in respiratory inhibition, ROS formation, dissipation of the transmembrane mitochondrial gradient and transient loss of mitochondrial membrane integrity (mitochondrial permeability transition (MPT)) [77] (Fig.2). This chain of events further results in the efflux of small molecules and metabolites from the mitochondria to the cytosol including: Ca2, NAD+/NADH, and cytochrome c. After reperfusion, depending on the degree of ATP depletion and cytochrome c release, different downstream cell-death pathways can be activated and cells can undergo either apoptosis or necrosis [78].
Energy Homeostasis and Neuronal Vulnerability
Although the mechanisms behind the increased vulnerability to ischemia associated with aging are not well understood, investigators have focused their attention especially on mitochondrial dysfunction, a decrease in glucose utilization [27, 73, 79], increased oxidative stress, deregulation of ionic homeostasis, and compromised glial function. See table 2 for a summary of the cellular events that investigators have proposed contributing to increased neuronal vulnerability to hypoxic/ischemic insult with aging.
Despite the age-dependent deficiency in the electron transport chain (as discussed above), ATP production during resting conditions does not appear to be compromised, as several investigators found no significant age related changes in the concentration of high energy substrates under normoxic condition [23, 27, 80]. For example, measurements conducted using the whole brain or acutely isolated brain slices have found that ATP and PCr levels were not altered in either middle aged (14–19 months old) or aged (24–29 month) rats, compared to younger rats. However, reports have suggested that a drop in ATP levels may occur earlier after exposure to hypoxia in aged subjects, leading to earlier failure of the membrane ion gradient and depolarization. Roberts et al., reported that during anoxia in hippocampal tissue slices isolated from aged rats the latency for developing anoxic depolarization (AD) or hypoxic spreading depression (HSD) was shorter in aged rats compared to younger rats [27, 73]. In this case, the investigators suggested that aging tissue has a decreased ability to up-regulate anaerobic glycolysis to provide ATP for ion pumping, leading to a faster increase of extracellular K+ during hypoxia. These findings, however, are in conflict with a more recent study that did not find a significant difference in the time to HSD between >22 months old and younger animals [26]. Consistent with the notion that aging compromises the ability to respond to increased metabolic demand, slices of older rodents show a slower rate of ATP recovery after they are exposed to transient global ischemia [81], which is accompanied by a slower recovery of the pH level in the tissue.
Reperfusion/Reoxygenation Events
Early during reoxygenation, mitochondria accelerate the rate of oxidative metabolisms utilizing all the available oxygen to generate ATP needed to restore the ionic homeostasis and the membrane potential. Tissue Po2 measurements demonstrate that in young animals following hypoxia of 2.5 min duration (post HSD) tissue oxygen levels remained severely depressed after reoxygenation due to increased oxygen uptake, and the recovery of tissue Po2 to pre-hypoxic levels is slow [82]. However, in 22-month old rats exposed to the same insult, the tissue Po2 recovered earlier after reoxygenation, reaching levels that were significantly higher than the pre-hypoxic baseline levels [26]. These observations suggest that in aged rats, tissue oxygen uptake (and mitochondrial oxygen utilization) was decreased probably due to the inability of the mitochondria to fully restore respiratory function after reoxygenation in spite of the immediate availability of an excess of oxygen.
In young rats, mitochondrial respiratory capacity is impaired during ischemia and recovers toward control levels within the first hours after reperfusion. Oxygen uptake measurements performed in brain homogenates collected from the focal and perifocal tissue at different time points after exposure to middle cerebral artery occlusion (in young rats), showed a progressive decline of ADP stimulated and uncoupled respiration; in contrast, basal respiration was preserved. Similar experiments also showed that mitochondria develop lower membrane potential during ischemia and early reperfusion. The extent of mitochondrial recovery during reperfusion depends on the severity of the insult; in mitochondria collected from the core of the ischemic lesion, the recovery is slower and sometimes incomplete [83]. Despite a transient recovery, later during reperfusion mitochondria undergo a secondary deterioration of the respiratory function which precedes and contributes to the development of irreversible neuronal injury. For example, in vulnerable brain regions, such as the area CA1 in the hippocampus, mitochondrial respiratory capacity declines later after exposure to transient global ischemia, leading to delayed cell death [84]. Because of underlying dysfunction, exposure to hypoxia/ischemia may have more detrimental effects in mitochondria of aged rats, resulting in a more severe impairment of the respiratory function. If mitochondrial dysfunction persists during reperfusion, the rate of ATP production may fall below ATP demand resulting in metabolic failure.
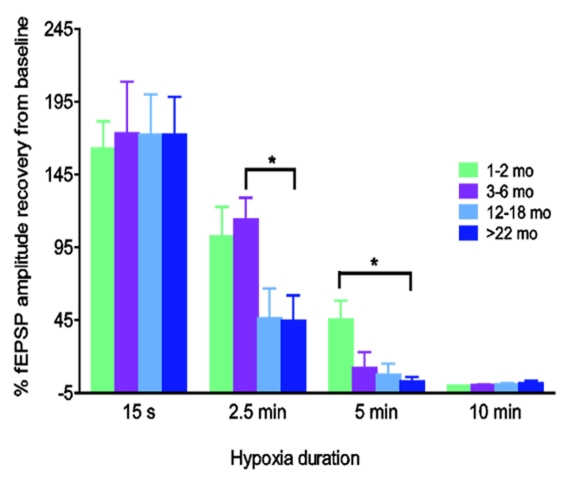
Foster et al. [26] showed that synaptic responses in hippocampal slices isolated from aging rats were more vulnerable to hypoxia compared to younger rats; for example, after hippocampal slices were exposed to hypoxia and reoxygenated at 2.5 min post-HSD occurrence, the fractional recovery of orthodromic field potentials (fEPSPs) was significantly lower in slices from 22-month old rats compared to younger rats (Fig. 3). Note that the time to onset of HSD was similar in young and aged rats, but slices from aged rats were much more sensitive to prolonged periods of post-HSD hypoxia (ie, > 30 sec) indicating much less reserve metabolic function in the aged tissue slices in the persistent absence of oxygen.
Fig.3:
The vulnerability of synaptic responses to hypoxia increases with age. Hippocampal slices from 1–2, 3–6, 12–20, >22 months old rats were exposed to varying lengths of hypoxia following the induction of hypoxic spreading depression (HSD) (0.25, 2.5, 5 and 10 min). Slices from >22 months old rats (45 +/− 49%, n=8) showed less fEPSP recovery after 2.5 mins compared to 3–6 months old rats (114 +/− 45%, n=9) (* p<0.05). Five minutes of hypoxia post-HSD resulted in partial fEPSP recovery in 1–2 month old rats but was lethal in all older age groups (modified from Foster et al. 2008).
During reoxygenation, as the tissue Po2 recovers there is a progressive switch of the NADH/NAD+ redox state toward oxidation due to elevated respiration, and eventually NADH fluorescence falls below baseline levels, indicating hyperoxidation and mitochondrial damage. Because the decrease of NADH fluorescence is partially irreversible, several investigators have proposed that a net loss of NAD+ from the mitochondrial pool due to induction of MPT may contribute to the hyperoxidation [26, 85]. Aged rats were more vulnerable to hyperoxidation after hypoxia compared to young rats exposed to similar insult [26] consistent with the hypothesis that the sensitivity to permeability transition pore (PTP) opening increases with age [86]. Several factors that contribute to MPT and hyperoxidation may be exacerbated by aging, in particular increased ROS production, altered calcium buffer ability and mitochondrial dysfunction.
As described earlier, calcium accumulation during ischemia and ROS bursts occurring during reperfusion appears to facilitate the opening of mitochondria PTP, which facilitates the diffusion of NAD+ from the mitochondria to the cytosol. This event is usually accompanied by a transient collapse of the mitochondrial membrane potential and proton gradient, which result in the uncoupling of the oxidative phosphorylation and interruption of ATP synthesis. Despite reperfusion and recovery of the mitochondrial membrane potential, the induction of MPT initiates a cascade of events that contribute to irreversible neuronal injury. Consistent with the hypothesis that these events may be exacerbated by aging, investigators have reported that cellular markers of degeneration were higher in hippocampal slices from 24-month old rats, which release higher levels of LDH after exposure to analogous OGD insults compared to middle aged or young adult animals [87].
Recently, investigators have demonstrated that induction of MPT exacerbated ROS and NO generation after ischemia [88], which are both key mediators of apoptotic cell death. Moreover, oxidative stress after ischemia contributes to further cytosolic/nuclear NAD loss via the activation of poly-ADP ribose polymerase-1(PARP-1); this enzyme hydrolyses its substrate nicotinamide adenine dinucleotide (NAD+) to nicotinamide and transfers poly (ADP-ribose) chains to a variety of other nuclear proteins to repair ROS-induced DNA damage during reperfusion [26]. Availability of metabolic cofactors, such as NAD+, is critical during reperfusion, when the mitochondria accelerate the rate of oxidative metabolism to rapidly restore ATP levels and to maintain cellular function. Depletion of NAD+ causes inhibition of several energy pathways including glycolysis, TCA cycle, and oxidative phosphorylation. Rats treated with nicotinamide after stroke had elevated NAD+ levels in the brain several hours after the injury [89] and attenuated neuronal death.
Limiting the loss of ATP levels is very important for neuronal function when energy production is impaired; for example, during hypoxia, which results in a rapid loss ionic homeostasis [90]. Maintenance of the electrochemical gradient across the membrane largely depends on the work of the NA+/K+ ATP-ase and is critical for brain function, such as generating, processing and transmitting impulses. Investigators have reported that the activity of the ATP-dependent membrane pump is enhanced after ischemia both in vivo [91] and in vitro [92], and the activation persists for several hours to allow for the recovery of transmembrane ion gradient. In aging rats, investigators reported a deficit in the up-regulation of the activity of the NA+/K+ ATP-ase 1hr after ischemia compared to younger rats, possibly due to insufficient levels of ATP. Failure to restore ionic homeostasis early after an ischemic/hypoxic insult delays the recovery of synaptic transmission, exacerbates the accumulation of intracellular Ca2+ and can contribute to the activation of a downstream cell death signal [91] such as the release of cytochrome c from the mitochondria.
Interventions during energy deprivation that limit ATP depletion or promote ATP recovery can also result in long-term protection by preventing the release of cytochrome-c and further mitochondrial dysfunction that may take place during reoxygenation [92], probably by restoring earlier ionic homeostasis and limiting intracellular and intra-mitochondrial calcium overload [92]. Especially in an aging model, restricting Ca2+ uptake during hypoxia prevented hyperoxidation and improved the recovery of synaptic transmission in hippocampal slices of 22-month old rat exposed to hypoxia and reoxygenated 2.5 min post HSD [26].
Metabolic and aging interactions with cognitive alterations and long-term potentiation
Another example of aging related events is cognitive decline, which can commonly occur even in the absence of clear pathological conditions such as Alzheimer’s disease; such decline may be related indirectly to deterioration of metabolic activity, cerebrovascular changes, cellular signaling, antioxidant defense mechanisms and blood electrolyte regulation [7]. In the search for a cellular correlate of cognition, long-term potentiation (LTP) has been intensely debated as a possible candidate, due to many similarities between LTP and learning, and indeed a tight correlation exists between learning and acquisition in vivo and LTP both in vivo and in vitro, particularly in brain slice preparations. We will first discuss the correlation between memory and LTP, then metabolic alterations affecting LTP during aging and how glycogen may relate to be involved with LTP.
Memory and LTP Correlation
The first evidence of a long lasting increase in synaptic strength was described in 1973 [93], based on recorded synaptic strength from granule cell layers of dentate gyrus of hippocampus following stimulation of the perforant path. Later it was eventually termed as long term potentiation (LTP) [94]. LTP is often differentiated into early phase LTP (short term memory; <1hr) and late phase LTP (long term memory; > 3hr).
In general, both phases of LTP are characterized by a series of three events: LTP induction, LTP maintenance, and LTP expression. LTP induction mechanisms are focused mainly on activation of postsynaptic NMDA and AMPA receptors by glutamate through repetitive high frequency stimulation of presynaptic terminals and postsynaptic processes; the critical interaction includes activation of synapses while post-synaptic depolarization occurs. Initially, glutamate activates AMPA receptor and causes depolarization of the postsynaptic membrane by enhancing the permeability to Na+, and K+ ions. The resulting depolarization of the postsynaptic membrane can be sufficient to displace the Mg2+ block inherent to NMDA receptors, and allow Ca2+ to flow into the cell through NMDA receptors, beginning a cascade of events initiated by Ca2+ for maintaining LTP. The early phase of LTP is dependent on posttranslational modifications such as protein phosphorylation, whereas later phases of LTP require new gene transcription and protein synthesis [95, 96]. Once LTP is consolidated then LTP can be expressed by synaptic stimulation. Eventually LTP demonstrates a decrease in the potentiation of synaptic strength, generally termed as LTP decay, over a time period of days to weeks in vivo, which is enhanced in aged animals [3]. The major differences in LTP induction and LTP maintenance with aging are described in the table below (Table 3).
Table 3.
Differences in LTP induction and maintenance during aging
| LTP events | Young adult Animal | Aged animal |
|---|---|---|
|
LTP Induction Robust High intensity stimulation protocol (50 μA at 100 Hz for 1sec) |
Normal induction | Normal induction [3, 103] Deficit in induction [196] |
| Lower amplitude current (fewer stimulus) | Normal | Deficits in induction in CA1 region [133] Normal in dentate gyrus [102] |
| LTP Maintenance and decay | L-LTP is normal | Deficits in maintenance over longer time scale in vivo [3] and 3hr after induction in vitro [106] |
| Robust high intensity stimulation protocol (50 μA at 100 Hz for 1sec) | E-LTP-normal | E-LTP is normal with robust stimulation [133] |
| NMDA dependent LTP | Normal | Decreased [197] |
| NMDA independent LTP (vdccLTP) Voltage dependent L-type Ca2+channels (vdcc) |
Normal [198] | Increased [197] |
| 1 Hz paired pulse (vdcc) | Induced early phase of LTP | Induced both E-LTP and L-LTP [108] |
Experiments performed to study whether the induction of LTP in the Schaffer collateral fibers to the CA1 pyramidal cells is affected by aging have produced mixed results due to several factors, including differences in rat ages, strains, experimental preparation and stimulation parameters used to induce LTP [97, 98]. There are many ways in which age-related deficits in memory, such as a spatial learning task, can be studied. Spatial learning in rodents can be studied using an eight arm radial maze [99], Barnes circular platform [97], T-maze [100], or the widely used Morris water maze. Correlation between LTP and memory deficits in aged animals was first observed with experiments using the Barnes maze [97] showing that aged rats took longer path lengths and a greater amount of time to solve a spatial memory task. Subsequent studies [98, 100] also demonstrated that aged rats showed increased rate of decay for the memory of the escape box and LTP was nearly twice as fast as young rats. Moreover, aged rats consistently show LTP induction deficits at the Schaffer collateral CA1 synapse and the perforant path-granule cell synapse [9, 101, 102].
Although there are age-related deficits in enhancement of the population spike observed in the CA1 region during high frequency stimulation, high frequency LTP induction threshold above the normal level shows no difference in LTP induction in aged rats [103, 104]. However, long term LTP (L-LTP) decayed twice as fast in aged compared to young rats [3,100, 105] under these conditions. Furthermore, Bach et al [106] uncovered age related LTP maintenance deficits in CA1 of old mice after 3h using the in vitro slice preparation. Correspondingly, spatial memory studies with 8-arm radial maze in aged rats showed faster decay rates for hippocampal LTP [107]. Taken together, the current available data indicate that spatial learning and memory deficits reported in aged rats and mice parallel the deficits observed during induction and maintenance of LTP. The different protocols applied to study the change in LTP with aging may give rise to distinct changes in metabolic activity with aging. In addition, a recent study also indicated a different form of age related enhancement of L-LTP induced by paired pulse stimulation (PP-1 Hz, 1min) and this form of L-LTP appears to be inversely correlated with memory ability [108].
Metabolic Interactions in Hippocampal LTP
Long-term potentiation in the hippocampus involves synaptic activation of afferent fibers (by high frequency stimulation), resulting in a long lasting increased efficacy of synaptic transmission [109], which includes the elevation of intracellular Ca2+ concentrations in the postsynaptic cell [110, 111], particularly via NMDA glutamate receptors [112]. The surge in calcium influx through NMDA receptors stimulates a series of cellular signaling cascades involving calmodulin, calpain and other gene regulation pathways [15, 18, 62, 63, 113–115] (Fig.1). Most importantly the induction of LTP also depends on the neuronal energy metabolic state. Studies have shown that inhibition of LTP induction occurs during glucose deprivation in slices [116–118] and in hypoglycemic rats [119]. One point of interaction between insufficient energy supply, low ATP levels and altered LTP induction may be due to changes in the phosphorylation of key proteins [120]. Other interactions include nitric oxide [121] and insufficient LTP consolidation [122].
Changes in LTP characteristics were related to several alterations observed with aging brain. For example this may be due to reduction in the actual number of perforant-path synaptic contacts on granule cells [102, 123]. The loss of functional synaptic contacts in CA1 was connected to decrease in Shaffer collateral induced fEPSP in CA1 region [100, 124, 125]. The changes in the dynamics of neuronal assembly that occur with aging was similar to that of blockade of NMDA receptor in young rats [126]. Typically NMDAR dependent LTP was severely affected in aged rats, however NMDAR independent LTP occurs distinctly in aged rats [127].
NMDA Receptor Function
Induction of LTP depends on the release of glutamate from the presynaptic terminal and its binding to postsynaptic NMDA and AMPA receptors [128, 129], and leads to depolarization of postsynaptic membrane. The NMDARs are glutamate-gated ion channels that are pivotal in the regulation of synaptic function in the CNS and are mainly localized to postsynaptic densities (PSD), where they are structurally organized in a large macromolecular signaling complex of synaptic scaffolding and adaptor proteins which are physically linked to the kinases, phosphatases and downstream signaling proteins and group I metabotrophic glutamate receptors. NMDARs are heterogenic assemblies of NR1, NR2 and NR3 subunits, which are co-translationally assembled in the endoplasmic reticulum (ER) to form functional channels with differing physiological and pharmacological properties, and distinct patterns of synaptic targeting. Normal NMDAR activity requires accurate delivery and targeting to the synapses. However, in older ages, in vitro, when the majority synapses are formed, NMDAR subunits are recruited more gradually in the form of clusters containing a small number of receptors to nascent synapses [130]. Thus, dendritic transport and synaptic recruitment of NMDARs might occur via distinct mechanisms at differing ages in vitro [131].
NMDARs are highly permeable to Ca2+ and Ca2+ influx through NMDARs is essential for synaptogenesis during synaptic remodeling and long lasting changes in synaptic efficacy such as long term potentiation and long term depression. An age- related decreased expression of the NMDA receptor subtypes has been noted in the CA1 region of the brain. A decreased ability to mobilize the surface expression of the NMDA receptors is one of the possible reasons for these events [132]. Young animals show a significant increase in the surface expression of NR1 and NR2A subunits, which is not seen in aged animals. Similarly, aged animals fail to show an increase in NMDA receptor mediated current associated with LTP stimulation. Furthermore, in aged rats induction of LTP has been repeatedly observed in the hippocampus when, robust high frequency stimulation protocols well above the minimum threshold for the LTP were used [3, 104]. However, use of fewer stimulus pulses and lower amplitude stimulus currents to induce LTP in old rats do show LTP induction deficits in the Schaffer collateral-CA1 synapse compared to their younger counter part [9, 133]. Furthermore, at the perforant path granule cell synapse, larger amplitude current injection is necessary for LTP to be induced in old rats when weak presynaptic stimulation is paired with direct depolarization of the postsynaptic granule cells [102].
Apart from changes in the expression of the isoforms of NMDA receptor subunits, deterioration of cellular energy metabolism is also involved in the alteration in the LTP process. Most of the NMDARs receptors dysfunction in the hippocampus of aged animals has been demonstrated by multiple groups using variety of techniques [134, 135]. Depolarization of the postsynaptic membrane (Na2+/K+ conductance) through AMPA receptors would be required to displace the Mg2+ blockage on NMDAR activity to allow Ca2+ influx for LTP events [2, 136–139]. NR2A subunit confers lower affinity for glutamate, distinctly faster kinetics, greater channel open probability and more prominent Ca2+ dependent desensitization than does the NR2B subunit, which confers the slower channel kinetics and reduced open probability [140]. NR2C and NR2D subunits are characterized by low conductance opening and reduced sensitivity to Mg2+ block and NR3 subunit confers reduced Ca2+ permeability and reduced surface expression.
CaMKII and ROS
LTP induction in the CA1 region requires elevation of the intracellular Ca2+ concentrations in the postsynaptic cell, as shown in Fig. 1 [110, 111] through the N-methyl-D-aspartate glutamate receptor [112] stimulating cellular signaling cascade system involving calmodulin, calpain and gene regulation [15, 18, 62, 63, 113–115]. Landfield and his colleagues [63] have found deficits in several aspects of calcium regulation during aging and also an increased density of L-type calcium channels in the old rats (schematized in Fig. 2). This age related change in calcium channel expression may contribute to enhanced long term depression (LTD). Aging animals also have more difficulty to strengthen the synapses as well as to form new memories [141]. Moreover, evidence also suggests that depotentiation is easier to induce in hippocampal slices from old rats than from adult rats [142]. Transient calcium influx into cells is a critical mediator of cellular metabolism, but increased deregulation of calcium homeostasis during aging may result in deficit in cellular function.
Ca2+/Calmodulin dependent protein kinase II (CaMKII) [143] is highly enriched in postsynaptic densities (10–30% of protein content), and regulates some of the key events responsible for LTP. CaMKII is a multimeric enzyme composed of several catalytic subunits (CaMKII α &β) which are activated by Ca2+/CaM through phosphorylation. Recent models of LTP show that CaMKII, together with other protein kinases (protein kinase C, tyrosine kinases), affect the functioning of postsynaptic AMPA receptors either by direct phosphorylation or by regulating the synaptic targeting or trafficking of AMPA receptors at the membrane [144]. Recently, a study showed that CaMKII is very susceptible to oxidative damage, which decreased CaMKII activity in hippocampal slices [145]. Autophosphorylation of CaMKII and its binding to NMDA receptors is also affected by the oxidation of calmodulin by ROS [146]. Therefore cellular redox potential plays a critical role in regulating the memory performance during aging.
The major antioxidant in the brain is glutathione, which is readily oxidized by H2O2 into three forms of oxidized glutathione derivatives: oxidized glutathione, glutathione disulfide dioxide, or monoxide. These glutathione derivatives can alter the cysteine residues of many proteins in vitro, including CaMKII [145, 147]. Moreover, the aged brain also exhibits an increase in oxidative damage [148, 149] with higher level of reactive oxygen species [150] and decreased oxidative buffering capacity [149, 151], due to decrease in the cellular glutathione [152, 153]. Furthermore, a recent study also demonstrated that the age related shift in intracellular redox states contributes to the decline in NMDAR response through CaMKII and altered synaptic plasticity [61]. Although several studies have showed a relationship between reactive oxygen species, cellular metabolism, and memory in aged animals, a direct effect of ROS on NMDA receptor mediated cellular signaling is still unclear. Increased ROS production together with increased activity of L-type voltage gated channels and the rise of Ca2+ during postsynaptic potential generation observed with aging animals have a direct implication with functional aspects of the CaMKII. These functional differences in aging can be related to abnormal posttranslational modifications (phosphorylation) rather than the number of NMDA subunits with aging.
Interestingly, cyclin-dependent kinase 5 (Cdk5), a member of cdk family expressed only during postnatal development, is known to be hyper-activated during oxidative stress due to cleavage of its regulator p35 (p39) by calpain [154] (Fig.2). Peroxiredoxin I and II are family of peroxidases efficiently involved in scavenging reactive oxygen species was regulated by phosphorylation by Cdk5 [155]. Phosphorylation of these peroxidases by Cdk5 efficiently compromises the ROS scavenging system, thereby increasing neuronal toxicity during neurodegenerative conditions [155, 156]. However, the expression of the p39/p35 complex was decreased during aging, leading to uncontrolled cleavage of p35 by calpain, hyperactivating the Cdk5 function during aging [157]. Moreover, hyperactivation of Cdk5 will result in altered phosphorylation of both NMDA receptor and CaMKII therefore altering synaptic potentiation.
Glycogen and LTP
The brain has a very low capability of storage of carbohydrates in the form of glycogen and is highly dependents upon oxidative metabolism. Glycogen is stored exclusively in astrocytes (3–6 μmol glycosyl units/g) [158], but can act as a buffer against hypoglycemic effects on neurons [116, 159, 160] but may be insufficient during severe hypoglycemic conditions. Like ATP, there is no significant difference in glycogen levels observed during aging. Although the brain accounts for 2% of the total body weight it depends on 20 % of the total systemic oxygen consumption. When the cellular oxygen supply is reduced to a critical level, as occurs in hypoxia and ischemia, damage to brain cells occurs and can also lead to depletion of the brain glycogen storage. However the detailed study of the effects of glycogen stores on induction and expression of LTP during aging is still unclear. A more pertinent question focuses on the impact of energy metabolism during aging on LTP
Summary and treatment approaches – caloric restriction
We have highlighted a number of metabolic changes during aging, which can alter the ability to respond to physiological increase of metabolic demand or metabolic insults such as ischemia-reperfusion injury. Several treatment approaches, including administration of dietary supplements such as vitamins and antioxidants, have been tested to prevent age-related alteration of cellular function and to improve outcome after hypoxic/ischemic events. Moreover, dietary interventions such as caloric restriction and intermittent fasting which alter the substrates underlying metabolism and its efficiency, have gained a lot of attention as life-span extending strategy.
Strategies to Improve Hypoxic/Ischemic tolerance in Aging
Pyruvate can be easily transported into neurons via high affinity MCT and can be used effectively as an alternate metabolic substitute for glucose to prevent neuronal damage caused by hypoglycemia [161] or oxygen-glucose deprivation [162]. However, its application as a potential supplement for enhancing mitochondrial function in neurons during aging remain to be analyzed [76]. Creatine is also very important in energy metabolism, and augmentation of creatine levels has been suggested to be neuroprotective [163–165]. Metabolic neuroprotection may be one aspect of clinical stroke treatment, depending on critical mechanisms. For example, an improvement of cellular energetics can be achieved with administration of L-carnitine, coenzyme Q10, and nicotinamide, which can ameliorate cellular energy deficits [29, 166, 167]. Interestingly, under resting conditions, levels of energy stores such ATP and glycogen are not significantly altered in aging individuals, but this alteration may become relevant in condition of sustained metabolic demand, and alter the ability of neurons to respond to functional and metabolic stress [64]. Studies in isolated cortical neurons, for example, reported that aging was correlated with a larger drop of ATP during oxygen and/or glucose deprivation.
Mechanism of Caloric Restriction
Caloric or dietary restriction (CR) of food intake has consistently enhanced lifespan across a number of species, through a variety of mechanisms [168–172]. Caloric restriction may be achieved through as little as a 20% decrease in daily caloric intake, or alternatively, through intermittent fasting (IF) (ie, food intake every other day). This lowered substrate diet appears to result in ketosis, decreased fat stores, and possibly acts through both altered gene expression (i.e., sirtuins and Foxo) as well as simply decreasing metabolic needs, perhaps leading to decreased ROS and secondary damage [173]. A number of mechanisms have been proposed as underlying this effect.
For example, possible mechanisms of CR converge on changes in mitochondrial function. ROS signaling may change considerably, with CR inducing Sirt3, which in turn enhances glutathione via IDH2 [173]. Additional effects may include changes in insulin/IGF-1 signaling, mTOR responses and changes associated with decreased glucose consumption [168]. The major effects of the intermittent fasting includes reduced blood glucose levels and enhanced fat utilization leading to ketosis, drastically affecting how the brain is supplied with nutrients, particularly ketone bodies rather than glucose. Thus neurons, rely less on glucose utilization via glycolysis, but oxidize ketone bodies directly in the mitochondria to support their activity. In addition, the induction of mitochondrial uncoupling during CR/IF can reduce ROS formation by increasing proton leakage across the mitochondrial membrane and thus disconnecting oxidative phosphorylation from the electron transport chain [174, 175].
But, there are some limitations of CR. If the caloric restriction is begun too late in life then it has little effect (possibly after age 60 in humans), so this needs to be a life-long approach. Likewise, since part of the efficacy of the diet comes from fasting, then muscle and bone loss appear to occur with the caloric restriction, unless offset by changes in exercise. In some ways, exercise may recreate some of the effects, particularly in humans over age 60, where the side effects of the actual caloric restriction may be deleterious on overall health.
Aging related changes occur in gene regulation and secondary DNA damage occur as well [176], including an enhanced “stress” response, leading to decreased secondary sprouting and axonal repair mechanisms. One example in Drosophila suggests a trade-off between reproduction and lifespan, with CR (or even specific amino acid enhancement/reduction) leading to longer lifespan but reduced fecundity, and greater caloric intake enhancing fecundity but lowering life span [170]. There is considerable interest in locating effects of CR which might be reduced to altering protein or genetic targets with pharmaceuticals, hence providing some of the benefit of CR but without the necessary fasting required.
Although CR leads to an improvement in lifespan of the individual, the precise mechanisms of CR induced lifespan extension and longevity are not very well understood. Therefore, finding the molecular mechanisms whereby CR regulates lifespan has attractive potential in aging studies. Recently it has been reported that neuroprotective effects of CR and exercise are mediated, in part, by changes in gene transcriptions in which down-regulation of microRNAs (miRNAs) has been observed. They target the mRNAs encoding cell survival proteins in the beneficial effects of CR on the aging brain. These miRNAs (miR-34a, miR30e and miR181-a-1*) are short non-coding RNAs that typically bind to the transcripts and inhibit translation of the targeted mRNA. These miRNAs are significantly lower in brain tissue samples from old mice (24–28 months-old) that had been maintained on a CR diet (40% CR beginning at 4 months of age) compared to mice on the usual ad libitum diet. Interestingly, all three of these miRNAs were predicted to have at least one target site for Bcl2, an anti-apoptotic protein previously been shown to increase with CR. CR also results in decreases in pro-apoptotic genes such as Bax and several caspases [177, 178]. Recent studies also highlighted the p16INK4a gene, a cyclin-dependent kinase inhibitor, which is believed to play an important role in tumor growth suppression and cell senescence. p16INK4a suppression by glucose restriction contributes to human cellular lifespan extension through SIRT1-mediated epigenetic and genetic mechanisms [179]. The accumulation of p16 contributes to senescence by negatively regulating the cell cycle in vitro and in vivo.
Caloric Restriction and Cerebrovascular Disease
Hypertension is a major risk factor for both coronary artery disease and stroke. There are many studies with CR documenting an improvement in healthy life span in rodents, monkey and humans. When rats are maintained on CR they exhibit enhanced cardiovascular adaptation to stress by rapid recovery of blood pressure (BP) and heart rate (HR) upon removal from the stress. When rats are maintained with CR, their resting BPs are significantly decreased whereas monkeys on the CR diet exhibit improved glucose regulation, decreased body fat, and reduced BP. Rats maintained on intermittent fasting (IF) are similar to those on a CR diet in exhibiting an increased resistance to kainic acid induced degeneration of neurons [180]. Additionally, CR leads to decreased low density lipoprotein (LDL) cholesterol and increased high density lipoprotein cholesterol suggesting one mechanism associated with improved cardiovascular function [181]. CR also reduced inflammatory processes that likely contribute to atherosclerosis, as indicated by the reduced levels of leukocytes and circulating levels of tumor necrosis factor and other inflammatory cytokines [182]. Moreover studies have also showed that CR can suppress inflammatory responses to damage to vascular endothelial cells, neurons and cardiac myocytes. CR also decreased pro-inflammatory cytokines [183]. CR also led to reduced levels of oxidative stress in the cardiovascular system as indicated by decreased oxidative modifications of protein (The heart oxidative protein markers Nepsilon-(carboxyethyl) lysine (CEL), Nepsilon-(carboxymethyl) lysine (CML), Nepsilon-(malondialdehyde)lysine (MDA-lys), and glutamic semialdehyde) and DNA and decreased levels of lipid peroxidation in the heart [184, 185].
Caloric Restriction and Nervous System Function
CR imposes a mild stress on brain cells, which appears to enhance their ability to resist more severe stress, possibly through increased brain-derived neurotrophic factor (BDNF) levels. Therefore, neurons from rats on a CR diet are more resistant to being killed by oxidative, metabolic and excitotoxic insults, presumably by the increasing in stress resistant proteins and BDNF [186], and interestingly, administration of 2-Deoxy-D-glucose also increases the production of stress resistant protein. These results suggest the possibility that reduced intake of glucose would increase the resistance of neurons to stroke. Apart from increasing BDNF, CR also causes decreased activity of the sympathetic nervous system in both rodents and humans, which may play an important role in the ability of CR to decrease BP and cardiovascular morbidity [187]. Measurements of concentration of ACTH and corticosterone under non stress and stress conditions revealed differences in activity of the hypothalamic pituitary adrenal axis; for example, basal levels of ACTH and corticosterone are increased with an IF diet, however these levels are significantly attenuated with stress compared to controls [188].
Food intake and active life style also play important roles with age associated cognitive decline. Reports show, for example, that improving the diet of older people rich with B-vitamins, antioxidants and omega-3s delay the onset of age associated cognitive decline [189–191]. Studies also showed an active and socially integrated lifestyle in late life may protect against dementia [192] notably by decreasing the risk factors associated with aging. Participation with activities that stimulate brain function has been shown to reduce cognitive decline. NMDA dependent LTP deficit seen in aged animals may be at least in part due to a decrease in expression of the NR1 subunit. However, lifelong caloric restriction can prevent this loss of NMDA subunit expression and may also prevent age-related deficits in LTP [135]. Enhanced newly generated neurons from stem cells and other factors may also collectively increase synaptic plasticity and promote increased survival of neurons [193].
Summary
There are several similarities in the two examples we have provided of specific alterations in age-related responses to ischemia/hypoxia and reduced cognitive enhancement possible with aging, which are mirrored at the whole animal level. A number of programmed and acquired abnormalities can affect metabolism in aged brain tissue, including changes at the vascular, glial and neuronal aspects of the metabolic unit. Normal functioning of the tissue for ongoing brain perception and interaction with the environment continues in most individuals with successful aging, but clearly in some there are pathological consequences.
Acknowledgments
This work was supported by grants from NIA (R01 AG037599-01) and a VA Merit Review award.
References
- [1].Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–94. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–79. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- [3].Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- [4].Lynch MA, Voss KL. Membrane arachidonic acid concentration correlates with age and induction of long-term potentiation in the dentate gyrus in the rat. Eur J Neurosci. 1994;6:1008–14. doi: 10.1111/j.1460-9568.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- [5].McGahon B, Clements MP, Lynch MA. The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience. 1997;81:9–16. doi: 10.1016/s0306-4522(97)00116-4. [DOI] [PubMed] [Google Scholar]
- [6].Shetty AK. Reelin Signaling, Hippocampal Neurogenesis, and Efficacy of Aspirin Intake & Stem Cell Transplantation in Aging and Alzheimer’s disease. Aging Dis. 2010;1:2–11. [PMC free article] [PubMed] [Google Scholar]
- [7].Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–35. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–63. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Moore CI, Browning MD, Rose GM. Hippocampal plasticity induced by primed burst, but not long-term potentiation, stimulation is impaired in area CA1 of aged Fischer 344 rats. Hippocampus. 1993;3:57–66. doi: 10.1002/hipo.450030106. [DOI] [PubMed] [Google Scholar]
- [10].Turner DA, Adamson DC. Neuronal-astrocyte metabolic interactions: understanding the transition into abnormal astrocytoma metabolism. J Neuropathol Exp Neurol. 2011;70:167–76. doi: 10.1097/NEN.0b013e31820e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zarchin N, Meilin S, Rifkind J, Mayevsky A. Effect of aging on brain energy-metabolism. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:117–20. doi: 10.1016/s1095-6433(01)00537-2. [DOI] [PubMed] [Google Scholar]
- [12].Zhang X, Liu H, Wu J, Liu M, Wang Y. Metabonomic alterations in hippocampus, temporal and prefrontal cortex with age in rats. Neurochem Int. 2009;54:481–7. doi: 10.1016/j.neuint.2009.02.004. [DOI] [PubMed] [Google Scholar]
- [13].Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–58. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- [14].Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271:165–70. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- [15].Disterhoft JF, Moyer JR, Jr, Thompson LT. The calcium rationale in aging and Alzheimer’s disease. Evidence from an animal model of normal aging. Ann N Y Acad Sci. 1994;747:382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- [16].Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- [17].Jin K. Modern Biological Theories of Aging. Aging Dis. 2010;1:72–74. [PMC free article] [PubMed] [Google Scholar]
- [18].Thibault O, Porter NM, Chen KC, Blalock EM, Kaminker PG, Clodfelter GV, Brewer LD, Landfield PW. Calcium dysregulation in neuronal aging and Alzheimer’s disease: history and new directions. Cell Calcium. 1998;24:417–33. doi: 10.1016/s0143-4160(98)90064-1. [DOI] [PubMed] [Google Scholar]
- [19].Boveris A, Navarro A. Brain mitochondrial dysfunction in aging. IUBMB Life. 2008;60:308–14. doi: 10.1002/iub.46. [DOI] [PubMed] [Google Scholar]
- [20].Raffaello A, Rizzuto R. Mitochondrial longevity pathways. Biochim Biophys Acta. 2011;1813:260–8. doi: 10.1016/j.bbamcr.2010.10.007. [DOI] [PubMed] [Google Scholar]
- [21].Petit-Taboue MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7:176–84. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- [22].Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- [23].Hoyer S, Krier C. Ischemia and aging brain. Studies on glucose and energy metabolism in rat cerebral cortex. Neurobiol Aging. 1986;7:23–9. doi: 10.1016/0197-4580(86)90022-9. [DOI] [PubMed] [Google Scholar]
- [24].Gold PE. Glucose and age-related changes in memory. Neurobiol Aging. 2005;26(Suppl 1):60–4. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [25].McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A. 2000;97:2881–5. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Foster KA, Margraf RR, Turner DA. NADH hyperoxidation correlates with enhanced susceptibility of aged rats to hypoxia. Neurobiol Aging. 2008;29:598–613. doi: 10.1016/j.neurobiolaging.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Roberts EL, Jr, Chih CP. Age-related alterations in energy metabolism contribute to the increased vulnerability of the aging brain to anoxic damage. Brain Res. 1995;678:83–90. doi: 10.1016/0006-8993(95)00168-p. [DOI] [PubMed] [Google Scholar]
- [28].Yager JY, Thornhill JA. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neurosci Biobehav Rev. 1997;21:167–74. doi: 10.1016/s0149-7634(96)00006-1. [DOI] [PubMed] [Google Scholar]
- [29].Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am J Clin Nutr. 2007;86:1738–44. doi: 10.1093/ajcn/86.5.1738. [DOI] [PubMed] [Google Scholar]
- [30].Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107:18670–5. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Santos RX, Correia SC, Wang X, Perry G, Smith MA, Moreira PI, Zhu X. Alzheimer’s disease: diverse aspects of mitochondrial malfunctioning. Int J Clin Exp Pathol. 2010;3:570–81. [PMC free article] [PubMed] [Google Scholar]
- [32].Aliev G, Liu J, Shenk JC, Fischbach K, Pacheco GJ, Chen SG, Obrenovich ME, Ward WF, Richardson AG, Smith MA, Gasimov E, Perry G, Ames BN. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med. 2009;13:320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Navarro A, Lopez-Cepero JM, Bandez MJ, Sanchez-Pino MJ, Gomez C, Cadenas E, Boveris A. Hippocampal mitochondrial dysfunction in rat aging. Am J Physiol Regul Integr Comp Physiol. 2008;294:R501–9. doi: 10.1152/ajpregu.00492.2007. [DOI] [PubMed] [Google Scholar]
- [34].Kilbride SM, Telford JE, Davey GP. Age-related changes in H2O2 production and bioenergetics in rat brain synaptosomes. Biochim Biophys Acta. 2008;1777:783–8. doi: 10.1016/j.bbabio.2008.05.445. [DOI] [PubMed] [Google Scholar]
- [35].Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1244–9. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- [36].Navarro A, Sanchez Del Pino MJ, Gomez C, Peralta JL, Boveris A. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R985–92. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- [37].Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int. 2008;53:126–31. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- [38].Ferrandiz ML, Martinez M, De Juan E, Diez A, Bustos G, Miquel J. Impairment of mitochondrial oxidative phosphorylation in the brain of aged mice. Brain Res. 1994;644:335–8. doi: 10.1016/0006-8993(94)91699-3. [DOI] [PubMed] [Google Scholar]
- [39].Xu K, Puchowicz MA, Sun X, LaManna JC. Decreased brainstem function following cardiac arrest and resuscitation in aged rat. Brain Res. 2010;1328:181–9. doi: 10.1016/j.brainres.2010.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Harmon HJ, Nank S, Floyd RA. Age-dependent changes in rat brain mitochondria of synaptic and non-synaptic origins. Mech Ageing Dev. 1987;38:167–177. doi: 10.1016/0047-6374(87)90076-5. [DOI] [PubMed] [Google Scholar]
- [41].Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J Neurochem. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- [42].Jones TT, Brewer GJ. Age-related deficiencies in complex I endogenous substrate availability and reserve capacity of complex IV in cortical neuron electron transport. Biochim Biophys Acta. 2010;1797:167–76. doi: 10.1016/j.bbabio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Navarro A, Bandez MJ, Lopez-Cepero JM, Gomez C, Boveris A. High doses of vitamin E improve mitochondrial dysfunction in rat hippocampus and frontal cortex upon aging. Am J Physiol Regul Integr Comp Physiol. 2011;300:R827–34. doi: 10.1152/ajpregu.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- [45].Reeve AK, Krishnan KJ, Turnbull D. Mitochondrial DNA mutations in disease, aging, and neurodegeneration. Ann N Y Acad Sci. 2008;1147:21–9. doi: 10.1196/annals.1427.016. [DOI] [PubMed] [Google Scholar]
- [46].Rossignol R, Faustin B, Rocher C, Malgat M, Mazat JP, Letellier T. Mitochondrial threshold effects. Biochem J. 2003;370:751–62. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- [48].Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–20. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- [49].Fearnley JM, Lees AJ. Agein and Parkinson’s Disease: Substantia Nigra Regional Selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- [50].Stuart JA, Bourque BM, de Souza-Pinto NC, Bohr VA. No evidence of mitochondrial respiratory dysfunction in OGG1-null mice deficient in removal of 8-oxodeoxyguanine from mitochondrial DNA. Free Radic Biol Med. 2005;38:737–45. doi: 10.1016/j.freeradbiomed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- [51].Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [52].Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–72. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xu J-X. Radical Metabolism Is Partner to Energy Metabolism in Mitochondria. Ann N Y Acad Sci. 2004;1011:57–60. doi: 10.1007/978-3-662-41088-2_6. [DOI] [PubMed] [Google Scholar]
- [54].Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98:10469–74. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34:609–16. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- [56].Sanz A, Caro P, Ibanez J, Gomez J, Gredilla R, Barja G. Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. J Bioenerg Biomembr. 2005;37:83–90. doi: 10.1007/s10863-005-4131-0. [DOI] [PubMed] [Google Scholar]
- [57].Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–14. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–20. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–5. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- [61].Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:1914–24. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Disterhoft JF, Thompson LT, Moyer JR, Jr, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–20. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- [63].Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–92. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- [64].Toescu EC, Verkhratsky A. Neuronal ageing from an intraneuronal perspective: roles of endoplasmic reticulum and mitochondria. Cell Calcium. 2003;34:311–23. doi: 10.1016/s0143-4160(03)00142-8. [DOI] [PubMed] [Google Scholar]
- [65].Verkhratsky A, Toescu EC. Calcium and neuronal ageing. Trends Neurosci. 1998;21:2–7. doi: 10.1016/s0166-2236(97)01156-9. [DOI] [PubMed] [Google Scholar]
- [66].Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;6:307–17. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Popa-Wagner A, Buga AM, Kokaia Z. Perturbed cellular response to brain injury during aging. Ageing Res Rev. 2011;10:71–9. doi: 10.1016/j.arr.2009.10.008. [DOI] [PubMed] [Google Scholar]
- [68].Barnett H. Stroke prevention in the elderly. Clin Exp Hypertens. 2002;24:563. doi: 10.1081/ceh-120015333. [DOI] [PubMed] [Google Scholar]
- [69].Van Kooten F, Koudstaal PJ. Epidemiology of post-stroke dementia. Haemostasis. 1998;28:124–33. doi: 10.1159/000022424. [DOI] [PubMed] [Google Scholar]
- [70].Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- [71].Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–18. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- [72].Floyd RA, Carney JM. Age influence on oxidative events during brain ischemia/reperfusion. Arch Gerontol Geriatr. 1991;12:155–77. doi: 10.1016/0167-4943(91)90025-l. [DOI] [PubMed] [Google Scholar]
- [73].Roberts EL, Jr, Rosenthal M, Sick TJ. Age-related modifications of potassium homeostasis and synaptic transmission during and after anoxia in rat hippocampal slices. Brain Res. 1990;514:111–8. doi: 10.1016/0006-8993(90)90441-d. [DOI] [PubMed] [Google Scholar]
- [74].Benzi G, Pastoris O, Marzatico F, Villa RF, Dagani F, Curti D. The mitochondrial electron transfer alteration as a factor involved in the brain aging. Neurobiol Aging. 1992;13:361–8. doi: 10.1016/0197-4580(92)90109-b. [DOI] [PubMed] [Google Scholar]
- [75].Martin RL, Lloyd HG, Cowan AI. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci. 1994;17:251–7. doi: 10.1016/0166-2236(94)90008-6. [DOI] [PubMed] [Google Scholar]
- [76].Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- [77].Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010;38:841–60. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- [78].Scarlett JL, Murphy MP. Release of apoptogenic proteins from the mitochondrial intermembrane space during the mitochondrial permeability transition. FEBS Lett. 1997;418:282–6. doi: 10.1016/s0014-5793(97)01391-4. [DOI] [PubMed] [Google Scholar]
- [79].Sato E, Inoue A, Kurokawa T, Ishibashi S. Early changes in glucose metabolism in the cerebrum of senescence accelerated mouse: involvement of glucose transporter. Brain Res. 1994;637:133–8. doi: 10.1016/0006-8993(94)91226-2. [DOI] [PubMed] [Google Scholar]
- [80].Benzi G, Pastoris O, Villa RF, Giuffrida AM. Effect of aging on cerebral cortex energy metabolism in hypoglycemia and posthypoglycemic recovery. Neurobiology of Aging. 1984;5:205–212. doi: 10.1016/0197-4580(84)90064-2. [DOI] [PubMed] [Google Scholar]
- [81].Funahashi T, Floyd RA, Carney JM. Age effect on brain pH during ischemia/reperfusion and pH influence on peroxidation. Neurobiol Aging. 1994;15:161–7. doi: 10.1016/0197-4580(94)90107-4. [DOI] [PubMed] [Google Scholar]
- [82].Foster KA, Beaver CJ, Turner DA. Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices. Neuroscience. 2005;132:645–57. doi: 10.1016/j.neuroscience.2005.01.040. [DOI] [PubMed] [Google Scholar]
- [83].Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- [84].Sims NR, Pulsinelli WA. Altered mitochondrial respiration in selectively vulnerable brain subregions following transient forebrain ischemia in the rat. J Neurochem. 1987;49:1367–74. doi: 10.1111/j.1471-4159.1987.tb01001.x. [DOI] [PubMed] [Google Scholar]
- [85].Soane L, Kahraman S, Kristian T, Fiskum G. Mechanisms of impaired mitochondrial energy metabolism in acute and chronic neurodegenerative disorders. J Neurosci Res. 2007;85:3407–15. doi: 10.1002/jnr.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Toman J, Fiskum G. Influence of aging on membrane permeability transition in brain mitochondria. J Bioenerg Biomembr. 2011;43:3–10. doi: 10.1007/s10863-011-9337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Siqueira IR, Cimarosti H, Fochesatto C, Salbego C, Netto CA. Age-related susceptibility to oxygen and glucose deprivation damage in rat hippocampal slices. Brain Research. 2004;1025:226–230. doi: 10.1016/j.brainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- [88].Martin LJ, Adams NA, Pan Y, Price A, Wong M. The Mitochondrial Permeability Transition Pore Regulates Nitric Oxide-Mediated Apoptosis of Neurons Induced by Target Deprivation. J Neurosci. 2011;31:359–370. doi: 10.1523/JNEUROSCI.2225-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–48. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- [91].Villa RF, Gorini A, Hoyer S. ATPases of synaptic plasma membranes from hippocampus after ischemia and recovery during ageing. Neurochem Res. 2002;27:861–70. doi: 10.1023/a:1020381829107. [DOI] [PubMed] [Google Scholar]
- [92].Galeffi F, Sah R, Pond BB, George A, Schwartz-Bloom RD. Changes in intracellular chloride after oxygen-glucose deprivation of the adult hippocampal slice: effect of diazepam. J Neurosci. 2004;24:4478–88. doi: 10.1523/JNEUROSCI.0755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Douglas RM, Goddard GV. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975;86:205–15. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- [95].Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- [96].Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–7. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- [97].Barnes CA, McNaughton BL. Neurophysiological comparison of dendritic cable properties in adolescent, middle-aged, and senescent rats. Exp Aging Res. 1979;5:195–206. doi: 10.1080/03610737908257198. [DOI] [PubMed] [Google Scholar]
- [98].Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–8. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- [99].Potegal M. Role of the caudate nucleus in spatial orientation of rats. J Comp Physiol Psychol. 1969;69:756–64. doi: 10.1037/h0028168. [DOI] [PubMed] [Google Scholar]
- [100].Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–85. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Deupree DL, Turner DA, Watters CL. Spatial performance correlates with in vitro potentiation in young and aged Fischer 344 rats. Brain Research. 1991;554:1–9. doi: 10.1016/0006-8993(91)90164-q. [DOI] [PubMed] [Google Scholar]
- [102].Barnes CA, Rao G, Houston FP. LTP induction threshold change in old rats at the perforant path--granule cell synapse. Neurobiol Aging. 2000;21:613–20. doi: 10.1016/s0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- [103].Landfield PW, Lynch G. Impaired monosynaptic potentiation in in vitro hippocampal slices from aged, memory-deficient rats. J Gerontol. 1977;32:523–33. doi: 10.1093/geronj/32.5.523. [DOI] [PubMed] [Google Scholar]
- [104].Landfield PW, McGaugh JL, Lynch G. Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Res. 1978;150:85–101. doi: 10.1016/0006-8993(78)90655-8. [DOI] [PubMed] [Google Scholar]
- [105].Barnes CA. Long-term potentiation and the ageing brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:765–72. doi: 10.1098/rstb.2002.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rossi MA, Mash DC, deToledo-Morrell L. Spatial memory in aged rats is related to PKCgamma-dependent G-protein coupling of the M1 receptor. Neurobiol Aging. 2005;26:53–68. doi: 10.1016/j.neurobiolaging.2004.02.029. [DOI] [PubMed] [Google Scholar]
- [108].Huang YY, Kandel ER. Age-related enhancement of a protein synthesis-dependent late phase of LTP induced by low frequency paired-pulse stimulation in hippocampus. Learn Mem. 2006;13:298–306. doi: 10.1101/lm.166906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nicoll RA. Expression mechanisms underlying long-term potentiation: a postsynaptic view. Philos Trans R Soc Lond B Biol Sci. 2003;358:721–6. doi: 10.1098/rstb.2002.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–21. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- [111].Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–4. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- [112].Nicoll RA, Kauer JA, Malenka RC. The current excitement in long-term potentiation. Neuron. 1988;1:97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- [113].Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998;18:3171–9. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci. 2002;22:9932–40. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25:2609–16. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Abdelmalik PA, Shannon P, Yiu A, Liang P, Adamchik Y, Weisspapir M, Samoilova M, McIntyre Burnham W, Carlen PL. Hypoglycemic seizures during transient hypoglycemia exacerbate hippocampal dysfunction. Neurobiol Dis. 2007;26:646–660. doi: 10.1016/j.nbd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [117].Izumi Y, Katsuki H, Zorumski CF. Monocarboxylates (pyruvate and lactate) as alternative energy substrates for the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1997;232:17–20. doi: 10.1016/s0304-3940(97)00567-3. [DOI] [PubMed] [Google Scholar]
- [118].Sadgrove MP, Beaver CJ, Turner DA. Effects of relative hypoglycemia on LTP and NADH imaging in rat hippocampal slices. Brain Res. 2007;1165:30–9. doi: 10.1016/j.brainres.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yamada KA, Rensing N, Izumi Y, De Erausquin GA, Gazit V, Dorsey DA, Herrera DG. Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatr Res. 2004;55:372–9. doi: 10.1203/01.PDR.0000110523.07240.C1. [DOI] [PubMed] [Google Scholar]
- [120].Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–90. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- [121].Izumi Y, Zorumski CF. Involvement of nitric oxide in low glucose-mediated inhibition of hippocampal long-term potentiation. Synapse. 1997;25:258–62. doi: 10.1002/(SICI)1098-2396(199703)25:3<258::AID-SYN4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [122].Lynch G, Rex CS, Gall CM. LTP consolidation: substrates, explanatory power, and functional significance. Neuropharmacol. 2007;52:12–23. doi: 10.1016/j.neuropharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- [123].Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci. 2001;21:5568–73. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–53. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Barnes CA, Rao G, Foster TC, McNaughton BL. Region-specific age effects on AMPA sensitivity: electrophysiological evidence for loss of synaptic contacts in hippocampal field CA1. Hippocampus. 1992;2:457–68. doi: 10.1002/hipo.450020413. [DOI] [PubMed] [Google Scholar]
- [126].Ekstrom AD, Meltzer J, McNaughton BL, Barnes CA. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal “place fields”. Neuron. 2001;31:631–8. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- [127].Boric K, Munoz P, Gallagher M, Kirkwood A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J Neurosci. 2008;28:8034–9. doi: 10.1523/JNEUROSCI.2036-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Collingridge GL, Kehl SJ, McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–9. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- [131].Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253–64. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Grosshans DR, Browning MD. Protein kinase C activation induces tyrosine phosphorylation of the NR2A and NR2B subunits of the NMDA receptor. J Neurochem. 2001;76:737–44. doi: 10.1046/j.1471-4159.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- [133].Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–58. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- [134].Barnes CA, Rao G, McNaughton BL. Increased electrotonic coupling in aged rat hippocampus: a possible mechanism for cellular excitability changes. J Comp Neurol. 1987;259:549–58. doi: 10.1002/cne.902590405. [DOI] [PubMed] [Google Scholar]
- [135].Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res. 2000;78:154–62. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- [136].Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–4. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- [137].Gustafsson B, Wigstrom H. Physiological mechanisms underlying long-term potentiation. Trends Neurosci. 1988;11:156–62. doi: 10.1016/0166-2236(88)90142-7. [DOI] [PubMed] [Google Scholar]
- [138].Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- [139].Gustafsson B, Wigstrom H, Abraham WC, Huang YY. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–80. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lau CG, Zukin RS, Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature Reviews Neuroscience. 2007;8:413–26. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- [141].Lister JP, Barnes CA. Neurobiological changes in the hippocampus during normative aging. Arch Neurol. 2009;66:829–33. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- [142].Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–92. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Fukunaga K, Muller D, Miyamoto E. CaM kinase II in long-term potentiation. Neurochem Int. 1996;28:343–58. doi: 10.1016/0197-0186(95)00097-6. [DOI] [PubMed] [Google Scholar]
- [144].Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–88. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- [145].Shetty PK, Huang FL, Huang KP. Ischemia-elicited oxidative modulation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2008;283:5389–401. doi: 10.1074/jbc.M708479200. [DOI] [PubMed] [Google Scholar]
- [146].Robison AJ, Winder DG, Colbran RJ, Bartlett RK. Oxidation of calmodulin alters activation and regulation of CaMKII. Biochem Biophys Res Commun. 2007;356:97–101. doi: 10.1016/j.bbrc.2007.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Huang KP, Huang FL, Shetty PK, Yergey AL. Modification of protein by disulfide S-monoxide and disulfide S-dioxide: distinctive effects on PKC. Biochemistry. 2007;46:1961–71. doi: 10.1021/bi061955i. [DOI] [PubMed] [Google Scholar]
- [148].Foster TC, Foster TC. Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs. 2006;20:153–66. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- [149].Poon HF, Shepherd HM, Reed TT, Calabrese V, Stella AM, Pennisi G, Cai J, Pierce WM, Klein JB, Butterfield DA. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging. 2006;27:1020–34. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- [150].Parihar MS, Brewer GJ. Simultaneous age-related depolarization of mitochondrial membrane potential and increased mitochondrial reactive oxygen species production correlate with age-related glutamate excitotoxicity in rat hippocampal neurons. J Neurosci Res. 2007;85:1018–32. doi: 10.1002/jnr.21218. [DOI] [PubMed] [Google Scholar]
- [151].Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J Neuroscie Res. 2008;86:2339–52. doi: 10.1002/jnr.21679. [DOI] [PubMed] [Google Scholar]
- [152].Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res. 2007;1127:10–8. doi: 10.1016/j.brainres.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Liu RM, Liu R-M. Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J Neurosci Res. 2002;68:344–51. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- [154].Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–4. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- [155].Qu D, Rashidian J, Mount MP, Aleyasin H, Parsanejad M, Lira A, Haque E, Zhang Y, Callaghan S, Daigle M, Rousseaux MW, Slack RS, Albert PR, Vincent I, Woulfe JM, Park DS. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- [156].Sun KH, de Pablo Y, Vincent F, Shah K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J Neurochem. 2008;107:265–78. doi: 10.1111/j.1471-4159.2008.05616.x. [DOI] [PubMed] [Google Scholar]
- [157].Kim MJ, Oh SJ, Park SH, Kang HJ, Won MH, Kang TC, Park JB, Kim JI, Kim J, Lee JY. Neuronal loss in primary long-term cortical culture involves neurodegeneration-like cell death via calpain and p35 processing, but not developmental apoptosis or aging. Exp Mol Med. 2007;39:14–26. doi: 10.1038/emm.2007.3. [DOI] [PubMed] [Google Scholar]
- [158].Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol. 1986;15:511–24. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- [159].Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res. 2003;72:25–32. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res. 2005;79:74–80. doi: 10.1002/jnr.20335. [DOI] [PubMed] [Google Scholar]
- [161].Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA. Pyruvate Administered After Severe Hypoglycemia Reduces Neuronal Death and Cognitive Impairment. Diabetes. 2005;54:1452–1458. doi: 10.2337/diabetes.54.5.1452. [DOI] [PubMed] [Google Scholar]
- [162].Shen H, Hu X, Liu C, Wang S, Zhang W, Gao H, Stetler RA, Gao Y, Chen J. Ethyl pyruvate protects against hypoxic-ischemic brain injury via anti-cell death and anti-inflammatory mechanisms. Neurobiol Dis. 2010;37:711–722. doi: 10.1016/j.nbd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Balestrino M, Rebaudo R, Lunardi G. Exogenous creatine delays anoxic depolarization and protects from hypoxic damage: dose-effect relationship. Brain Res. 1999;816:124–30. doi: 10.1016/s0006-8993(98)01131-7. [DOI] [PubMed] [Google Scholar]
- [164].Brustovetsky N, Brustovetsky T, Dubinsky JM. On the mechanisms of neuroprotection by creatine and phosphocreatine. J Neurochem. 2001;76:425–34. doi: 10.1046/j.1471-4159.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- [165].Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann Neurol. 2001;49:561–74. [PubMed] [Google Scholar]
- [166].Calabrese V, Colombrita C, Sultana R, Scapagnini G, Calvani M, Butterfield DA, Stella AM. Redox modulation of heat shock protein expression by acetylcarnitine in aging brain: relationship to antioxidant status and mitochondrial function. Antioxid Redox Signal. 2006;8:404–16. doi: 10.1089/ars.2006.8.404. [DOI] [PubMed] [Google Scholar]
- [167].Liu D, Pitta M, Mattson MP. Preventing NAD(+) depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann N Y Acad Sci. 2008;1147:275–82. doi: 10.1196/annals.1427.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Duan W, Ross CA. Potential therapeutic targets for neurodegenerative diseases: lessons learned from calorie restriction. Curr Drug Targets. 2010;11:1281–92. doi: 10.2174/1389450111007011281. [DOI] [PubMed] [Google Scholar]
- [170].Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Morley JE, Chahla E, Alkaade S. Antiaging, longevity and calorie restriction. Curr Opin Clin Nutr Metab Care. 2010;13:40–5. doi: 10.1097/MCO.0b013e3283331384. [DOI] [PubMed] [Google Scholar]
- [172].Smith DL, Jr, Nagy TR, Allison DB. Calorie restriction: what recent results suggest for the future of ageing research. Eur J Clin Invest. 2010;40:440–50. doi: 10.1111/j.1365-2362.2010.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–12. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch. 2010;459:277–89. doi: 10.1007/s00424-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Mookerjee SA, Divakaruni AS, Jastroch M, Brand MD. Mitochondrial uncoupling and lifespan. Mech Ageing Dev. 2010;131:463–72. doi: 10.1016/j.mad.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–91. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- [177].Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–37. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- [178].Khanna A, Muthusamy S, Liang R, Sarojini H, Wang E. Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging (Albany NY) 2011;3:223–36. doi: 10.18632/aging.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Li Y, Tollefsbol TO. p16 Suppression by Glucose Restriction Contributes to Human Cellular Lifespan Extension through SIRT1-Mediated Epigenetic and Genetic Mechanisms. PLoS One. 2011;6:e17421. doi: 10.1371/journal.pone.0017421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- [181].Kendall DM, Harmel AP. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: understanding the role of insulin resistance. Am J Manag Care. 2002;8:S635–53. quiz S654–7. [PubMed] [Google Scholar]
- [182].Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- [183].Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–9. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- [184].Pamplona R, Portero-Otin M, Requena J, Gredilla R, Barja G. Oxidative, glycoxidative and lipoxidative damage to rat heart mitochondrial proteins is lower after 4 months of caloric restriction than in age-matched controls. Mech Ageing Dev. 2002;123:1437–46. doi: 10.1016/s0047-6374(02)00076-3. [DOI] [PubMed] [Google Scholar]
- [185].Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–24. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- [186].Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–9. [PubMed] [Google Scholar]
- [187].Young JB, Mullen D, Landsberg L. Caloric restriction lowers blood pressure in the spontaneously hypertensive rat. Metabolism. 1978;27:1711–4. doi: 10.1016/0026-0495(78)90256-1. [DOI] [PubMed] [Google Scholar]
- [188].Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–9. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- [189].Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, Penke L, Rafnsson SB, Starr JM. Age-associated cognitive decline. Br Med Bull. 2009;92:135–52. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- [190].Van Dyk K, Sano M. The impact of nutrition on cognition in the elderly. Neurochem Res. 2007;32:893–904. doi: 10.1007/s11064-006-9241-5. [DOI] [PubMed] [Google Scholar]
- [191].Parrott MD, Greenwood CE. Dietary influences on cognitive function with aging: from high-fat diets to healthful eating. Ann N Y Acad Sci. 2007;1114:389–97. doi: 10.1196/annals.1396.028. [DOI] [PubMed] [Google Scholar]
- [192].Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- [193].Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- [194].Tan SE, Wenthold RJ, Soderling TR. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J Neurosci. 1994;14:1123–9. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Nicholls DG. Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol. 2002;34:1372–1381. doi: 10.1016/s1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- [196].Tielen AM, Mollevanger WJ, Lopes da Silva FH, Hollander CF. Neuronal plasticity in hippocampal slices of extremely old rats. In: Gipsen WH, Traber J, editors. Aging of the brain. Elsevier; Amsterdam: 1983. pp. 73–84. [Google Scholar]
- [197].Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J Neurophysiol. 1998;79:334–41. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- [198].Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–9. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]