Abstract
Objectives
The purpose of this study was to compare the vocal fold vibratory characteristics of ex vivo bovine, canine, ovine, and porcine larynges to human male and female vocal fold vibrations to determine the best model organism for laryngeal studies concerning vibratory and kinetic characteristics.
Study Design
Prospective experimental
Methods
High speed videos were gathered at 4000 frames per second (fps) in the animal models and human high-speed endoscopy data were gathered at 2000 fps. Videos were converted into kymograms and the amplitude, oscillation frequency, and phase difference of vocal fold vibration were measured.
Results
No statistically significant differences were found with respect to frequency, amplitude, or phase difference between canines and humans. Swine were not significantly different from human females but did have a significantly different oscillation frequency than human males. Ovine vibrational amplitudes were significantly different from humans, and bovine frequency and amplitude differed significantly from humans.
Conclusion
Canine and porcine larynges are the most appropriate model specimens for laryngeal studies contingent on vibratory or kinetic properties of phonation.
Keywords: High speed digital imaging, vocal fold vibration, mucosal wave, interspecies, excised larynx, human endoscopy
INTRODUCTION
The purpose of medical research is to develop innovations that improve treatment outcomes and the quality of life of patients. Because new treatment modalities and techniques are frequently tested on experimental models before human subjects, it is in the interest of medical researchers to determine which models are appropriate for a given application. In laryngology, this involves mitigating the difference between an ideal experimental model, such as an in vivo human larynx, and the available experimental models, such as ex vivo animal models. Candidate animal models have included species such as dog, sheep, pig, rabbit, rat, and cow. These laryngeal models have differential similarity to humans based on various dimensions, but none of them is a perfect representation. Researchers have investigated the degree to which these models resemble the human larynx using measures of anatomy(1–5), acoustics(6), aerodynamics(6, 7), immunology(5), and histology(8, 9). However, none have compared the vibratory characteristics of the vocal folds in these models using high-speed videokymographic analysis. This is an important measurement, as the parameters that are derived are a useful metric of vocal fold vibration and function; vocal fold vibration is the source of voice. Because pathologies such as vocal fold scarring and paralysis affect the vibration of the vocal folds, a study of the comparative vibratory characteristics of various animal models is essential to the determination of an appropriate experimental model of the human larynx and valuable to the development of effective treatment strategies for laryngeal disease.
Normal vocal fold vibration is characterized by a traveling wave of the mucosa that propagates superiorly from the inferior edge. Mathematically, it can be described by the one-dimensional wave equation:
| (1) |
where c is the mucosal wave velocity, x is the time-dependent displacement of the vocal fold edge from the glottal midline, and z is the displacement from the midpoint of the glottis in the direction of flow. This equation has sinusoidal solutions; for a given frontal plane, the glottal half-widths at the superior and inferior margins of the vocal fold cover are
| (2) |
where g1(t) and g2(t) are the time-dependent superior and inferior glottal half-widths, g01 and g02 are the superior and inferior prephonatory glottal half-widths, a1 and and a2 are the superior and inferior mucosal wave amplitudes, f is the frequency, and ϕ is the phase delay between the superior and inferior vocal fold edges(10). The phase difference can be written explicitly as
| (3) |
where z is the distance between the upper and lower vocal fold margins and c is the mucosal wave velocity. Thus, the vibration of a vocal fold edge can be described using four variables: amplitude, frequency, phase delay, and mucosal wave velocity. Applied to both vocal folds at the superior and inferior margins, this yields a set of sixteen variables which kinetically quantify phonation.
Digital videokymography (DKG) is a robust and useful measure of vocal fold vibration. The method involves digitally recording a high-speed video with a sampling rate of about an order of magnitude higher than the frequency of vibration and using computer programming to select a single line of pixels through time to produce a kymograph. Aberrant vocal fold vibration is easy to visualize in the kymograph, making this a clinically useful indicator of laryngeal function. Movement of the vocal fold mucosa follows a wave function that propagates in the superior direction along the mucosa margin; this vertical propagation is the result of the top and bottom lips of the vocal fold edge oscillating at the same frequency but out of phase. As a result, the top and bottom vocal fold edge are visible in the kymograph differentially in the time domain, and computer processing programs can calculate sinusoidal regressions of the glottal edge using image processing techniques (11). Physiologically relevant parameters can be extracted, such as the mucosal wave amplitude, frequency, and phase difference. One important limitation of DKG analysis is that the analysis is performed on the vocal fold movement at the glottal midpoint only. The single-pixel line images do not provide a complete picture of the vocal folds. However, because the amplitude of vocal fold vibration is maximal at the glottal midpoint, these measurements can be used to qualitatively characterize vocal fold vibration at other points with the knowledge that amplitude will diminish as the point in consideration approaches an endpoint of the vocal fold.
Hirano (12) developed the body cover model of the vocal folds to explain the properties of the mucosal wave based on its histological and rheological characteristics. In humans, the “body” of this model is the deep layer of the lamina propria (LP) and the thyroarytenoid (TA) muscle and the “cover” is the intermediate and superficial layers of the LP and the vocal fold epithelium. Kakita et al. (13) showed in excised canine larynges that the transverse forces acting on the vocal fold act almost entirely on the LP. This suggests that mucosal wave properties are predominately determined by the LP and the underlying TA muscle. Organisms or species with histological differences between the LP and TA muscle may have different mucosal wave properties. The human LP has three layers: the superficial layer (SLLP), intermediate layer (ILLP), and the deep layer (DLLP) (14). Canine LP also has three layers with similar tissue composition as the human LP (8). Porcine LP has two layers that are less distinct than the human or canine LP, but the layers have similar histological compositions to those of the human SLLP and DLLP (8). Happak et al. compared the histochemistry of human and sheep laryngeal muscles, and concluded that the two species’ laryngeal muscles are similar (9).
The purpose of the present study was to investigate the quantitative vibratory characteristics of ex vivo pig, dog, sheep, and cow larynges. These data were then compared to the same parameters measured from in vivo human larynges to determine which animal models are most suitable for simulating the human larynx.
MATERIALS AND METHODS
Experimental Design
This study followed a prospective two-independent variable experimental design. One independent variable, the species of the ex vivo laryngeal specimen, contained four categorical levels: canine (N=7), porcine (N=7), ovine (N=5), and bovine (N=6). The other independent variable, the subglottal pressure at which high-speed videos were recorded of the phonation, contained three ordinal levels. Because the phonation pressure range differs by species, these levels differed by species, as seen in Table I. At least five larynges of each species were included in the sample population, and ten replicates of data were collected for each unique combination of larynx and subglottal pressure. Previous, unpublished human endoscopy data was collected with IRB approval from females (N=17) and males (N=18) and was analyzed in the same manner as the ex vivo data. The vocal fold length was estimated using the height and gender of the subjects and the equations proposed by Filho (15).
Table I.
The levels of subglottal pressure used for each ex vivo species.
| Species | Subglottal pressures (cm H2O) |
|---|---|
| Canine | {15, 20, 25} |
| Porcine | {15, 20, 25} |
| Ovine | {5,10,15}∨{15, 20, 25} |
| Bovine | {5,10,15}∨{15, 20, 25} |
Specimen Preparation
Larynges were obtained immediately post mortem and were stored frozen at −12° C in a 0.9% saline solution and thawed slowly in a cold water bath before use. Larynges were dissected to provide an unobstructed superior view of the vocal folds. The epiglottis, corniculate, and cuneiform cartilages were dissected away along with the associated tissues. The posterosuperior aspects of the thyroid cartilage were dissected away to allow insertion of three-pronged micromanipulators into the lateral aspects of the arytenoid cartilages. Because of the interspecies anatomical differences, the dissection procedure differed slightly among species.
Ovine and bovine larynges lack a distinct border between superior tissue and vocal fold mucosa, so superior tissue was conservatively removed to a point at which the vocal folds could be clearly visualized for high speed analysis.
The superior vocal folds of the porcine larynx were removed, despite the fact that they have been shown to be an active oscillator during phonation (6). This was done to facilitate more accurate quantification of the vocal fold vibration in the kymograph and to more closely model the human larynx, which only has one set of true vocal folds.
Experimental Procedure
Each larynx was mounted to an excised larynx phonation system (Figure 1) using a metal hose clamp. The experiments were conducted in a triple-walled sound attenuated room to reduce extraneous noise and help maintain constant humidity. The arytenoid cartilages were adducted using three pronged micromanipulators which allowed for three-dimensional control of the vocal folds. A suture in the laryngeal prominence of the anterior thyroid cartilage was fastened to an anterior micromanipulator to precisely control vocal fold elongation. The arytenoids were adducted to the point of approximation of the vocal processes, and the vocal folds were elongated to 105% of their anatomically resting vocal fold length to provide slight tension for phonation. Symmetry was maintained across the midsagittal plane using the arytenoid micromanipulators such that the superior and inferior margins of the contralateral vocal folds were aligned.
Figure 1.
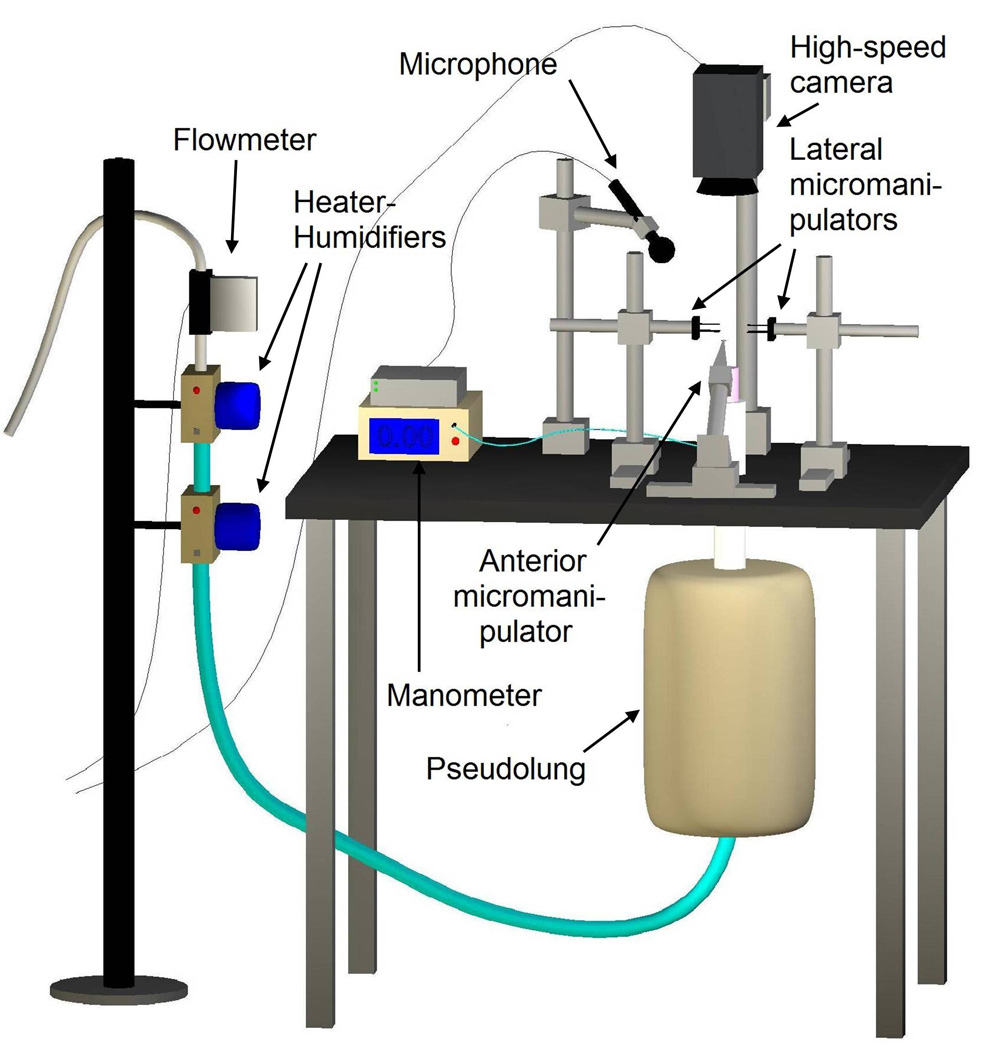
Diagram of the excised larynx phonation system.
The excised larynx phonation system was designed to mimic the human respiratory system; air from an internal building source was passed through two Concha Therm III heater-humidifiers (Fisher & Paykel Healthcare Inc., Laguna Hills, CA) and a pseudolung before entering the larynx. Pressure was measured directly below the larynx using a Heise digital pressure meter (901 series; Ashcroft Inc., Stratford, CT), and flow was measured using an Omega airflow meter (model FMA-1601A; Omega Engineering Inc., Stamford, CT). Acoustic data were measured using a Sony microphone (model ECM-88; Sony Electronics Inc., New York, NY) placed 10.0 cm from the glottis and positioned at a 45° angle to plane of the glottis. A symetrix pre-amplifier (model 302; Symetrix Inc., Mountlke Terrace, WA) was used to amplify the acoustic signal. All aerodynamic and acoustic data were acquired using a National Instruments data acquisition board (model AT-MIO-16; National Instruments Corp., Austin, TX); data were recorded at sampling rate of 100 Hz using custom designed LabVIEW 8.2.1 software (National Instruments Corp., Austin, TX). High-speed videos (HSVs) were recorded using a Photron FastCam –ultima APX (Photron, San Diego, CA) in grayscale at size of 256×512 pixels. The videos were 0.1919 seconds in duration and were captured at 4000 frames per second. All data were stored on an Intel-based personal computer.
The vocal fold edge, measured as the distance between the vocal process of the arytenoids and the anterior commissure of the vocal folds, was recorded before each trial. This distance reflected the length of the visibly conspicuous vocal fold edge as seen in the HSVs. Ten HSVs were recorded at each of three subglottal pressure levels. A total of thirty HSVs were recorded per larynx, and the data set of the sample population of candidate models contained 750 HSVs. A sample population of 35 HSVs were analyzed from humans.
Data Analysis
HSVs were analyzed using custom MATLAB R2006a (The Mathworks, Inc., Natic, MA) software. A sinusoidal regression was calculated using a Fourier series for each of the four vocal fold edges that appear in the kymograph: the left superior (LS), left inferior (LI), right superior (RS), and right inferior (RI) (Figure 2). A second-order sinusoidal regression was written:
| (4) |
where gi(t) is the glottal half-width of a given vocal fold margin (i={LS, LI, RS, RI}), a0 is a physiologically irrelevant value representing the horizontal shift of the function based on the predetermined MATLAB coordinate plane, aji is the amplitude of the curve (j={1, 2}, indicating the order of the sinusoid) in millimeters, fi is the frequency of the curve in Hertz, and pji is the phase shift of the curve in radians (11). For typical periodic phonation, a2 = 0 and the function reduces to a first-order sinusoid. Phase difference was determined using the equation:
| (5) |
where
| (6) |
j={1, 2} indicating the sinusoidal term number, and k={1, 2} indicating the left or right vocal fold. Phase difference is defined only in the half-open interval [0,2π). In the rare case of second order phonation, Eq. 5 ensures that the phase difference is that of the highest power sinusoidal term. Because power is directly proportional to the square of amplitude, the phase difference of the highest amplitude term was used.
Figure 2.
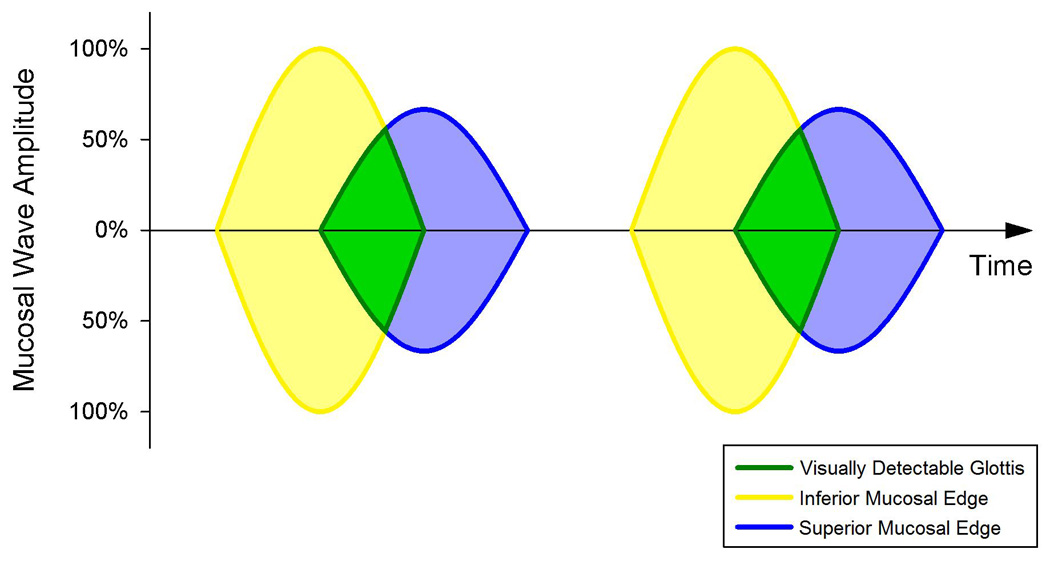
The procedure for mucosal wave parameter extraction. High-speed digital images of the glottal plane of phonating excised larynges provided pixels lines from the glottal midline, which were plotted as a function of time to produce a kymograph. Edge extraction methods allowed sinusoidal regressions to be calculated for the left and right superior and inferior vocal fold margins. An idealized kymograph is shown for two glottal cycles, where the origin of the visually detectable glottis is shown to be a function of the inferior and superior glottal widths.
Statistical comparisons were conducted between species on measures of amplitude, frequency, and phase difference. One-way ANOVA with respect to species was performed to determine if statistically significant differences existed between the means of each mucosal wave parameter across species, and post-hoc analyses provided pair-wise comparisons between every combination of two species.
RESULTS
The oscillation frequency, amplitude, and superior-inferior phase difference distributions are illustrated in Figures 3, 4, and 5. One-way ANOVA on ranks suggested that statistically significant differences existed between the median frequency, amplitude, and phase difference across species (p<0.001, p<0.001, p<0.001). Pair-wise comparisons using Dunn’s method are presented in Table II. The family-wise significance level was 0.05, and using the Bonferroni correction to prevent type I error inflation, the pair-wise significance level was 0.0033. Bolded p-values in Table II indicate a statistically significant difference between the medians of the two comparison groups. Asterisks after a species comparison indicate that no statistically significant difference existed between the median frequency, amplitude, or phase difference data for the pair.
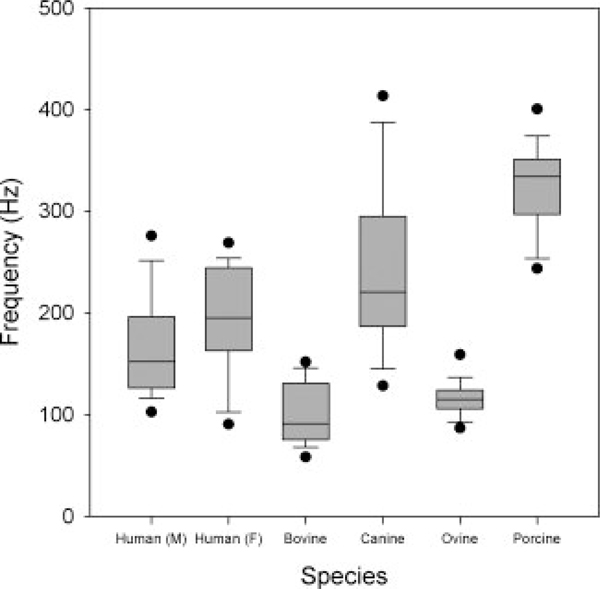
Figure 3.
The oscillation frequency across species.
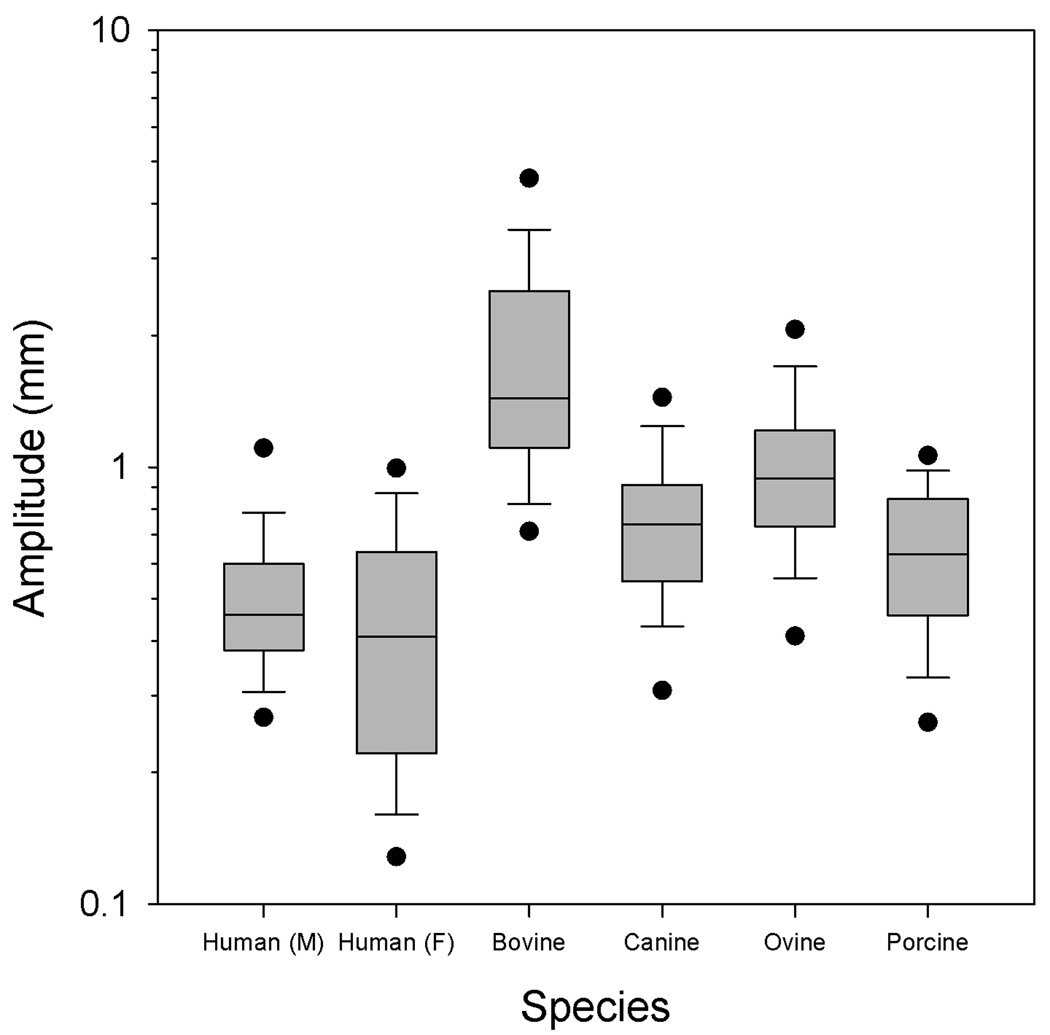
Figure 4.
The vibratory amplitude across species. Amplitude is plotted on a logarithmic scale.
Figure 5.
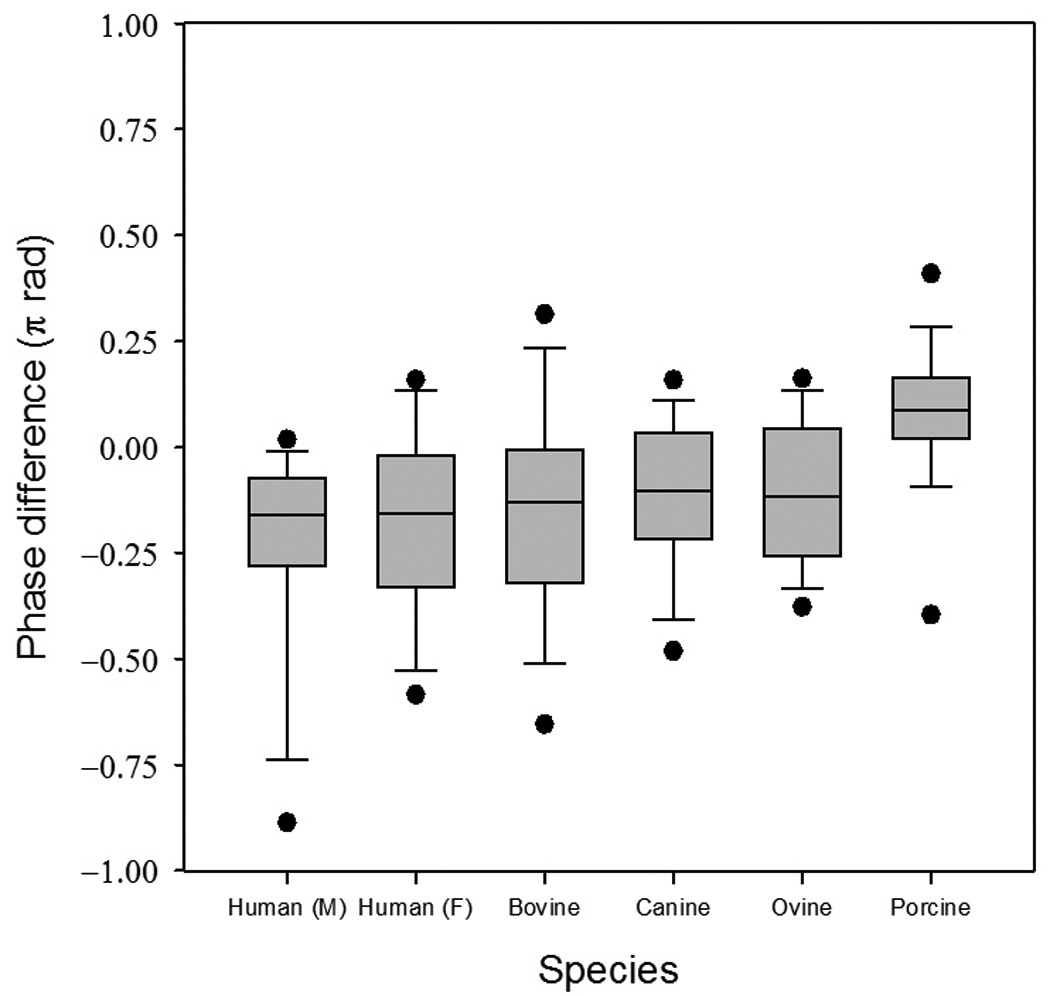
The superior-inferior oscillation phase difference across species.
Table II.
Post-hoc analysis of the aggregate frequency, amplitude, and phase difference data using Dunn’s method of unplanned comparisons among medians. Bolded p-values indicate statistical significance (p<0.0033). Species pairs with no statistical differences in any variable are indicated with an asterisk.
| Frequency | Amplitude | Phase difference | ||||
|---|---|---|---|---|---|---|
| Comparison | Q | p | Q | p | Q | p |
| Human (males) v. humans (females) * | 1.776 | 0.1826 | 0.398 | 0.5281 | 0.512 | 0.4743 |
| Humans (males) v. bovines | 7.922 | 0.0048 | 17.21 | <0.0001 | 1.722 | 0.1894 |
| Humans (males) v. canines * | 5.244 | 0.0220 | 5.884 | 0.0153 | 2.528 | 0.1118 |
| Humans (males) v. ovines | 5.719 | 0.0167 | 10.449 | 0.0012 | 2.451 | 0.1175 |
| Humans (males) v. porcines | 10.686 | 0.0011 | 3.400 | 0.0652 | 8.144 | 0.0043 |
| Humans (females) v. bovines | 10.488 | 0.0012 | 18.109 | <0.0001 | 1.066 | 0.3019 |
| Humans (females) v. canines * | 2.975 | 0.0845 | 6.537 | 0.0105 | 1.895 | 0.1686 |
| Humans (females) v. ovines | 8.190 | 0.0042 | 11.188 | 0.0008 | 1.822 | 0.1771 |
| Humans (females) v. porcines * | 8.534 | 0.0035 | 4.001 | 0.0455 | 7.627 | 0.0058 |
| Bovines v. canines | 27.785 | <0.0001 | 23.706 | <0.0001 | 1.744 | 0.1866 |
| Bovines v. ovines | 4.255 | 0.0391 | 13.314 | 0.0002 | 1.566 | 0.2108 |
| Bovines v. porcines | 39.026 | <0.0001 | 28.702 | <0.0001 | 13.573 | 0.0002 |
| Canines v. ovines | 21.705 | <0.0001 | 9.177 | 0.0025 | 0.109 | 0.7413 |
| Canines v. porcines | 11.06 | 0.0008 | 2.013 | 0.0252 | 11.402 | 0.0007 |
| Ovines v. porcines | 32.223 | <0.0001 | 13.95 | 0.0002 | 11.124 | 0.0009 |
DISCUSSION
Animal models of in vivo human vocal fold oscillation
One objective of this experiment was to determine which animal model most closely simulated the vibratory characteristics of in vivo human vocal fold oscillation. Three primary variables were considered important metrics of vibratory similarity: oscillation frequency, amplitude, and superior-inferior phase difference. For periodic and nearly periodic phonation, these parameters describe the physical vibratory nature of the vocal folds and indirectly describe the biomechanical properties of the larynx. These vibratory variables are dependent upon the constants used in low dimensional vocal fold models, which Titze has shown to be dependent upon physiologically relevant biomechanical properties (16). While investigators have sought to relate these variables to physiologically relevant parameters, the relationships are complex and still not well understood.
The frequency data are presented in Figure 3. The set of human female oscillation frequencies was higher than those of males, but not significantly. Bovines and ovines had characteristically low oscillation frequencies, which may be due to the larger vocal fold lengths. The set of porcine oscillation frequencies was relatively high, while the canines were found to have an oscillation frequency range most similar to that of human males and females (Table II).
The amplitude data (Figure 4) were plotted on a semi-logarithmic scale for ease of visualization. Most conspicuous from the results is the large oscillation amplitude of the bovines, presumably due to their large vocal fold lengths. Ovines were found to have the second largest amplitudes. Porcine and canine oscillation amplitudes were not found to be significantly different from one another, nor were either found to be significantly different from humans. While the porcines were found to have an oscillation amplitude range most similar to that of humans, both porcines and canines were found to have amplitude ranges similar to humans (Table II).
Of the three variables analyzed in the present study, phase difference is perhaps the most difficult to interpret. It represents the superior-inferior distance in radians that the mucosal wave travels along the vocal fold margin. Figure 5 illustrates the phase difference across species. No statistically significant differences were found among any species, with the exception of porcines. Porcine phase differences were found to be significantly different from bovines, canines, and ovines. Phase difference is a composite or lumped variable, as seen in Eq. 3, and reflects frequency, superior-inferior vocal fold thickness, and the mucosal wave velocity. The higher values of phase difference observed in porcines indicate that the frequency or thickness is greater relative to the mucosal wave velocity than the other species.
From a physical vibration standpoint, these results indicate that the canine and porcine larynges are best suited for modeling the in vivo human larynx. Previous research has indicated the use of canine models for aerodynamic studies of phonation and porcine models for anatomical studies of phonation (1). However, studies that aim to verify current theoretical or mathematical models in ex vivo specimens may benefit from the present study, which suggests that canines and porcines are both adequate models. In particular, studies which investigate pathological impacts on the mucosal wave mechanism of human voice production would benefit from using models most closely resembling humans.
Relationship to other interspecies studies
Numerous anatomical studies of the larynx provide an interpretive foundation for the results presented here. Anatomically, porcine vocal folds are most similar to humans due to their mucosal thickness, highly developed vocal ligament, and well-defined boundaries (1) (17). In the present study, porcines were found to be similar to humans by vibratory measurements. The presence of a vocal ligament is important, particularly for voice registers and high pitched singing (18). Canines lack a vocal ligament. This may be an important factor is deciding which animal model to use in studies investigating the role of pitch. Nevertheless, the canine laryngeal framework is comparable to humans (19). Vibrational characteristics are complex functions of the underlying anatomy. This is in agreement with the results from the present study, which suggest that canines are most similar to humans in terms of vibration. Vocal folds in both bovines and ovines are broad, pliable soft tissues without a discernable differentiation at the boundaries, making them less suitable for modeling the human larynx (7). The ovine cricoid, thyroid, and arytenoid cartilage measurements have been found to be significantly different from humans, while the canine laryngeal cartilage measurements were not found to significantly differ from humans (2). The large vocal fold length in bovines and ovines may contribute to the low frequency and high amplitude observed in the present study.
Data on interspecies histological comparison of the vocal fold is sparse, but some studies have been conducted which offer insight into the present study. Porcines, and ovines have a two-layered lamina propria structure similar to the three-layered lamina propria in humans (20). The density of the elastic fibers is greatest in the intermediate layer, and decreases in the superficial and deep directions. These trends were observed in pigs and somewhat in sheep. Other studies have noted that sheep laryngeal muscles are compositionally comparable humans (9).
Research has also investigated the aerodynamic similarities and differences between species. Alipour and Jaiswal measured the average glottal flow resistance in porcines, ovines, and bovines, but found no statistically significant differences between these species (7). They reported the average acoustic frequencies of porcines (194±105 Hz), ovines (114±33 Hz), and bovines (82±14.9 Hz). Our data agree with these results, which may be due to the anatomical differences between species. Bovine vocal folds have a higher stiffness and larger mass than porcine vocal folds, and oscillate at lower frequencies. Ovine vocal folds are broad in the superior-inferior direction and have relatively small vocal fold lengths, making them oscillate at a low frequency with comparatively greater consistency. These trends in acoustic frequency across species are in agreement with the oscillation frequency trends observed in the present study.
CONCLUSION
In the present study, we investigated the differences in vibratory characteristics among ex vivo bovine, canine, ovine, porcine, and in vivo human larynges to determine which excised animal larynx was best suited for modeling the vibratory properties of in vivo human phonation. The ex vivo animal larynges were mounted on an excised larynx phonation system, and high-speed digital videos of the vocal folds were recorded during phonation. The oscillation frequency, amplitude, and superior-inferior phase difference were calculated. The results suggest that canine and porcine larynges are adequate models for simulating in vivo human phonation based on vibrational characteristics.
AKNOWLEDGEMENTS
The authors would like to thank Benjamin Schoepke for his assistance with Matlab programming and Amanda Ni for assistance with data analysis. This work was supported by NIH grant number R01 DC005522-09 from the Institute of Deafness and Other Communication Disorders.
REFERENCES
- 1.Jiang JJ, Raviv JR, Hanson DG. Comparison of the phonation-related structures among pig, dog, white-tailed deer, and human larynges. Ann Otol Rhinol Laryngol. 2001;110:1120–1125. doi: 10.1177/000348940111001207. [DOI] [PubMed] [Google Scholar]
- 2.Kim MJ, Hunter EJ, Titze IR. Comparison of human, canine, and ovine laryngeal dimensions. Ann Otol Rhinol Laryngol. 2004;113:60–68. doi: 10.1177/000348940411300114. [DOI] [PubMed] [Google Scholar]
- 3.Zrunek M, Happak W, Hermann M, et al. Comparative anatomy of human and sheep laryngeal skeleton. Acta Otolaryngol. 1988;105:155–162. doi: 10.3109/00016488809119460. [DOI] [PubMed] [Google Scholar]
- 4.Effat KG. The laryngeal dissection laboratory. J Laryngol Otol. 2005;119:981–984. doi: 10.1258/002221505775010841. [DOI] [PubMed] [Google Scholar]
- 5.Gorti GK, Birchall MA, Haverson K, et al. A preclinical model for laryngeal transplantation: anatomy and mucosal immunology of the porcine larynx. Transplantation. 1999;68:1638–1642. doi: 10.1097/00007890-199912150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Alipour F, Jaiswal S. Phonatory characteristics of excised pig, sheep, and cow larynges. J Acoust Soc Am. 2008;123:4572–4581. doi: 10.1121/1.2908289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alipour F, Jaiswal S. Glottal airflow resistance in excised pig, sheep, and cow larynges. J Voice. 2009;23:40–50. doi: 10.1016/j.jvoice.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Garrett CG, Coleman JR, Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000;110:814–824. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Happak W, Zrunek M, Pechmann U, et al. Comparative histochemistry of human and sheep laryngeal muscles. Acta Otolaryngol. 1989;107:283–288. doi: 10.3109/00016488909127510. [DOI] [PubMed] [Google Scholar]
- 10.Titze I, Alipour F. The myoelastic aerodynamic theory of phonation. Iowa City, IA: National Center for Voice and Speech, Denver, CO; 2006. [Google Scholar]
- 11.Jiang JJ, Yumoto E, Lin SJ, et al. Quantitative measurement of mucosal wave by high-speed photography in excised larynges. Ann Otol Rhinol Laryngol. 1998;107:98–103. doi: 10.1177/000348949810700203. [DOI] [PubMed] [Google Scholar]
- 12.Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr (Basel) 1974;26:89–94. doi: 10.1159/000263771. [DOI] [PubMed] [Google Scholar]
- 13.Kakita Y, Hirano M, Ohmaru K. Physical properties of the vocal fold tissue: measurements on excised larynges. Vocal fold physiology. 1981:377–396. [Google Scholar]
- 14.Titze IR. Principles of voice production. Englewood Cliffs, N.J: Prentice Hall; 1994. [Google Scholar]
- 15.Filho J, Madruga De Melo E, De Giacomo Carneiro C, et al. Length of the human vocal folds: Proposal of mathematical equations as a function of gender and body height. The Annals of otology, rhinology & laryngology. 2005;114:390–392. doi: 10.1177/000348940511400510. [DOI] [PubMed] [Google Scholar]
- 16.Titze IR, Story BH. Rules for controlling low-dimensional vocal fold models with muscle activation. J Acoust Soc Am. 2002;112:1064–1076. doi: 10.1121/1.1496080. [DOI] [PubMed] [Google Scholar]
- 17.Titze IR, Hunter EJ. Normal vibration frequencies of the vocal ligament. J Acoust Soc Am. 2004;115:2264–2269. doi: 10.1121/1.1698832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Den Berg J. Myoelastic-aerodynamic theory of voice production. J Speech Hear Res. 1958;1:227–244. doi: 10.1044/jshr.0103.227. [DOI] [PubMed] [Google Scholar]
- 19.Tayama N, Chan RW, Kaga K, et al. Geometric characterization of the laryngeal cartilage framework for the purpose of biomechanical modeling. Ann Otol Rhinol Laryngol. 2001;110:1154–1161. doi: 10.1177/000348940111001213. [DOI] [PubMed] [Google Scholar]
- 20.Kurita S, Nagata K, Hirano M. A comparative study of the layer structure of the vocal fold. College-Hill: Vocal fold physiology San Diego; 1983. pp. 3–21. [Google Scholar]