Abstract
Campylobacter lari is a phenotypically and genotypically diverse species that comprises the classical nalidixic acid-resistant thermophilic campylobacters (NARTC) and the biochemical C. lari variants, including the urease-positive campylobacters (UPTC), the nalidixic acid-susceptible campylobacters (NASC), and the urease-producing nalidixic acid-susceptible campylobacters. To study the taxonomic and epidemiological relationships among strains of the C. lari variants, amplified fragment length polymorphism (AFLP) profiling and whole-cell protein profile analysis were performed with 55 C. lari strains. Great genetic heterogeneity in AFLP and protein profiles was observed. Numerical analysis of AFLP profiles and of partial protein profiles allowed discrimination of four distinct genogroups. AFLP cluster I included nearly homogeneous patterns for C. lari NARTC strains (genogroup I). UPTC strains together with non-urease-producing NASC strains produced highly diverse patterns and were placed in genogroup II. The genogroup III strains had the NASC phenotype and produced more homogeneous patterns. Finally, genogroup IV strains had the classical NARTC phenotype and produced AFLP patterns that were very distinct from those of other genogroups. One UPTC strain had aberrant patterns and clustered separately, which may indicate that there is an additional genogroup. Preliminary DNA-DNA hybridization experiments suggested that genogroups I and III represent a single genomic species and that genogroup IV represents a distinct species. The detection of moderate levels of DNA-DNA hybridization between a genogroup II reference strain and genogroup I and III reference strains highlights the need for further DNA-DNA hybridization experiments to clarify the taxonomic status of the former group. No correlation of genogroups with different sources of strains was identified. These data show that UPTC strains are genetically diverse and distinct from NARTC strains. In addition, they indicate that the classical NARTC phenotype encompasses at least two genogroups.
The most important Campylobacter species found in human infections are the thermophilic species C. jejuni, C. coli, C. upsaliensis, and C. lari (31). In humans, C. lari has been associated with diarrhea (6, 7, 31), with bacteremia in immunocompromised and immunocompetent patients (18, 21, 23), with urinary tract infections (3), with reactive arthritis (15), and, recently, with a prosthetic joint infection (35). Infections after consumption of contaminated shellfish, as well as a large outbreak due to a common waterborne source, have been reported (1, 23). Outbreaks caused by C. lari have been incidentally reported, and it is assumed that the number of C. lari infections is greatly underreported (12). This may result from the fact that C. lari is phenotypically difficult to distinguish from some other Campylobacter species, particularly C. jejuni and C. coli. As molecular typing methods are not always used in clinical diagnostic laboratories, identification is generally limited to the genus level. C. lari could easily be distinguished from other thermophilic Campylobacter species based on resistance to nalidixic acid until in the 1990s nalidixic acid-resistant C. jejuni and C. coli strains emerged (10, 22).
C. lari is widely distributed in the environment and can be isolated from a variety of sources, including water and animals. Seagulls have been shown to be a reservoir for C. lari, and it has been proposed that they contribute to contamination of water storage reservoirs and of mussels and oyster banks (5, 11, 25).
C. lari variants that differ from the primary nalidixic acid-resistant thermophilic Campylobacter (NARTC) strains have emerged, and these strains have been referred to as nalidixic acid-susceptible (NASC) strains, urease-producing (UPTC) strains, and urease-producing nalidixic acid-susceptible strains (19, 25, 33). UPTC strains differ from C. lari in producing urease and have been isolated worldwide from water, mussels, and oysters banks (5, 11, 25), and human infections (19). The clinical relevance of the UPTC strains, as well as of other C. lari variants, is not yet fully understood. Moreover, differences in virulence among the biochemical C. lari variants have not been identified yet.
C. lari can be isolated from several environmental sources and is an infrequent, but possibly underrecognized, cause of human infection. Whether strains associated with human infections have specific characteristics which differ from those of strains from environmental sources is still unknown. In this study the highly discriminating genotyping method amplified fragment length polymorphism analysis (AFLP) (16, 30), analysis of whole-cell protein profiles (34), and DNA-DNA hybridization studies were combined to establish the phylogeny of C. lari variants from several sources.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A total of 55 C. lari strains were analyzed. The strain designations and available information about the organisms are shown in Table 1. Fourteen reference strains of C. lari, three UPTC strains (LMG 7791, LMG 19453, and R-11304) and five clinical isolates (R-3341, M595a, R-1189, R-1638, and R-4681) were obtained from the BCCM/LMG Culture Collection (Ghent, Belgium). Thirty-three strains were isolated from oysters and mussels (11).
TABLE 1.
C. lari strains used in this study
| Genogroup | Straina | Other designationa | Source | Country |
|---|---|---|---|---|
| I | LMG 9887 | CCUG 15035 | Seagull | Canada |
| LMG 9889 | CCUG 19528 | Human | Canada | |
| LMG 7929 | CCUG 23948 | Dog, feces | United Kingdom | |
| LMG 9913 | CCUG 20581 | Human | ||
| LMG 8845 | CCUG 23949 | Child, feces | United Kingdom | |
| LMG 8846T | NCTC 1352T | Gull | United Kingdom | |
| LMG 9152 | CCUG 24266 | Horse, intestine | Sweden | |
| LMG 9914 | CCUG 20593 | Human | Canada | |
| LMG 14338 | CA 53 | Human, feces | Belgium | |
| LMG 19175 | M595b | Human, feces | Belgium | |
| R3341 | Brug 425 | Human, feces | Belgium | |
| II | LMG 19453 | CCUG 22396 | Human | France |
| LMG 7791 | CCUG 18267 | Water | United Kingdom | |
| R-11304 | CDC D5450 | Human | United States | |
| 4870-41 | Shellfish | The Netherlands | ||
| 2315BVA | Shellfish | The Netherlands | ||
| 3363-41 | Shellfish | The Netherlands | ||
| LMG 17563 | 2767A1VAP | Shellfish | The Netherlands | |
| 3365-41 | Shellfish | The Netherlands | ||
| LMG 17562 | 2576PW | Shellfish | The Netherlands | |
| LMG 17560 | 2316A3 | Shellfish | The Netherlands | |
| 2665PW | Shellfish | The Netherlands | ||
| LMG 17558 | 2314RG | Shellfish | The Netherlands | |
| 3437-41 | Shellfish | The Netherlands | ||
| 4698A2 | Shellfish | The Netherlands | ||
| LMG 17559 | 2315RG | Shellfish | The Netherlands | |
| LMG 17561 | 2323BVA | Shellfish | The Netherlands | |
| 3363-36 | Shellfish | The Netherlands | ||
| 2352PW | Shellfish | The Netherlands | ||
| 2897P | Shellfish | The Netherlands | ||
| M595a | Human, feces | Belgium | ||
| 4207 | Shellfish | The Netherlands | ||
| 3439-36 | Shellfish | The Netherlands | ||
| 2352BVA | Shellfish | The Netherlands | ||
| 4870-36 | Shellfish | The Netherlands | ||
| III | LMG 8844 | NCTC 11844 | Seawater | United Kingdom |
| LMG 11760 | LCDC 12273 | Human | Canada | |
| R-1189 | CA6022 | Human, feces | Belgium | |
| 2897R | Shellfish | The Netherlands | ||
| 2664BVA | Shellfish | The Netherlands | ||
| 2575PW | Shellfish | The Netherlands | ||
| IV | LMG 9253 | D-411 | Human, feces | United Kingdom |
| LMG 11251 | CA 4129 | Human, feces | Belgium | |
| R-1638 | Brug 251 | Dialysis fluid | Belgium | |
| R-4681 | 3968 | Human feces | Belgium | |
| 4604BVA | Shellfish | The Netherlands | ||
| 2898R | Shellfish | The Netherlands | ||
| 2314BVA | Shellfish | The Netherlands | ||
| 3365-36 | Shellfish | The Netherlands | ||
| 3437-36 | Shellfish | The Netherlands | ||
| 4604A3 | Shellfish | The Netherlands | ||
| 2663BVA | Shellfish | The Netherlands | ||
| LMG 17564 | 2665BVA | Shellfish | The Netherlands | |
| 3364-36 | Shellfish | The Netherlands | ||
| None | 4698PWb | Shellfish | The Netherlands |
LMG, BCCM/LMG Bacteria Collection, Laboratory of Microbiology, University of Ghent, Ghent, Belgium; CCUG, Culture Collection of the University of Göteborg, Göteborg, Sweden.
Strain 4698PW does not belong to any of the groups.
Bacteria were grown on blood agar plates supplemented with 5% sheep blood at 37°C for 2 to 3 days under microaerobic conditions. An Anoxomat system was used to prepare a final gas mixture consisting of 6% O2, 7% CO2, 80% N2, and 7% H2 (Mart B.V., Lichtenvoorde, The Netherlands). Bacterial cultures were stored at −80°C in 15% glycerol in heart infusion broth. Routine biochemical tests were performed to determine the hydrolysis of urease and the susceptibility to nalidixic acid (2).
AFLP analysis.
The AFLP analysis was performed by using the previously described AFLP method for Campylobacter genotyping, which is a protocol adapted from the AFLP microbial fingerprinting method of PE Applied Biosystems (9). Briefly, isolated chromosomal DNA was digested with HindIII and HhaI, and in a simultaneous reaction the fragments were ligated to restriction site-specific adapters for 2 h at 37°C. Then a preselective PCR with the adapter-specific HindIII primer (5′GACTGCGTACCAGCTT) and HhaI primer (5′GATGAGTCCTGATCGC) was performed. Next, an aliquot was subjected to a selective PCR in which both primers contained an additional A at the 3′ end (HindIII primer, 5′GACTGCGTACCAGCTTA; and HhaI primer, 5′ GATGAGTCCTGATCGCA). The 5′ end of the HindIII primer contained a fluorescent label. The final products were electrophoresed on a 7.3% denaturing acrylamide sequencing gel for 5 h by using an ABI 373A automated DNA sequencer.
Data processing.
After electrophoresis, the banding pattern data were collected with the ABI Genescan software (PE Applied Biosystems). Each gel track was then imported into the GelCompar 4.2 software package (Applied Maths, Kortrijk, Belgium) with the program ABICON (Applied Maths). Gels were normalized by using an internal standard (Genescan-500, labeled with the red fluorophore 6-carboxy-x-rhodamine [ROX]; PE Applied Biosystems) that was added to each sample. After normalization of the gels, the levels of genetic similarity between AFLP patterns were calculated with the Pearson product-moment correlation coefficient. For cluster analysis of AFLP banding patterns the unweighted pair group method using average linkages was used.
Polyacrylamide gel electrophoresis of whole-cell proteins.
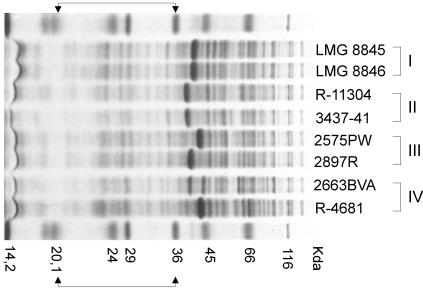
The whole-cell protein profiles of the 14 C. lari reference strains and the 33 oyster and mussel isolates have been reported in previous studies (11, 33, 34). In the present study, whole-cell protein extracts were prepared from the eight additional isolates. Extract preparation and polyacrylamide gel electrophoresis were performed as described previously (28). However, only the 20,100- to 36,000-molecular-weight region of the whole-cell protein profile (see Fig. 2) was used for numerical analysis with the GelCompar 4.2 software (Applied Maths).
FIG. 2.
Whole-cell protein profiles of two representative strains for each C. lari genogroup. The roman numerals are AFLP cluster numbers, as explained in the text. The molecular mass markers used (top and bottom lanes) were β-galactosidase (116 kDa), bovine albumin (66 kDa), egg albumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), trypsinogen (24 kDa), trypsin inhibitor (20.1 kDa) (observed as a double band), and lysozyme (14.2 kDa). The brackets indicate the region used in the numerical analysis.
Determination of the DNA base composition.
DNA was enzymatically degraded into nucleosides as described by Mesbah et al. (20). The nucleoside mixture obtained was then separated by high-performance liquid chromatography by using a Waters SymmetryShield C8 column kept at 37°C. The solvent was 0.02 M NH4H2PO4 (pH 4.0) with 1.5% acetonitrile. Nonmethylated lambda phage (Sigma, St. Louis, Mo.) was used as the calibration reference.
DNA-DNA hybridization.
The levels of DNA-DNA hybridization for strains LMG 8846 (type strain of C. lari), R-11304, LMG 11760, and LMG 9253, representatives of genogroups I through IV, respectively, were determined. Chromosomal DNA was isolated by the method described by Pitcher et al. (27). One milligram of DNA was used for DNA-DNA reassociation in a microtiter well by the method described by Ezaki et al. (13). The hybridization temperature was 30°C.
RESULTS
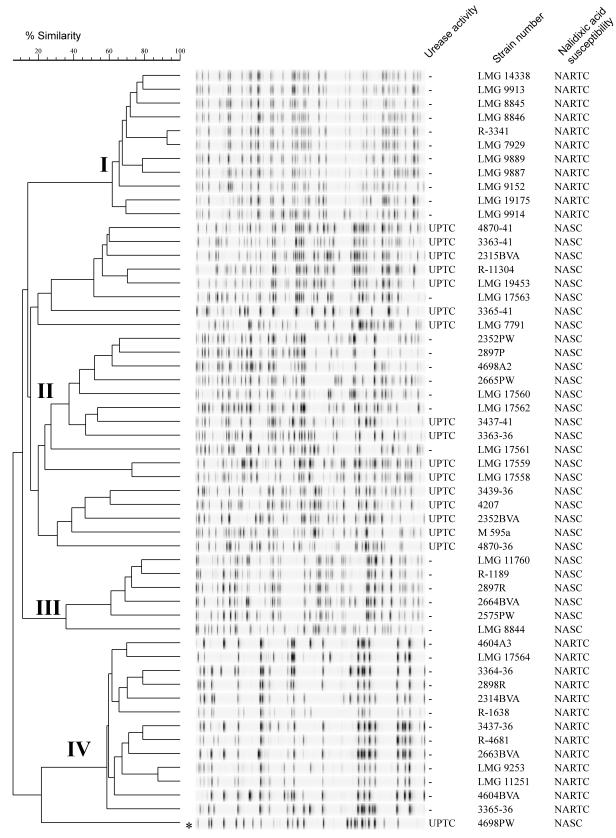
AFLP analysis with restriction endonucleases HindIII and HhaI revealed a considerable degree of genomic heterogeneity among the 55 C. lari strains tested. The dendrogram in Fig. 1 shows the genotypic similarities among the AFLP banding patterns obtained, which contained clearly separated bands that varied in size from 100 to 500 bp. Numerical analysis differentiated the AFLP patterns into four distinct clusters, representing genogroups I to IV, and grouped one strain (4698PW) separately. The latter strain may belong to an additional genogroup. The banding patterns of different clusters showed less than 20% similarity, and the pattern of AFLP cluster IV consisted of a significantly lower number of bands (Fig. 1). The reproducibility of the AFLP analysis was determined by performing the whole assay, starting with isolation of chromosomal DNA, four times for each sample. The similarity between patterns obtained repeatedly was at least 90%, as determined by the Pearson product-moment correlation, and the results correlated perfectly with previously described reproducibility values (9, 24).
FIG. 1.
Dendrogram derived from unweighted pair group method using average linkage cluster analysis of AFLP fingerprints of C. lari strains analyzed in this study. The scale bar indicates levels of linkage between patterns. The roman numerals indicate the distinct clusters to which the strains belong. The asterisk indicates a highly diverse AFLP pattern that clusters separately.
The classical C. lari strains, which are nalidixic acid resistant and urease negative, clustered in AFLP cluster I. The AFLP banding patterns of these strains were homogeneous, exhibiting more than 60% similarity. Two strains, R-3341 and LMG 7929, had patterns which were 90% similar. The patterns of AFLP cluster II strains were highly diverse, and the UPTC reference strains LMG 19453, LMG 7791, and R-11304 grouped in this cluster. The strains in cluster II were all nalidixic acid sensitive, whereas strains that were both positive and negative for urease activity were present. AFLP cluster III consisted of six additional strains that were nalidixic acid sensitive and urease negative. The banding patterns in this cluster were more homogeneous than the patterns in cluster II, and cluster III was distinguished from cluster II by patterns that exhibited less than 20% similarity (Fig. 1). Strain LMG 8844 produced a highly divergent banding pattern that exhibited only 39% similarity with the other patterns in cluster III. This similarity level corresponded with the intraspecies diversity demonstrated in cluster analysis when AFLP patterns from other Campylobacter species were used (8).
The AFLP banding patterns for cluster IV were considerably different from the patterns obtained for the other clusters. The AFLP profiles had only 16 to 24 bands and revealed interstrain genetic diversity (Fig. 1). Biochemical analysis identified these strains as members of a group of non-urease-producing classical NARTC C. lari strains.
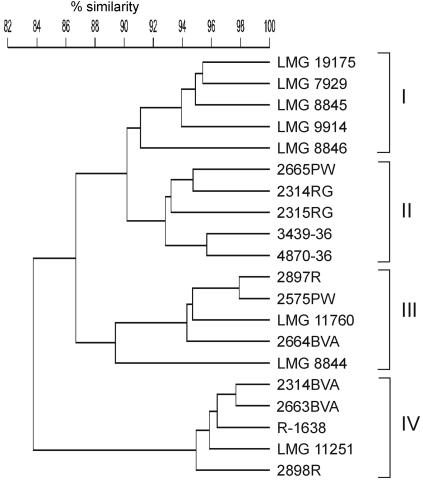
The whole-cell protein profiles of all of the strains examined were visually very similar but included a variable dense band region, as described previously for C. lari and other thermotolerant Campylobacter species (4, 25, 34). When this variable dense band region was omitted from the numerical analysis, all isolates examined formed a single tight cluster, as reported previously (34). However, the low-molecular-mass region of the profile (Fig. 2) had additional heterogeneity. If numerical analysis was used only for this region, it resulted in delineation of the same four groups that were observed by AFLP analysis. A dendrogram illustrating this for a subset of the isolates (five randomly chosen strains per cluster) is shown in Fig. 3. The protein patterns of two representative strains for each of the four genogroups are shown in Fig. 2. Strain 4698PW clustered separately as determined by AFLP analysis (Fig. 1) and was identified as C. lari by using a whole-cell protein profile without the variable dense band region; however, subsequent analysis of the low-molecular-mass region excluded this organism from the four main genogroups (data not shown). Whole-cell protein electrophoresis and AFLP confirmed that this strain was slightly aberrant and may have indicated that this strain belongs to an additional unidentified distinct genogroup of C. lari.
FIG. 3.
Dendrogram derived from numerical analysis of partial protein profiles (20,100- to 36,000-molecular-weight region) of five randomly chosen strains from each C. lari genogroup. The roman numerals are AFLP cluster numbers, as explained in the text.
The AFLP and protein profiles revealed no substantial differences between C. lari strains isolated from humans and C. lari strains isolated from birds, oysters, and mussels. Strains from these sources were present in all AFLP and protein profile groups identified.
A preliminary DNA-DNA hybridization study was performed in order to assess the genomic divergence of the four genogroups. The levels of hybridization among four strains representing the four genogroups are shown in Table 2. A high level of hybridization (80%) between the genogroup I and III isolates was observed. Both of these isolates exhibited low levels of hybridization (55 and 62%) with the representative of genogroup IV. The levels of hybridization of the genogroup II isolate with the other isolates were low to intermediate (59 to 76%). The average G+C content of the strains was determined to be 29%.
TABLE 2.
DNA-DNA binding values for strains examined
| Genogroup | Strain | DNA binding values with:
|
|||
|---|---|---|---|---|---|
| Genogroup I strain | Genogroup II strain | Genogroup III strain | Genogroup IV strain | ||
| I | LMG 8846T | 100 | |||
| II | R-11304 | 62 | 100 | ||
| III | LMG 11760 | 80 | 76 | 100 | |
| IV | LMG 9253 | 55 | 59 | 62 | 100 |
DISCUSSION
Genotypic and phenotypic analyses in which numerical analysis of AFLP and whole-cell protein profiles of a set of biochemical variants of C. lari (NARTC, NASC, and UPTC strains) was used showed that the variants were separated in four groups and a single separate strain with a unique profile. The NARTC strains were divided into two clusters (clusters I and IV). The second cluster (cluster II) contained UPTC and non-urease-producing strains that were nalidixic acid sensitive (NASC strains). Cluster III contained only non-urease-producing NASC strains.
It has been shown previously that there is extensive heterogeneity at the genetic and protein levels among C. lari strains and between different biochemical variants (11, 25, 26). AFLP analysis of C. lari detected considerably more diversity in this species than was observed with AFLP analysis of a range of other Campylobacter species (8, 24). By using the low-molecular-weight region of the protein profiles the same four groups that were observed with AFLP were observed. Although AFLP genomic fingerprinting analysis has recently been shown to be an accurate approach for determining bacterial taxonomy and the phylogenetic structure of bacteria, no general threshold levels for delineation of bacterial species have been established (29). Therefore, we performed a preliminary DNA-DNA hybridization experiment to quantify the degrees of genomic divergence among the four taxa. The results suggested that genogroups I and III represent a single genomic species and that genogroup IV represents a distinct species. Detection of low to moderate levels of DNA-DNA hybridization between a genogroup II reference strain and genogroup I and III reference strains highlights the need for further DNA-DNA hybridization experiments to clarify the taxonomic status of the former genogroup.
UPTC C. lari strains can hydrolyze urea. Whether the UPTC C. lari strains deserve separate taxonomic status, either as a distinct species closely related to C. lari or as a subspecies or biovar of C. lari, is unclear. By using semiquantitative DNA-DNA hybridization, three UPTC isolates have been classified as C. lari (19). Whole-cell protein electrophoresis based on analysis of complete protein profiles identified UPTC strains as C. lari, and therefore it was proposed that these organisms should be considered distinct biovars of C. lari (25). Recently, On and Harrington (24) showed that there are substantial differences between the AFLP patterns of UPTC and NARTC strains. Our data support this observation. However, these authors also found that the cluster comprising most of the UPTC isolates (cluster II) also comprised urease-negative isolates, indicating that urease activity is not uniformly present in this taxon. In addition, one of the UPTC strains, 4698PW, represented another genogroup within C. lari, demonstrating that the capacity to produce urease activity is present in multiple C. lari-like campylobacters. These results indicate that the UPTC phenotype is not confined to a single taxon and that it is a variable characteristic within the taxon.
The AFLP patterns of UPTC strains were extremely heterogeneous and confirmed the previously observed variations in randomly amplified polymorphic DNA patterns and GTPase gene sequences of these strains (11, 32). The previously described indistinguishable pulsed-field gel electrophoresis patterns obtained for three UPTC strains from seagulls are inconsistent with these results (17), although this finding may have been affected by sampling bias and the low number of strains tested.
A remarkable observation is the uniform distribution of nalidixic acid susceptibility among the genogroups (Fig. 1). However, as nalidixic acid resistance results from reversible single DNA point mutations in the gyrA or parC genes (14), this characteristic may be inappropriate for use in classification of C. lari strains (23). In this case, it seems likely that the identical nalidixic acid susceptibilities of strains within a genogroup are due to coincidence.
The epidemiology and natural habitat of C. lari are largely unknown. Initially, most strains were isolated from gulls, but C. lari has now been isolated from a variety of environmental and animal sources. C. lari is infrequently isolated from humans, but it is associated with severe diseases in both immunocompetent and immunocompromised hosts. It is still not known whether strains from these sources contain determinants that may contribute to specialization for a niche or whether hydrolysis of urease is an important determinant for human infection. In the present study no correlation between AFLP or protein profiles and a source (human, shellfish, animal) could be identified.
In conclusion, C. lari shows extensive genetic diversity. By using AFLP and whole-cell protein analysis, the C. lari population studied could be divided into four clearly distinct genogroups and an additional unique isolate. Preliminary DNA-DNA hybridization experiments indicated that two genogroups (genogroups I and III) represent a single genomic species and suggested that one of the genogroups (genogroup IV) deserves separate species rank. The taxonomic relationships within genogroup II and between genogroup II and the other taxa require further analysis by DNA-DNA hybridization. We obtained no evidence for host specificity of the genogroups. Molecular characterization of more C. lari isolates from various sources is needed to elucidate the epidemiology of C. lari genogroups in the environment and in human infection.
Acknowledgments
We thank J. S. Vliegenthart (Food Inspection Service, Rotterdam, The Netherlands) for providing the shellfish strains.
REFERENCES
- 1.Abeyta, C., F. G. Deeter, C. A. Kaysner, R. F. Stott, and M. M. Wekell. 1993. Campylobacter jejuni in a Washington sate shellfish growing bed associated with illness. J. Food. Prot. 56:323-325. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin, J., S. Leaper, R. J. Owen, and M. B. Skirrow. 1983. Description of Campylobacter laridis, a new species comprising the nalidixic acid resistant thermophilic Campylobacter (NARTC) group. Curr. Microbiol. 8:231-238. [Google Scholar]
- 3.Bezian, M. C., G. Ribou, C. Barberis-Giletti, and F. Megraud. 1990. Isolation of a urease positive thermophilic variant of Campylobacter lari from a patient with urinary tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 9:895-897. [DOI] [PubMed] [Google Scholar]
- 4.Blaser, M. J., J. A. Hopkins, R. M. Berka, M. L. Vasil, and W.-L. L. Wang. 1983. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect. Immun. 42:176-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton, F. J., A. V. Holt, and D. N. Hutchinson. 1985. Urease-positive thermophilic campylobacters. Lancet i:1217-1218. [DOI] [PubMed]
- 6.Broczyk, A., S. Thomson, D. Smith, and H. Lior. 1987. Water-borne outbreak of Campylobacter laridis-associated gastroenteritis. Lancet i:164-165. [DOI] [PubMed] [Google Scholar]
- 7.Chiu, C. H., C. Y. Kuo, and J. T. Ou. 1995. Chronic diarrhea and bacteremia caused by Campylobacter lari in a neonate. Clin. Infect. Dis. 21:700-701. [DOI] [PubMed] [Google Scholar]
- 8.Duim, B., P. R. Vandamme, A. Rigter, S. Laevens, J. R. Dijkstra, and J. A. Wagenaar. 2001. Differentiation of Campylobacter species by AFLP fingerprinting. Microbiology 147:2729-2737. [DOI] [PubMed] [Google Scholar]
- 9.Duim, B., T. M. Wassenaar, A. Rigter, and J. A. Wagenaar. 1999. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with AFLP fingerprinting. Appl. Environm. Microbiol. 65:2369-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endtz, H. P., G. J. Ruijs, B. van Klingeren, W. H. Jansen, T. van der Reyden, and R. P. Mouton. 1991. Quinolone resistance in campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J. Antimicrob. Chemother. 27:199-208. [DOI] [PubMed] [Google Scholar]
- 11.Endtz, H. P., J. S. Vliegenthart, P. Vandamme, H. W. Weverink, N. P. van den Braak, H. A. Verbrugh, and A. Van Belkum. 1997. Genotypic diversity of Campylobacter lari isolated from mussels and oysters in The Netherlands. Int. J. Food Microbiol. 34:79-88. [DOI] [PubMed] [Google Scholar]
- 12.Engberg, J., S. L. W. On, C. S. Harrington, and P. Gerner-Smidt. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J. Clin. Microbiol. 38:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezaki, T., Y. Hashimoto, H. Yamamoto, M. L. Lucida, S. L. Liu, S. Kusunoki, K. Asano, and E. Yabuuchi. 1990. Evaluation of the microplate hybridization method for rapid identification of Legionella species. Eur. J. Clin. Microbiol. Infect. Dis. 9:213-217. [DOI] [PubMed] [Google Scholar]
- 14.Gibreel, A., E. Sögren, B. Kaijser, B. Wretlind, and O. Sköld. 1998. Rapid emergence of high-level resistance to quinolones in Campylobacter jejuni associated with mutational changes in gyrA and parC. Antimicrob. Agents Chemother. 42:3276-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goudswaard, J., L. Sabbe, and W. te Winkel. 1995. Reactive arthritis as a complication of Campylobacter lari enteritis. J. Infect. 31:171. [DOI] [PubMed] [Google Scholar]
- 16.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko, A., M. Matsuda, M. Miyajima, J. E. Moore, and P. G. Murphy. 1999. Urease-positive thermophilic strains of Campylobacter isolated from seagulls (Larus spp.). Lett. Appl. Microbiol. 29:7-9. [DOI] [PubMed] [Google Scholar]
- 18.Martinot, M., B. Jaulhac, R. Moog, S. De Martino, P. Kehrli, H. Monteil, and Y. Piemont. 2001. Campylobacter lari bacteremia. Clin. Microbiol. Infect. 7:96-97. [DOI] [PubMed] [Google Scholar]
- 19.Mégraud, F., D. Chevrier, N. Deplaces, A. Sedallian, and J. L. Guesdon. 1988. Urease-positive thermophilic campylobacters (Campylobacter laridis variant) isolated from an appendix and from human feces. J. Clin. Microbiol. 26:1050-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesbah, M., U. Premachandran, and W. B. Whitman. 1989. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int. J. Syst. bacteriol. 39:159-167. [Google Scholar]
- 21.Morris, C. N., B. Scully, and G. J. Garvey. 1998. Campylobacter lari associated with permanent pacemaker infection and bacteremia. Clin. Infect. Dis. 27:220-221. [DOI] [PubMed] [Google Scholar]
- 22.Nachamkin, I., J. Engberg, and F. M. Aarestrup. 2000. Diagnosis and antimicrobial susceptibility of Campylobacter species, p. 45-66. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 23.Nachamkin, I., C. Stowell, D. Skalma, A. M. Jones, R. Hoop, I. I., and R. M. Smibert. 1984. Campylobacter laridis causing bacterimia in an immunosuppressed patient. Ann. Intern. Med. 101:55-57. [DOI] [PubMed] [Google Scholar]
- 24.On, S. L., and C. S. Harrington. 2000. Identification of taxonomic and epidemiological relationships among Campylobacter species by numerical analysis of AFLP profiles. FEMS. Microbiol. Lett. 193:161-169. [DOI] [PubMed] [Google Scholar]
- 25.Owen, R. J., M. Costas, L. L. Sloss, and F. J. Bolton. 1988. Numerical analysis of electrophoretic protein patterns of Campylobacter laridis and allied thermophilic campylobacters from the natural environment. J. Appl. Bacteriol. 65:69-78. [DOI] [PubMed] [Google Scholar]
- 26.Owen, R. J., M. Desai, and S. Garcia. 1993. Molecular typing of thermotolerant species of Campylobacter with ribosomal RNA gene patterns. Res. Microbiol. 144:709-720. [DOI] [PubMed] [Google Scholar]
- 27.Pitcher, D. G., N. A. Saunders, and R. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 28.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprinting, p. 493-521. In M. Goodfellow and A. G. O’Donnell (ed.), Modern microbial methods: chemical methods in bacterial Systematics. Wiley, Chichester, United Kingdom.
- 29.Rademaker, J. L. W., B. Hoste, F. J. Louws, K. Kersters, J. Swings, L. Vauterin, P. Vauterin, and F. J. de Bruijn. 2000. Comparison of AFLP and rep-PCR genomic fingerprinting with DNA-DNA homology studies: Xanthomonas as a model system. Int. J. Syst. Evol. Microbiol. 50:665-677. [DOI] [PubMed] [Google Scholar]
- 30.Savelkoul, P. H., H. J. Aarts, J. de Haas, L. Dijkshoorn, B. Duim, M. Otsen, J. L. Rademaker, L. Schouls, and J. A. Lenstra. 1999. Amplified fragment length polymorphism analysis: the state of an art. J. Clin. Microbiol. 37:3083-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauxe, R. V. 1992. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized coutries, p. 9-19. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, D.C.
- 32.van Doorn, L.-J., A. Verschuuren-van Haperen, A. Van Belkum, H. P. Endtz, J. S. Vliegenthart, P. Vandamme, and W. G. V. Quint. 1998. Rapid identification of diverse Campylobacter lari strains isolated from mussels and oysters using a reverse hybridization line probe assay. J. Appl. Microbiol. 84:545-550. [DOI] [PubMed] [Google Scholar]
- 33.Vandamme, P., P. Bot, and K. Kersters. 1991. Differentiation of campylobacters and Campylobacter-like organisms by numerical analysis of one-dimensional electrophoretic protein patterns. Syst. Appl. Microbiol. 14:57-66. [Google Scholar]
- 34.Vandamme, P., D. Dewettinck, and K. Kerstens. 1992. Application of numerical analysis of electrophoretic protein profiles for the identification of thermophilic Campylobacter. Syst. Appl. Microbiol. 15:402-408. [Google Scholar]
- 35.Werno, A. M., J. D. Klena, G. M. Shaw, and D. R. Murdoch. 2002. Fatal case of Campylobacter lari prosthetic joint infection and bacteremia in an immunocompetent patient. J. Clin. Microbiol. 40:1053-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]