Experimental autoimmune encephalomyelitis as a model for demyelinating disease challenges the mindset that β2-integrins are redundant in function and potential therapeutic targets for multiple sclerosis.
Keywords: adhesion molecules, neuroimmunology, experimental autoimmune encephalomyelitis, multiple sclerosis, T cells
Abstract
The β2-integrins are a subfamily of integrins expressed on leukocytes that play an essential role in leukocyte trafficking, activation, and many other functions. Studies in EAE, the animal model for multiple sclerosis, show differential requirements for β2-integrins in this disease model, ranging from critical in the case of LFA-1 (CD11a/CD18) to unimportant in the case of CD11d/CD18. Importantly, expression of β2-integrins on T cell subsets provides some clues as to the function(s) these adhesion molecules play in disease development. For example, transferred EAE studies have shown that Mac-1 (CD11b/CD18) expression on αβ T cells is critical for disease development, and the absence of LFA-1 on Tregs in recipient mice results in exacerbated disease. In this review, we summarize recent findings regarding the role of β2-integrins in demyelinating disease and new information about the role of β2-integrins with respect to alterations in Treg numbers and function. In addition, we discuss the potential for targeting β2-integrins in human demyelinating disease in light of the recent animal model studies.
Introduction
The β2-integrins are members of a large family of integrin molecules that play critical roles in cell adhesion, tissue-specific homing, and the trafficking of many cell types during development and in infection [1,2,3,4,5]. There are four members in the β2-integrin family, and they are best known for their roles in leukocyte trafficking during inflammation and for their contributions to leukocyte activation in immune responses and phagocytosis [4,5,6]. The most studied of these adhesion receptors CD11a/CD18 (αLβ2, LFA-1) and CD11b/CD18 (αMβ2, Mac-1, CR3) participate at various steps in a well-defined cascade that results in transmigration of leukocytes into secondary lymphoid organs (an important component of immunosurveillance) and into sites of infection and inflammation. CD11c/CD18 (αXβ2, p150,95, CR4) along with Mac-1 are important in complement-mediated phagocytosis and also serve as frequently used markers for DCs, although their function in DC biology remains unclear [7, 8]. CD11d (a.k.a., αDβ2) remains the least functionally characterized of the β2-integrins [9,10,11] and as will be described below, is phenotypically unimportant in demyelinating disease [12]. The use of β2-integrin-deficient mice and antibodies has in recent years demonstrated unique functions for each family member, but a direct comparison of phenotypic outcome in a single defined disease model has not been performed until now. In this review, we discuss recent data about the role of the β2-integrins in EAE, the animal model for MS. The clinical course of EAE is distinct in each β2-integrin-deficient mouse, and differential expression of these integrins on T subsets indicates important roles on lymphocytes as well as myeloid cells that may contribute to the development of demyelinating disease.
β2-INTEGRINS—STRUCTURE, FUNCTION, LIGANDS, SIGNALING
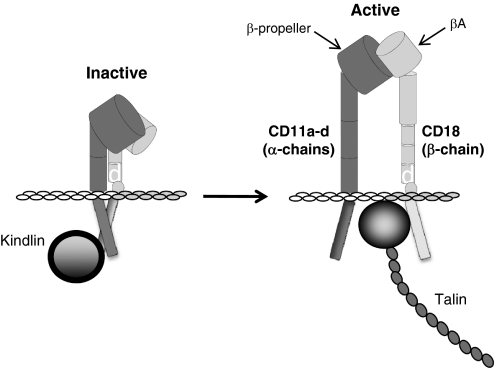
The β2-integrins are heterodimeric receptors that share a common β-chain (CD18). The α- and β-chains are composed of multiple protein domains that contribute to ligand binding and signaling events (Fig. 1). The so-called I domain (also known as the von Willebrand factor A domain) of the α-chain and the A domain of the β-chain form the ligand-binding site when the integrin is in the active, high-affinity conformation [13,14,15,16]. In the inactive state, integrins are in a “folded” conformation and not capable of interacting with their ligands. Recent studies have shown that the cytoplasmic domains of integrins are also in a “closed” conformation, held together via a salt bridge and thus, unable to interact with talin and kindlin, components of the so-called “adhesion plaque” that couples integrins to the actin cytoskeleton. On cellular activation by inflammatory agonists (cytokines, chemokines, complement anaphylatoxins, bacterial by-products, etc.), the intracellular domains also undergo a conformation change to an open, active state, allowing interaction with the actin cytoskeleton and signaling through multiple pathways [16,17,18]. Once the intracellular domains have become “activated,” the extracellular portion of the integrin undergoes conformational change. This complex series of events is called inside-out signaling and occurs rapidly (<1 s), allowing for firm adhesion between integrin-expressing leukocytes and APCs, endothelial cells, target cells (in the case of cytotoxic T cells), or the ECM. Mutations in CD18 result in a complete loss of β2-integrin expression and the life-threatening immunodeficiency, LAD I. Another syndrome, LAD III, is functionally similar to LAD I in terms of immunodeficiency but is caused by mutations in kindlin 3 or diacylglycerol-regulated guanine nucleotide exchange factor 1, resulting in failed inside-out signaling [19,20,21].
Figure 1.
Schematic structure of the β2-integrins. A representative structure for the α- and β-chains of β2-integrins in the inactive, folded, and active, extended conformation is shown. Both polypeptide chains are composed of multiple protein domains, and the β-propeller and βA domains form the ligand-binding site in the active conformation. Both chains undergo significant conformational change on leukocyte activation through “inside-out signaling.” The intracellular domains also change conformation to allow interaction with kindlin and talin and subsequently, the actin cytoskeleton.
β2-Integrins bind a wide variety of ligands, including several of the ICAM molecules, VCAM, iC3b (a proteolytic fragment derived from C3 on activation of complement), ECM components (e.g., fibrinogen), and some CHOs (see Table 1) [22,23,24,25]. The affinity of integrins for their ligands depends in part on the conformational state of the integrin, with lowest affinity in the bent conformation and highest affinity in the extended, active conformation. Some studies suggest that ligand binding occurs prior to and is required for fully extended conformation [14, 16, 26, 27]. Activation of the β2-integrins also leads to rapid multimerization of these receptors and the assembly of the intracellular signaling platform that results in gene expression via signaling through Akt, Erk, and Jnk pathways. Furthermore, activation leads to changes in cytoskeletal organization, in part through activation of Rho GTPases [17, 18]. The latter set of mechanisms is largely independent of changes in gene expression but is critical for firm adhesion and transmigration in leukocyte trafficking and in the engulfment process during phagocytosis.
TABLE 1.
β2-Integrin Family Members: Nomenclature and Biological Functions
| CD11a αLβ2, LFA-1 | CD11b αMβ2, Mac-1, CR3 | CD11c αXβ2, p150,95, CR4 | CD11d αDβ2 | |
|---|---|---|---|---|
| Ligands | ICAM-1-3 | iC3b, CHOs, ICAM-1, ECM | C3bi, ICAM-1/2, VCAM-1, ECM | VCAM-1, ICAM-3 |
| Expression | Leukocytes | Myeloid cells, B cells, T cell subsets | Myeloid cells, DCs, T cell subsets | Myeloid, T, B, and NK cells |
| Functions | Leukocyte trafficking, Signaling | Phagocytosis, Trafficking, Tolerance | Phagocytosis, Trafficking | Phagocytosis? Trafficking? |
β2-INTEGRINS IN EAE
EAE is the commonly used animal model for the human demyelinating disease, MS. Although this model does not replicate many aspects of MS pathology and clinical presentation, one hallmark feature they share is infiltration of multiple leukocyte subsets into the CNS [28]. These leukocyte subsets contribute to the pathophysiology of demyelinating disease, and their expression of adhesion molecules, including the β2-integrins and their ligands, is considered critical for their trafficking into the brain and spinal cord during development and progression of disease. Aside from a host of publications demonstrating changes in expression of β2-integrins (primarily LFA-1, Mac-1, and p150,95) on a variety of cell types, the most informative studies demonstrating a role for these receptors in demyelinating disease are those in which anti-β2-integrin antibodies or β2-integrin-deficient mice were used. Antibodies to the α-chains of LFA-1 and Mac-1 have provided conflicting results in EAE studies. In both cases, antibody treatment provided varying degrees of protection from disease, primarily by attenuating disease severity rather than completely blocking disease development [29,30,31,32,33,34]. In some studies, however, treatment with antibody exacerbated disease severity [30]. In our hands, treatment with anti-CD11c antibodies during MOG-induced EAE in C57BL/6 mice was unexpectedly and uniformly fatal in all treated mice (X. Hu, Don. Staunton, S. R. Barnum, unpublished observations). Treatment with anti-CD11d antibodies has not been performed in EAE; however, anti-CD11d antibody was protective in a spinal cord injury model, suggesting potential use in EAE [35, 36]. Although antibody treatment studies can be informative, their interpretation is complicated frequently by differences in disease induction (dosing with disease-causing, myelin-derived proteins or peptides and/or pertussis toxin), rodent species (rat vs. mouse), or MHC haplotype within species. Furthermore, the antibodies used in such studies have differences in affinity for their integrin ligands and variable biological half-lives. Undoubtedly, Fc-mediated effects come into play as well in these studies, based on observations in EAE and MS in which Igs are used as a treatment modality [37,38,39,40]. Clearly, the results of antibody treatment studies require cautious interpretation.
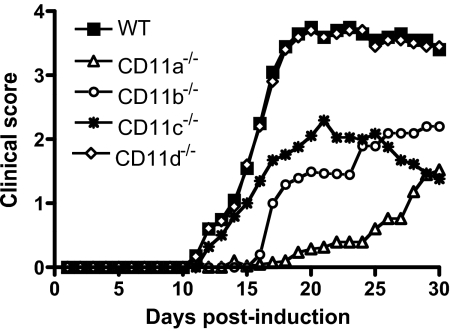
In the last few years, EAE has been performed using mice deficient in each of the β2-integrin α-chains, and an interesting phenotypic picture has emerged, indicating unique, functional roles for these molecules in this autoimmune disease model [12, 41,42,43,44]. Figure 2 shows the overall phenotypic differences between the β2-integrin mutant mice using MOG-induced EAE in C57BL/6 mice. Each mutant had a different overall course of disease with varying differences in disease onset and severity compared with wild-type mice, with the exception of CD11d−/− mice. In CD11d−/− mice, all disease parameters were identical to wild-type mice, including total leukocyte and T cell infiltration into the CNS, T cell proliferation, and cytokine production [12]. These studies do not preclude a role for CD11d/CD18 in demyelinating disease but suggest that it is not significant. Deletion of any of the remaining β2-integrins resulted in modest attenuation of disease (CD11c−/− mice) to near-complete absence of disease (CD11a−/− mice). The disease parameters among all four mutants are compared in Table 2. There was a clear gradient of reduction in disease severity moving from CD11a−/− to CD11d mice−/−. Interestingly, EAE in CD11a−/− mice, as reported by Wang and colleagues [43], developed more rapidly and was more severe than we observed, despite a reported deficit in Th17 cells. The reasons for this are unclear but may be a result, in part, of technical differences in EAE induction. In general, the reduced disease severity in CD11a–c-deficient mice can be attributed to diminished leukocyte trafficking, phagocytosis, and altered cytokine production (predominately IFN-γ and TNF-α but other cytokines as well). Although some of these contributions to disease development can be considered myeloid-specific, it was surprising to see that expression of the β2-integrins on T cell subsets was just as critical to disease outcome as phagocytic cells, based on transferred EAE experiments and T cell proliferation assays.
Figure 2.
Comparison of the disease phenotype of MOG-induced, active EAE in wild-type and β2-integrin-deficient mice. Representative disease curves for wild-type (WT), CD11a−/−, CD11b−/−, CD11c−/−, and CD11d−/− mice with active EAE over the course of 30 days are shown. Onset and progression of EAE symptoms were monitored using a clinical scale as follows: 0, asymptomatic; 1, loss of tail tone; 2, flaccid tail; 3, incomplete paralysis of one or two hind limbs; 4, complete hind-limb paralysis; 5, moribund. CD11a−/− and CD11b−/− mice have delayed disease onset and the least severe disease phenotype. CD11c−/− mice have an intermediate disease phenotype, and CD11d−/− mice present with disease identical to wild-type mice. (Adapted from refs. [12, 41, 42, 44].)
TABLE 2.
Comparison of EAE Parameters among β2-Integrin-Deficient Mice
| EAE parameter |
|||||
|---|---|---|---|---|---|
| Gene mutation | Onseta | Severityb | Incidencec | Cellular infiltration | Demyelination |
| CD11a−/− | Severely delayed | Attenuated (80%) | Reduced (∼40%) | Reduced | Reduced |
| CD11b−/− | Delayed | Attenuated (65%) | Reduced (∼15%) | Reduced | Reduced |
| CD11c−/− | Unchanged | Attenuated (45%) | Reduced (∼5%) | Reduced | Reduced |
| CD11d−/− | Unchanged | Unchanged | Unchanged | Unchanged | Unchanged |
Disease onset is defined as the 1st of 2 consecutive days with a clinical score of two or more. Mice with no signs of disease are assigned a score of 30 days.
Based on the percent change in cumulative disease index (the mean of the sum of daily clinical scores observed between Days 7 and 30) compared with wild-type mice.
Disease incidence is defined as the percent of mice that displayed any clinical signs of disease compared with wild-type mice.
In transferred EAE studies, encephalitogenic T cells derived from mice deficient in LFA-1, Mac-1, or p150,95 generated attenuated disease on transfer to wild-type mice compared with wild-type-to-wild-type transfers [41, 42, 44]. Unexpectedly, encephalitogenic T cells from Mac-1−/− mice were completely unable to induce disease [41]. Attenuated disease in this setting is likely a result of a combination of functional deficits, including poor T cell activation, altered trafficking capacity, and limited macrophage/microglia activation and phagocytosis. CD11b−/− T cells were restimulated effectively with MOG peptide and proliferated comparably with wild-type T cells, indicating that other functional deficits must predominate to account for the transferred EAE phenotype observed. In experiments in which wild-type encephalitogenic T cells were transferred to β2-integrin-deficient mice, attenuated disease also observed with one important exception: Transfers into LFA-1−/− mice resulted in markedly exacerbated disease with 30% mortality, a finding not observed in any EAE studies related to β2-integrins [44]. Bioluminescent studies of LFA-1−/− mice receiving luciferase-positive, encephalitogenic T cells revealed rapid and massive expansion of transferred T cells throughout the secondary lymphoid system and trafficking into the spinal cord and brain [44]. These studies suggested a regulatory deficit in LFA-1−/− mice that allowed unchecked proliferation of primed, antigen-specific T cells and led us to examine changes in numbers and functionality of Tregs in β2-integrin-deficient mice (see below). The results of the transfer studies demonstrated that expression of β2-integrins on T cells is critical for development and progression of EAE, although expression levels on T cell subsets are often low [45].
β2-INTEGRIN EXPRESSION ON T CELL SUBSETS
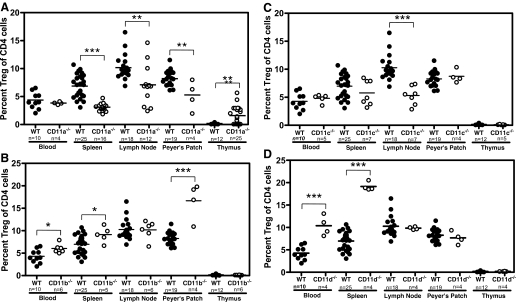
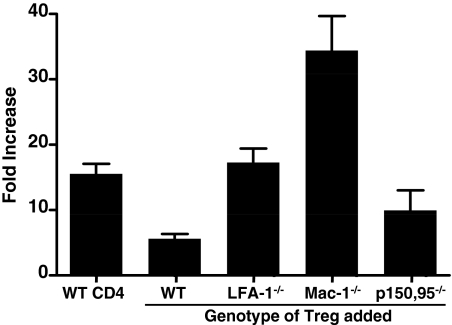
β2-Integrins are expressed on most leukocytes, and their expression on αβ and γδ T cells under homeostatic conditions and during EAE has been studied extensively [2, 45,46,47,48]. In contrast, the expression of β2-integrins on Tregs has received little attention, and until recently, nothing was known regarding the contribution of β2-integrins to Treg function(s). In recent studies comparing the percentage of Tregs (CD4+CD25+FoxP3+) as a total of CD4+ T cells, it was shown that Treg numbers were reduced significantly in spleen, lymph nodes, and Peyer’s patches but elevated in the thymus of LFA-1−/− compared with wild-type mice (Fig. 3). The elevated numbers of Tregs in the thymus were an intriguing contrast to other lymphoid tissues (and other β2-integrin mutant mice) and suggested alterations in Treg maturation; however, the single- and double-positive CD4/CD8 profile of Tregs was normal in LFA-1−/− mice, indicating no developmental aberration. Elevated thymic Treg numbers in LFA-1−/− mice may occur as a result of a homeostatic mechanism to repopulate the Treg-deficient secondary lymphoid tissues. This would be consistent with the observation that Treg numbers are not reduced in the secondary lymphoid tissues or thymi of remaining β2-integrin-deficient mice compared with wild-type mice (Fig. 3). LFA-1−/− Tregs also completely failed to suppress in vitro proliferation of CD4+CD25− cells stimulated with anti-CD3 and anti-CD28 antibodies (Fig. 4) and were less efficient than wild-type Tregs at suppressing colitis development in a Helicobacter hepaticus/recombination activating gene-1−/− colitis model [49]. These data suggest that a Treg defect in LFA-1−/− mice could account, at least in part, for the exacerbated EAE phenotype observed on transfer of wild-type encephalitogenic T cells into the LFA-1−/− background. Experiments using wild-type and LFA-1−/− Tregs in EAE are needed to verify this possibility.
Figure 3.
Treg frequency in lymphoid tissues in wild-type and β2-integrin-deficient mice. Blood, spleen, lymph nodes, Peyer’s patches, and thymus were harvested from C57BL/6 mice; cells were isolated using a Miltenyi Treg kit, as described previously [49]; and Tregs (CD4+CD25+FoxP3+) were analyzed using flow cytometry. Data shown are gated on CD4+ cells. Statistical analysis was performed for each wild-type and β2-integrin-deficient tissue using the Student’s t-test (**, P≤0.01; ***, P≤0.001). (A) CD11a−/−mice. (B) CD11b−/−mice. (C) CD11c−/−mice. (D) CD11d−/−mice.
Figure 4.
Capacity of β2-integrin-deficient Tregs to suppress CD4+effector cell proliferation. In vitro suppression assays were performed as described previously [49] using wild-type CD4+CD25− responder cells and wild-type CD4+CD25+ or β2-integrin-deficient CD4+CD25+ Tregs. Cultures were stimulated with soluble anti-CD3 and anti-CD28 antibodies for 72 h and were pulsed with 3H-thymidine for the last 18 h. The results are shown as fold-increase over baseline controls for wild-type CD4+CD25− cells alone (n=6), control CD4+CD25− cells with wild-type CD4+CD25+ cells (n=17), and wild-type CD4+CD25− cells with CD11a−/− (n=6), CD11b−/− (n=3), or CD11c−/− (n=3) CD4+CD25+ cells.
Treg numbers and function in the remaining β2-integrin mutant mice differed from that of LFA-1−/− mice (Fig. 3). Unlike CD11a−/− mice, CD11b−/− and CD11d−/− mice presented with elevated numbers of Tregs in several lymphoid tissues, including blood and spleen and in the case of CD11b−/− mice, the Peyer’s patches as well (Fig. 3). Despite elevated numbers of Tregs in CD11b−/− mice, these cells failed to suppress in vitro T cell responses; in fact, they appeared to potentiate them (Fig. 4). Tregs from CD11c−/− mice were similar in number to wild-type mice but were modestly effective in in vitro suppression assays (Fig. 4). CD11d−/− Tregs have not yet been evaluated in suppression assays. These data make it clear that β2-integrins play an important role in Treg immunobiology, although precisely how they modulate Treg functions remains unknown. They could influence trafficking of Tregs, adhesion to effector T cells, and/or APCs or modulation of intracellular signaling events that control Treg suppressor functions (cytokine production and/or sequestration, etc.) [50,51,52,53,54,55,56,57]. Given the differential roles of β2-integrins in the development of EAE, it would not be surprising if there were overlapping roles for these adhesion molecules in Treg function. Additional studies and perhaps additional mutant mice are required to determine possible inter-relationships.
β2-INTEGRINS—A THERAPEUTIC TARGET FOR MS?
Therapeutic approaches in autoimmune disease have targeted inhibition of several integrins as a potential treatment. With respect to MS, the best-known integrin target is α4β1, also known as VLA-4, an integrin expressed on effector T cells that migrate into the CNS and contribute to the pathophysiology of MS. Animal model studies in the early 1990s demonstrated that antibodies to VLA-4 provided significant protection from EAE [58, 59], and within a decade, clinical trials using humanized antibodies (natalizumab, marketed as Tyasbri) were also showing protective effects (increasing the time between disease relapses and slowing the overall disease progression in the relapsing-remitting population) [60]. Unfortunately, shortly after approval of natalizumab for treatment of MS, several individuals developed PML, an often fatal brain infection [61, 62]. Initially, PML in these patients was thought to have arisen as a result of the combination of natalizumab with other immunosuppressant medication; however, additional cases of PML have occurred in the absence of coadministered immunosuppressant drugs [63,64,65,66]. Importantly, efalizumab (Raptiva), an anti-LFA-1 mAb used to treat psoriasis, has also been pulled from the market as a result of confirmed cases of PML [67]. The trials and tribulations of natalizumab and efalizumab highlight the potential risk for anti-integrin therapeutic approaches. At face value, inhibition of β2-integrins appears an attractive therapeutic target, because of the possibility of blocking several key steps in the development and progression of MS, including leukocyte trafficking to the CNS, inhibition of T cell activation, and modulation of myeloid effector functions (myelin stripping, cytokine production, and possibly antigen presentation). Studies using β2-integrin-deficient mice support at least some potential therapeutic pitfalls. For example, blocking LFA-1 may reduce Treg numbers and functions in addition to inhibiting effector T cell trafficking and activation, possibly resulting in a profound immunosuppression. As mentioned previously, treatment with anti-CD11c antibodies proved fatal in EAE studies, indicating that this integrin plays multiple critical roles (perhaps on several leukocyte subsets) too important to inhibit in this manner. Based on what is known to date, inhibition of Mac-1 may offer the most useful β2-integrin therapeutic approach. Targeting Mac-1 will affect multiple leukocyte subsets, including T cells (αβ, γδ, and Treg), B cells, and myeloid cells, all of which contribute to MS pathophysiology and may avoid the functional deficits observed in CD11a−/− mice. How anti-Mac-1 antibodies would alter Treg immunobiology is unclear at this time. Although in vitro studies indicated that CD11b-deficient Tregs were poorly immunosuppressant, this has not been verified in any in vivo model system to date. Although mutant mice have provided a wealth of data about the potential for β2-integrin-based therapeutics, there may be sufficient differences between species at the level of Treg biology, in particular, indicating that caution is warranted before moving forward therapeutically.
CONCLUDING REMARKS
In the last few years, a wealth of new information about the role of β2-integrins in demyelinating disease has made it clear that this family of adhesion molecules plays a more critical role in disease development than appreciated previously. The low expression levels of β2-integrins, such as Mac-1 and p150,95 on some resting leukocytes, has led some to conclude that they played little to no significant role in immune responses. We now know that expression levels of all of the β2-integrins change throughout the course of EAE in a T cell-specific manner [45] and through studies using mutant mice, that the functions of multiple leukocyte subsets are affected by these receptors [12, 41,42,43,44]. Despite these advances, these approaches are still somewhat blunt, and we know few details of precisely how β2-integrins function separately or in combination in EAE or MS. The challenge now is to engineer better mice and tools to determine more precisely how these adhesion molecules modulate disease at different stages of disease development. Such tools will allow better insight into how β2-integrins may be targeted for demyelinating and other diseases.
AUTHORSHIP
All authors contributed equally to the writing of this review article.
ACKNOWLEDGMENTS
This work was supported by grants from the National Multiple Sclerosis Society (RG 3437-B-9) to S. R. B. and from the National Institutes of Health (T32 AI07051) to J. E. W. and K. J. D. The authors acknowledge the support of the Gnotobiotic and Genetically Engineered Mouse Core of the University of Alabama at Birmingham, Digestive Diseases Research Development Center (P30 DK064400). We thank Dr. Dan Bullard for critical reading of the manuscript.
Footnotes
Abbreviations: CHO=carbohydrate, DC=dendritic cell, EAE=experimental autoimmune encephalomyelitis, ECM=extracellular matrix, FoxP3+= forkhead box P3+, LAD I=leukocyte adhesion deficiency type I, Mac-1= macrophage-1 antigen, MOG=myelin oligodendrocytes glycoprotein, MS=multiple sclerosis, PML=progressive multi-focal leukoencephalopathy, Treg=regulatory T cell
References
- Hynes R. O. Specificity of cell adhesion in development: the cadherin superfamily. Curr Opin Genet Dev. 1992;2:621–624. doi: 10.1016/s0959-437x(05)80182-0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S. M., Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Luster A. D., Alon R., von Andrain U. H. Immune cell migration in inflammation: present and future therapeutics. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Davis D. M. Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat Rev Immunol. 2009;9:543–555. doi: 10.1038/nri2602. [DOI] [PubMed] [Google Scholar]
- Van Lookeren Campagne M., Wiesmann C., Brown E. J. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 2007;9:2095–2102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- Hume D. A. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- Wong D. A., Davis E. M., LeBeau M., Springer T. A. Cloning and chromosomal localization of a novel gene-encoding a human β 2-integrin α subunit. Gene. 1996;171:291–294. doi: 10.1016/0378-1119(95)00869-1. [DOI] [PubMed] [Google Scholar]
- Shelley C. S., Da Silva N., Georgakis A., Chomienne C., Arnaout M. A. Mapping of the human CD11c (ITGAX) and CD11d (ITGAD) genes demonstrates that they are arranged in tandem separated by no more than 115 kb. Genomics. 1998;49:334–336. doi: 10.1006/geno.1998.5232. [DOI] [PubMed] [Google Scholar]
- Noti J. D., Johnson A. K., Dillon J. D. Structural and functional characterization of the leukocyte integrin gene CD11d Essential role of Sp1 and Sp3. J Biol Chem. 2000;275:8959–8969. doi: 10.1074/jbc.275.12.8959. [DOI] [PubMed] [Google Scholar]
- Adams J. E., Webb M. S., Hu X., Staunton D., Barnum S. R. Disruption of the β(2)-integrin CD11d (α(D)β2) gene fails to protect against experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;184:180–187. doi: 10.1016/j.jneuroim.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Lupher M. L., Liddington R., Gallatin W. M. Targeting integrin structure and function in disease. Adv Immunol. 2006;91:111–157. doi: 10.1016/S0065-2776(06)91003-7. [DOI] [PubMed] [Google Scholar]
- Luo B. H., Carman C. V., Springer T. A. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B. H., Springer T. A. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18:579–586. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Legate K. R., Zent R., Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- Abram C. L., Lowell C. A. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate K. R., Wickstrom S. A., Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984;160:1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzioni A. Leukocyte adhesion deficiencies: molecular basis, clinical findings, and therapeutic options. Adv Exp Med Biol. 2007;601:51–60. doi: 10.1007/978-0-387-72005-0_5. [DOI] [PubMed] [Google Scholar]
- Etzioni A. Genetic etiologies of leukocyte adhesion defects. Curr Opin Immunol. 2009;21:481–486. doi: 10.1016/j.coi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Picker L. J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Williams M., Youngman K., Rott L., Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Von Andrian U. H., Mackay C. R. T-cell function and migration Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Xiong J. P., Stehle T., Goodman S. L., Arnaout M. A. New insights into the structural basis of integrin activation. Blood. 2003;102:1155–1159. doi: 10.1182/blood-2003-01-0334. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Goodman S. L., Xiong J. P. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M., Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Cannella B., Cross A. H., Raine C. S. Anti-adhesion molecule therapy in experimental autoimmune encephalomyelitis. J Neuroimmunol. 1993;46:43–55. doi: 10.1016/0165-5728(93)90232-n. [DOI] [PubMed] [Google Scholar]
- Welsh C. T., Rose J. W., Hill K. E., Townsend J. J. Augmentation of adoptively transferred experimental allergic encephalomyelitis by administration of a monoclonal antibody specific for LFA-1 α. J Neuroimmunol. 1993;43:161–167. doi: 10.1016/0165-5728(93)90087-f. [DOI] [PubMed] [Google Scholar]
- Gordon E. J., Myers K. J., Dougherty J. P., Rosen H., Ron Y. Both anti-CD11a (LFA-1) and anti-CD11b (MAC-1) therapy delay the onset and diminish the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1995;62:153–160. doi: 10.1016/0165-5728(95)00120-2. [DOI] [PubMed] [Google Scholar]
- Huitinga I., Damoiseaux J. G., Dopp E. A., Dijkstra C. D. Treatment with anti-CR3 antibodies ED7 and ED8 suppresses experimental allergic encephalomyelitis in Lewis rats. Eur J Immunol. 1993;23:709–715. doi: 10.1002/eji.1830230321. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Staykova M. A., Miyasaka M. Short term treatment with soluble neuroantigen and anti-CD11a (LFA-1) protects rats against autoimmune encephalomyelitis: treatment abrogates autoimmune disease but not autoimmunity. J Immunol. 1996;157:1973–1980. [PubMed] [Google Scholar]
- Brocke S., Piercy C., Steinman L., Weissman I. L., Veromaa T. Antibodies to CD44 and integrin α4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabon P. J., Weaver L. C., Dekaban G. A. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin αD: a potential new anti-inflammatory treatment. Exp Neurol. 2000;166:52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- Gris D., Marsh D. R., Oatway M. A., Chen Y., Hamilton E. F., Dekaban G. A., Weaver L. C. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Miron S. Immunoglobulins treatment in multiple sclerosis and experimental autoimmune encephalomyelitis. Mult Scler. 2000;6(Suppl. 2):S6–S8. [PubMed] [Google Scholar]
- Humle Jorgensen S., Sorensen P. S. Intravenous immunoglobulin treatment of multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;233:61–65. doi: 10.1016/j.jns.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Antel J., Bar-Or A. Roles of immunoglobulins and B cells in multiple sclerosis: from pathogenesis to treatment. J Neuroimmunol. 2006;180:3–8. doi: 10.1016/j.jneuroim.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Wiendl H., Toyka K. V., Rieckmann P., Gold R., Hartung H. P., Hohlfeld R. Basic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendations. J Neurol. 2008;255:1449–1463. doi: 10.1007/s00415-008-0061-1. [DOI] [PubMed] [Google Scholar]
- Bullard D. C., Hu X., Schoeb T. R., Axtell R. C., Raman C., Barnum S. R. Critical requirement of CD11b (Mac-1) on T cells and accessory cells for development of experimental autoimmune encephalomyelitis. J Immunol. 2005;175:6327–6333. doi: 10.4049/jimmunol.175.10.6327. [DOI] [PubMed] [Google Scholar]
- Bullard D. C., Hu X., Adams J. E., Schoeb T. R., Barnum S. R. p150,95 (CD11c/CD18) expression is required for the development of experimental autoimmune encephalomyelitis. Am J Pathol. 2007;170:2001–2008. doi: 10.2353/ajpath.2007.061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kai H., Chang F., Shibata K., Tahara-Hanaoka S., Honda S., Shibuya A., Shibuya K. A critical role of LFA-1 in the development of Th17 cells and induction of experimental autoimmune encephalomyelytis. Biochem Biophys Res Commun. 2007;353:857–862. doi: 10.1016/j.bbrc.2006.12.104. [DOI] [PubMed] [Google Scholar]
- Dugger K. J., Zinn K. R., Weaver C., Bullard D. C., Barnum S. R. Effector and suppressor roles for LFA-1 during the development of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;206:22–27. doi: 10.1016/j.jneuroim.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. S., Barnum S. R. Differential expression of β 2-integrins and cytokine production between γδ and αβ T cells in experimental autoimmune encephalomyelitis. J Leukoc Biol. 2008;83:71–79. doi: 10.1189/jlb.0407263. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol. 1997;9:643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- Archelos J. J., Previtali S. C., Hartung H. P. The role of integrins in immune-mediated diseases of the nervous system. Trends Neurosci. 1999;22:30–38. doi: 10.1016/s0166-2236(98)01287-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Wohler J., Bullard D., Schoeb T., Barnum S. LFA-1 is critical for regulatory T cell homeostasis and function. Mol Immunol. 2009;46:2424–2428. doi: 10.1016/j.molimm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhove G., de Heusch M., Urbain-Vansanten G., Urbain J., Maliszewski C., Leo O., Moser M. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–266. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarween N., Chodos A., Raykundalia C., Khan M., Abbas A. K., Walker L. S. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- Stassen M., Jonuleit H., Muller C., Klein M., Richter C., Bopp T., Schmitt S., Schmitt E. Differential regulatory capacity of CD25+ T regulatory cells and preactivated CD25+ T regulatory cells on development, functional activation, and proliferation of Th2 cells. J Immunol. 2004;173:267–274. doi: 10.4049/jimmunol.173.1.267. [DOI] [PubMed] [Google Scholar]
- DiPaolo R. J., Glass D. D., Bijwaard K. E., Shevach E. M. CD4+CD25+ T cells prevent the development of organ-specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J Immunol. 2005;175:7135–7142. doi: 10.4049/jimmunol.175.11.7135. [DOI] [PubMed] [Google Scholar]
- Sukiennicki T. L., Fowell D. J. Distinct molecular program imposed on CD4+ T cell targets by CD4+CD25+ regulatory T cells. J Immunol. 2006;177:6952–6961. doi: 10.4049/jimmunol.177.10.6952. [DOI] [PubMed] [Google Scholar]
- Sojka D. K., Huang Y. H., Fowell D. J. Mechanisms of regulatory T-cell suppression—a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka D. K., Hughson A., Sukiennicki T. L., Fowell D. J. Early kinetic window of target T cell susceptibility to CD25+ regulatory T cell activity. J Immunol. 2005;175:7274–7280. doi: 10.4049/jimmunol.175.11.7274. [DOI] [PubMed] [Google Scholar]
- Scheffold A., Murphy K. M., Hofer T. Competition for cytokines: T(reg) cells take all. Nat Immunol. 2007;8:1285–1287. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Kent S. J., Karlik S. J., Cannon C., Hines D. K., Yednock T. A., Fritz L. C., Horner H. C. A monoclonal antibody to α 4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:1–10. doi: 10.1016/0165-5728(94)00165-k. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Khan O. A., Sheremata W. A., Blumhardt L. D., Rice G. P., Libonati M. A., Willmer-Hulme A. J., Dalton C. M., Miszkiel K. A., O'Connor P. W. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A., Atlas S. W., Green A. J., Bollen A. W., Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters B. K., Tyler K. L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon β-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Goodin D. S., Cohen B. A., O'Connor P., Kappos L., Stevens J. C. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;71:766–773. doi: 10.1212/01.wnl.0000320512.21919.d2. [DOI] [PubMed] [Google Scholar]
- Stuve O., Gold R., Chan A., Mix E., Zettl U., Kieseier B. C. α4-Integrin antagonism with natalizumab: effects and adverse effects. J Neurol. 2008;255(Suppl. 6):58–65. doi: 10.1007/s00415-008-6011-0. [DOI] [PubMed] [Google Scholar]
- Major E. O. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2009 doi: 10.1146/annurev.med.080708.082655. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Carson K. R., Focosi D., Major E. O., Petrini M., Richey E. A., West D. P., Bennett C. L. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10:816–824. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- Pugashetti R., Koo J. Efalizumab discontinuation: a practical strategy. J Dermatolog Treat. 2009;20:132–136. doi: 10.1080/09546630902984596. [DOI] [PubMed] [Google Scholar]