Abstract
Nrf2-mediated activation of antioxidant response element is a central part of molecular mechanisms governing the protective function of phase II detoxification and antioxidant enzymes against carcinogenesis, oxidative stress and inflammation. Nrf2 is sequestered in the cytoplasm by its repressor, Keap1. We have designed and synthesized novel chalcone derivatives as Nrf2 activators. The potency of these compounds was measured by the expression of Nrf2 dependent antioxidant genes, GCLM, NQO1 and HO1, in human lung epithelial cells; while the cytotoxicity was analyzed using MTT assay. In vivo potency of identified lead compounds to activate Nrf2 was evaluated using mouse model. Our studies showed 2-trifluoromethyl-2’-methoxychalone (2b) to be a potent activator of Nrf2, both, in vitro and in mice. Additional experiments showed that the activation of Nrf2 by this compound is independent of reactive oxygen species or redox changes. We have discussed a quantitative structure-activity relationship and proposed a possible mechanism of Nrf2 activation.
INTRODUCTION
Nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) is a basic-leucine zipper (b-ZIP) transcription factor present in the cytoplasm of normal cells. Upon activation in response to inflammatory stimulus, environmental toxicant, oxidative and electrophilic stress, Nrf2 detaches from its cytosolic inhibitor, Kelch-like ECH-associated protein 1 (Keap1) and translocates to the nucleus and binds to the antioxidant response element (ARE) of target genes along with other binding partners leading to their transcriptional induction.1–4 The Keap1-Nrf2 system is the major regulatory pathway of cytoprotective gene expression against oxidative and/or electrophilic stresses. Keap1 acts as a stress sensor protein in this system. While Keap1 constitutively suppresses Nrf2 activity under unstressed conditions, oxidants or electrophiles provoke the repression of Keap1 activity, inducing the Nrf2 activation.5–7 In addition to Keap1, the activation of diferent protein kinases has been shown to activate Nrf2.8–12 The Nrf2-regulated genes include almost all of the relevant antioxidants and cytoprotective genes such as heme oxygenase-1 (HO-1), NAD (P)H:quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase modifier subunit (GCLM), γ-glutamyl cysteine synthase, glutathione peroxidase (GPx), and several members of the glutathione S-transferase family 6, 13–18 that express an ARE in their promoter.19 Small molecules that activate Nrf2 signaling are being investigated as potential anti-cancer or anti-inflammatory agents. A wide variety of dietary and synthetic compounds that function as potent inducers of ARE-regulated gene expression have been shown to exert chemopreventive activities, e.g., sulforaphane4, 20–22, dithiolethione23–25, curcumin26, and caffeic acid phenethyl ester (CAPE)26. It is notable that both curcumin and CAPE bear an α, β-unsaturated ketone moiety and can therefore act as Michael acceptors that are able to modify cysteine thiols present in Keap1.
Chalcones or 1,2-diphenyl-2-propen-1-ones are Michael acceptors and constitute an important group of natural products belonging to the flavonoid family.27, 28 They have been reported to possess many biological properties including anti-cancer29, 30, anti-malarial31, 32, anti-inflammatory33–35, antileishmanial33–35, anti-tuberclulosis36, nitric oxide inhibition37, 38, anti-mitotic39, analgesic, antipyretic, antioxidant40–43, antibacterial, anti-HIV44, antifungal45 and antiprotozoal activities.46–48 They are also reported to be gastric protectant49, anti-mutagenic, and anti-tumorogenic.50–52 Natural and synthetic chalcones have been reported to possess strong antiproliferative effects in primary and established ovarian cancer cells53 and in gastric cancer cells.52 Chalcones contain two aromatic rings separated by α, β -unsaturated ketone and this unique structure is responsible for various activities of these molecules.27 It is well known that α, β unsaturated carbonyl entity in chalcones is a soft electrophile and would attract soft nucleophiles like thiols, rather than hard nucleophiles like amino and hydroxyl groups. Chalcones are unlikely to react with the amino and hydroxyl groups on nucleic acids and thus would unlikely induce mutagenicity and carcinogenicity commonly associated with alkylating agents used in cancer chemotherapy.28
The remarkable biological potential of chalcones is due to their possible interactions with various proteins related to cell apoptosis and proliferation.54, 55 A number of recent studies have indicated that the anti-inflammatory effect of chalcones is due to the inhibition of the NF-κB pathway, which is mediated by IκB degradation and the phosphorylation of c-Jun N-terminal kinase (JNK) and c-Jun.56–58 It has been reported that electrophilic α, β-unsaturated carbonyl moiety on chalcone resulted in the activation of Nrf2/ARE pathway and the induction of phase II detoxifying enzyme expression.59, 60 This moiety acts as an electrophile and reacts with free sulfhydryl groups of thioredoxin and cysteine residues in proteins.58, 59, 61 It is also reported that electrophilic phytochemicals could give rise to thiyl radicals, which could also interact with sulfhydryl residues of intracellular targets, including Nrf2.62 These studies demonstrate that the endogenous electrophilic activity, through its α, β-unsaturated carbonyl moiety, is involved in the anti-oxidant and anti-inflammatory properties of chalcone. In the present study, we synthesized novel chalcone derivatives and tested their Nrf2 activating activity in human bronchial epithelial cells. Furthermore, to draw a structure-activity relationship, a possible mechanism for Nrf2 activation is proposed.
RESULTS AND DISCUSSION
Chemistry
Chaclones can be readily synthesized by the base-catalyzed Claisen-Schmidt condensation of an aldehyde and ketone in a polar solvent like ethanol or methanol. The traditional synthesis of chalcones involves the use of strong bases such as NaOH31, 35, 39, 63, KOH16, 64, Ba(OH)265, 66, hydrotalcites67, and LiHMDS68, calcined NaNO3/natural phosphate69. They can also be synthesized by acid-catalyzed aldol condensations, e.g., AlCl370, BF371, dry HCl72, Zn(bpy)(OAc)273, Cp2ZrH2/NiCl274, and RuCl3 (for cyclic and acyclic ketones).75 Suzuki coupling has also been employed for the synthesis of chalcone derivatives.76 Several disadvantages of these procedures include long reaction time, high reaction temperature, complex reaction conditions and the use of expensive and non-commercial reagents. Recently, an efficient and facile synthesis of chalcones by condensation of aldehydes and ketones has been reported using LiOH.H2O as a dual activation catalyst under mild conditions.77 We employed similar conditions for the synthesis of all chalcone derivatives used in the present study. In short, the appropriately substituted acetophenone was dissolved in ethanol followed by addition of catalytic amount of LiOH.H2O. The reaction mixture was stirred at room temperature for 15 minutes and the desired substituted benzaldehyde was added. The reaction was carried out at room temperature until completion and the corresponding chalcone derivative (1a–5l) was isolated by crystallization or by silica gel flash chromatography (Table 1). All chalcone derivatives were characterized by 1H, 13C NMR, and HRMS. To the best of our knowledge, compounds 2b, 2f, 2i, 2k, 2l, 3b, 3c, 3f, 3i, 3k, 5f, 5g, and 5i have never been reported before, whereas compounds 2e, 2g, 2h, 3g, and 3h are commercially available as per the SciFinder search; however, there is no report on these compounds in mice and human lung epithelial cells.
Table 1.
Synthesis of chalcone derivatives by Claisen-Schmidt condensation and their Nrf2 induction activity
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Chalcones | Substituents on Ring A | Substituents on Ring B |
Relative fold Changea |
|||||||
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | GCLM | NQO1 | ||
| 1 | 1a | H | H | H | H | H | H | H | H | 0.7 | 1.8 |
| 2 | 1b | OMe | H | H | H | H | H | H | H | 3.6 | 3.2 |
| 3 | 1c | H | OMe | H | H | H | H | H | H | 1.5 | 2.4 |
| 4 | 1d | H | H | OMe | H | H | H | H | H | 1.9 | 2.8 |
| 5 | 1e | OMe | H | OMe | H | H | H | H | H | 2.7 | 2.8 |
| 6 | 1f | OMe | H | H | H | OMe | H | H | H | 2.5 | 1.9 |
| 7 | 1g | OMe | H | H | OMe | H | H | H | H | 3.0 | 2.6 |
| 8 | 1h | H | OMe | OMe | H | H | H | H | H | 2.3 | 2.5 |
| 9 | 1i | H | OMe | H | OMe | H | H | H | H | 3.0 | 2.4 |
| 10 | 1j | H | OMe | OMe | OMe | H | H | H | H | 0.7 | 0.7 |
| 11 | 1k | OMe | OMe | OMe | H | H | H | H | H | 2.4 | 2.4 |
| 12 | 1l | OMe | H | OMe | H | OMe | H | H | H | 3.1 | 1.8 |
| 13 | 2a | H | H | H | H | H | CF3 | H | H | 5.0 | 5.3 |
| 14 | 2b | OMe | H | H | H | H | CF3 | H | H | 4.5 | 4.6 |
| 15 | 2c | H | OMe | H | H | H | CF3 | H | H | 5.6 | 4.3 |
| 16 | 2d | H | H | OMe | H | H | CF3 | H | H | 5.4 | 4.6 |
| 17 | 2e | OMe | H | OMe | H | H | CF3 | H | H | 5.4 | 4.5 |
| 18 | 2f | OMe | H | H | H | OMe | CF3 | H | H | 5.7 | 5.3 |
| 19 | 2g | OMe | H | H | OMe | H | CF3 | H | H | 4.4 | 2.7 |
| 20 | 2h | H | OMe | OMe | H | H | CF3 | H | H | 4.3 | 2.7 |
| 21 | 2i | H | OMe | H | OMe | H | CF3 | H | H | 4.0 | 3.7 |
| 22 | 2j | H | OMe | OMe | OMe | H | CF3 | H | H | 3.0 | 3.1 |
| 23 | 2k | OMe | OMe | OMe | H | H | CF3 | H | H | 5.4 | 4.1 |
| 24 | 2l | OMe | H | OMe | H | OMe | CF3 | H | H | 4.4 | 4.3 |
| 25 | 3a | H | H | H | H | H | H | CF3 | H | 3.1 | 2.8 |
| 26 | 3b | OMe | H | H | H | H | H | CF3 | H | 3.9 | 2.9 |
| 27 | 3c | H | OMe | H | H | H | H | CF3 | H | 4.6 | 3.5 |
| 28 | 3d | H | H | OMe | H | H | H | CF3 | H | 2.7 | 2.8 |
| 29 | 3e | OMe | H | OMe | H | H | H | CF3 | H | 4.6 | 4.0 |
| 30 | 3f | OMe | H | H | H | OMe | H | CF3 | H | 1.8 | 0.6 |
| 31 | 3g | OMe | H | H | OMe | H | H | CF3 | H | 1.8 | 0.8 |
| 32 | 3h | H | OMe | OMe | H | H | H | CF3 | H | 4.1 | 2.9 |
| 33 | 3i | H | OMe | H | OMe | H | H | CF3 | H | 3.8 | 3.9 |
| 34 | 3j | H | OMe | OMe | OMe | H | H | CF3 | H | 1.7 | 0.8 |
| 35 | 3k | OMe | OMe | OMe | H | H | H | CF3 | H | 3.6 | 3.2 |
| 36 | 3l | OMe | H | OMe | H | OMe | H | CF3 | H | 1.4 | 0.7 |
| 37 | 4a | H | H | H | H | H | H | H | CF3 | 3.1 | 2.9 |
| 38 | 4b | OMe | H | H | H | H | H | H | CF3 | 4.3 | 5.4 |
| 39 | 4c | H | OMe | H | H | H | H | H | CF3 | 2.9 | 3.1 |
| 40 | 4d | H | H | OMe | H | H | H | H | CF3 | 3.5 | 4.6 |
| 41 | 4e | OMe | H | OMe | H | H | H | H | CF3 | 5.0 | 4.8 |
| 42 | 4f | OMe | H | H | H | OMe | H | H | CF3 | 1.4 | 0.6 |
| 43 | 4g | OMe | H | H | OMe | H | H | H | CF3 | 4.1 | 4.0 |
| 44 | 4h | H | OMe | OMe | H | H | H | H | CF3 | 3.5 | 2.7 |
| 45 | 4i | H | OMe | OMe | OMe | H | H | H | CF3 | 4.1 | 3.7 |
| 46 | 4j | OMe | OMe | OMe | H | H | H | H | CF3 | 4.1 | 3.9 |
| 47 | 4k | OMe | H | OMe | H | OMe | H | H | CF3 | 2.4 | 2.4 |
| 48 | 5a | H | H | H | H | H | NO2 | H | H | 2.1 | 1.6 |
| 49 | 5b | OMe | H | H | H | H | NO2 | H | H | 1.9 | 1.4 |
| 50 | 5c | H | OMe | H | H | NO2 | H | H | 2.9 | 2.7 | |
| 51 | 5d | H | H | OMe | H | H | NO2 | H | H | 4.6 | 4.3 |
| 52 | 5e | OMe | H | OMe | H | H | NO2 | H | H | 2.7 | 3.3 |
| 53 | 5f | OMe | H | H | H | OMe | NO2 | H | H | 4.1 | 3.7 |
| 54 | 5g | OMe | H | H | OMe | H | NO2 | H | H | 1.7 | 0.7 |
| 55 | 5h | H | OMe | OMe | H | H | NO2 | H | H | 2.3 | 2.7 |
| 56 | 5i | H | OMe | H | OMe | H | NO2 | H | H | 3.4 | 4.1 |
| 57 | 5j | H | OMe | OMe | OMe | H | NO2 | H | H | 1.2 | 0.7 |
| 58 | 5k | OMe | OMe | OMe | H | H | NO2 | H | H | 2.9 | 3.3 |
| 59 | 5l | OMe | H | OMe | H | OMe | NO2 | H | H | 2.6 | 2.0 |
| DMSO | 1.0 | 1.0 | |||||||||
| Sulforaphane | 2.7 | 3.6 | |||||||||
Data presented are representative of 3 independent experiments.
Values shown are mean ± SD of quadruplicate wells.
Biology
Potency of chalcone derivatives to activate the expression of Nrf2-regulated cytoprotective genes in human lung epithelial cells
To investigate the potency of chalcone derivatives to activate Nrf2, we measured the expression of antioxidant genes, GCLM and NADPH-NQO1, two well characterized transcriptional targets of Nrf2, as surrogate markers. Previously, we and others have shown that oxidants or small molecule activators of Nrf2 increase GCLM and NQO1 in cells or tissues of wild-type but not in Nrf2-deficient mice.78 In this study, to screen novel Nrf2 activators, we treated normal human bronchial epithelial cells (Beas-2B) with chalcone derivatives (10 µM) for 16 h and analyzed the expression of GCLM and NQO1 by quantitative RT-PCR (qRT-PCR). We included sulforaphane, a well known potent activator of Nrf2, as a positive control. We identified 59 chalcone derivatives that induce the expression of GCLM and NQO1 (Table 1). Concurrent with the gene expression analysis, we analyzed the cytotoxicity of the chalcone derivatives using the MTT assay. A total of 20 chalcones showed a higher induction of Nrf2-regulated transcriptional targets than the positive control i.e., sulforaphane (Table 2).
Table 2.
List of positive leads from primary screening and their effect on cell viability
| Entry | Compound | Relative Fold Changea | % Cell Viability |
|
|---|---|---|---|---|
| GCLM | NQO1 | |||
| 1 | DMSO | 1 | 1 | 100 |
| 2 | sulforaphane | 2.7 | 3.6 | 97.3 |
| 3 | 2a | 5 | 5.3 | 144.8 |
| 4 | 2b | 4.5 | 4.6 | 100.9 |
| 5 | 2c | 5.6 | 4.3 | 96.6 |
| 6 | 2d | 5.4 | 4.6 | 96 |
| 7 | 2e | 5.4 | 4.5 | 105 |
| 8 | 2f | 5.7 | 5.3 | 101.1 |
| 9 | 2i | 4 | 3.7 | 87.1 |
| 10 | 2k | 5.4 | 4.1 | 101.6 |
| 11 | 2l | 4.4 | 4.3 | 145.3 |
| 12 | 3c | 4.6 | 3.5 | 95.3 |
| 13 | 3i | 3.8 | 3.9 | 108.2 |
| 14 | 4b | 4.3 | 5.4 | 91 |
| 15 | 4d | 3.5 | 4.6 | 104.1 |
| 16 | 4e | 5 | 4.8 | 92.6 |
| 17 | 4g | 4.1 | 4 | 91.6 |
| 18 | 4j | 4.1 | 3.7 | 100.1 |
| 19 | 5d | 4.6 | 4.3 | 93.2 |
| 20 | 5f | 4.1 | 3.7 | 75.8 |
| 21 | 5i | 3.4 | 4.1 | 90.9 |
Data presented are representative of 3 independent experiments.
Values shown are mean ± SD of quadruplicate wells.
Preliminary structure-activity relationship
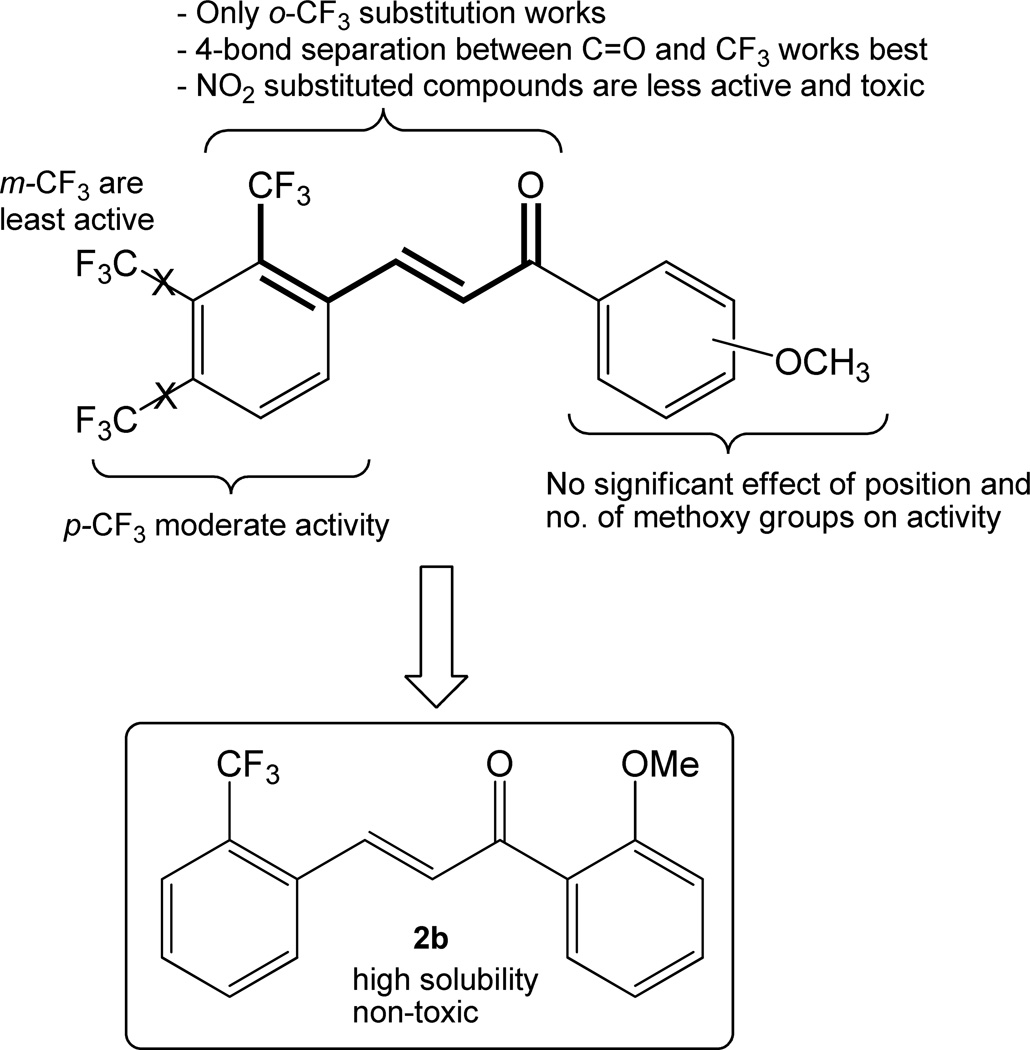
The structure-activity relationship analysis showed that the chalcone derivatives 1a–l without any substitution on ring B were not active. The activity of similar derivatives with trifluoromethyl (CF3) substitution on ring B enhanced the activity dramatically. The position of CF3 substitution was also crucial for the activity and cytotoxicity of these compounds. In general, the chalcone derivatives with CF3 substitution at ortho position on ring B were the most active compounds (entries 13–24, Table 1), followed by para (entries 37–47, Table 1), and meta (entries 25–36, Table 1) substitution. Also, the cytotoxicity data show that the ortho CF3-substituted chalcones were non cytotoxic. This shows that 4-bond separation between carbonyl and CF3 is crucial for the induction activity. Interestingly, with nitro (NO2) substitution at ortho position on ring B, the activity decreased and the toxicity increased significantly (entries 48–59, Table 1 and entries 19–21, Table 2). Thus, based on these data, we selected only those chalcone derivatives that showed > 4-fold induction of GCLM and NQO1 genes and > 95% cell viability. Based on these stringent criteria, of the 20 compounds that showed potency to increase Nrf2 activity, we selected compounds 2a–f, 2k, and 2l for further analysis.
In vivo potency of identified lead compounds to activate Nrf2 using mouse models
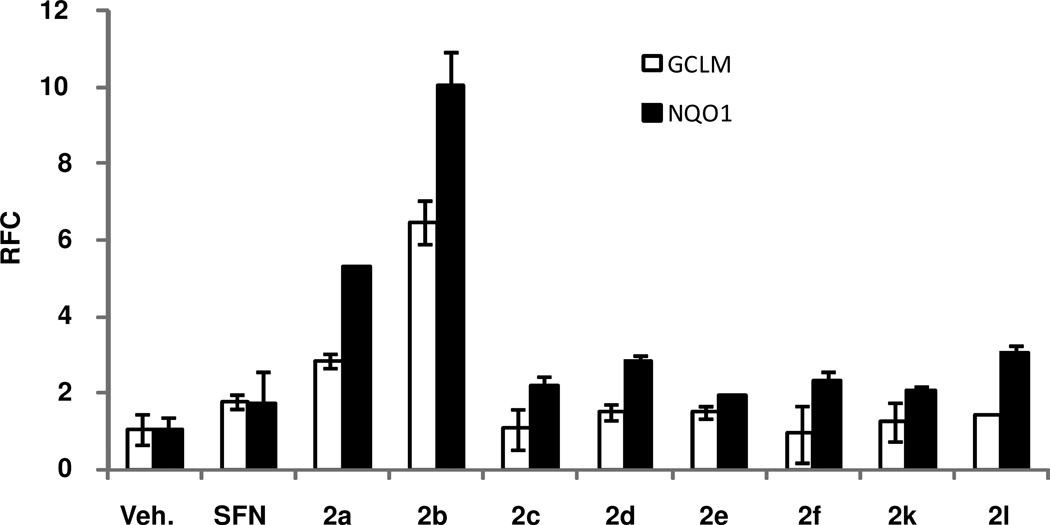
Next, we evaluated the potency of the 8 lead chalcones identified in the in vitro screening to activate Nrf2 pathway in mouse models. First, we tested various formulations to dissolve the compounds, and the DCP (10% DMSO+ 10% Cremophor EL+ 80% phosphate buffered saline) formulation offered the maximum solubility for delivery of these compounds by oral route. Mice (C57BL/6) were administrated with a single dose of vehicle or test compound(s) or sulforaphane as the positive control at a dose of 50 mg/kg body weight by gavage and small intestines were harvested 24 h later. The expression of Nrf2-regulated genes GCLM and NQO1 was analyzed in the tissue by qRT-PCR. All the 8 lead compounds increased the expression of GCLM and NQO1 in small intestine. However, 2b was the most potent inducer of Nrf2 activity (Figure 2). The expression of GCLM and NQO1 in the small intestine of mice treated with 2b was 6-fold and 10-fold higher compared to vehicle, respectively. Similarly, the expression of GCLM and NQO1 in the small intestine treated with 2b was 3-fold and 5-fold higher compared to sulforaphane, respectively. Taken together, we selected 2b as the most potent activator of Nrf2 for further studies.
Figure 2. Expression of Nrf2-regulated genes in small intestine after treatment with chalcone derivatives.
Mice (n=4) were fed with vehicle (DCP-10% DMSO + 10% Cremophor + 80% phosphate buffered saline) or chalcone derivatives or sulforaphane (50 mg/kg body weight) by gavage, and the small intestines were harvested 24 h later. The expression of Nrf2-regulated genes GCLM and NQO1 was analyzed in the tissues by qRT-PCR as a surrogate marker of Nrf2 activity. β-actin was used for normalization. Data are representative of 3 independent experiments (P ≤ 0.05).
Nrf2 is essential for induction of antioxidant genes by compound 2b
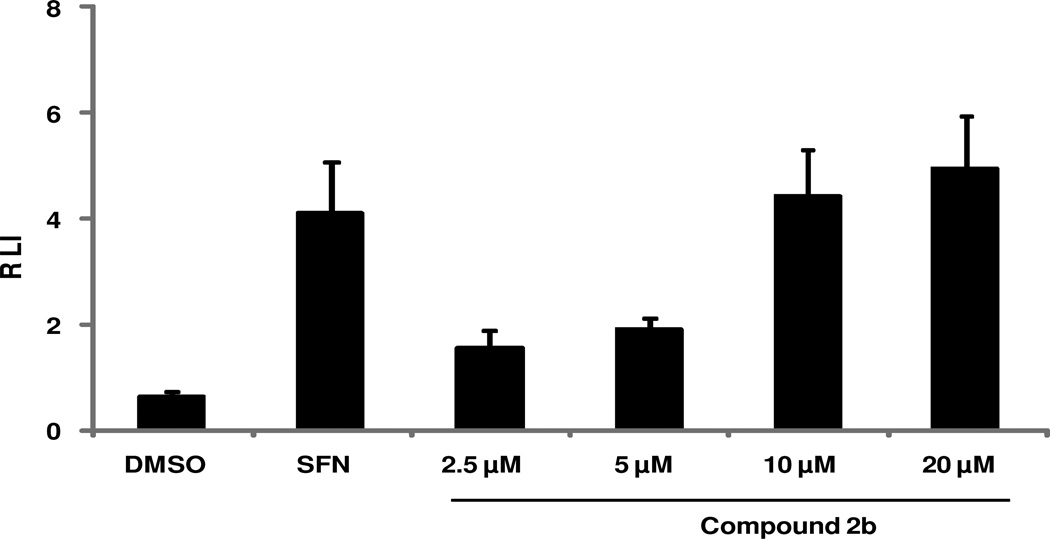
We further characterized Nrf2 induction by 2b using cell-based assays. Nrf2 increases the expression of NQO1 and GCLM by binding to the ARE present in the promoter region of these genes.79 We determined whether the ARE mediates the transcriptional regulation of NQO1 by 2b. We measured the expression of the luciferase gene under the control of NQO1-ARE sequence using stably transfected Beas-2B cells treated with 2b. The exposure to compound 2b resulted in a significant concentration-dependent increase in luciferase activity as measured by the chemiluminescence-based assay (Figure 3). These results implicate the ARE element in the induction of NQO1 gene by compound 2b. The transcriptional activation of antioxidant genes through an ARE is largely dependent upon Nrf2, suggesting that 2b upregulates antioxidant genes via Nrf2 activation.
Figure 3. Levels of NQO1-ARE luciferase activity after treatment with compound 2b.
NQO1-ARE luciferase activity was measured by using stably transfected Beas-2B cells after treatment with compound 2b or sulforaphane (SFN) or dimethyl sulfoxide (DMSO). The exposure to compound 2b resulted in a significant concentration-dependent increase in luciferase activity as relative luminescence intensity (RLI). Data are representative of 3 independent experiments. Values shown are mean ± SD of quadruplicate wells (P ≤ 0.05).
Concentration and time-course studies
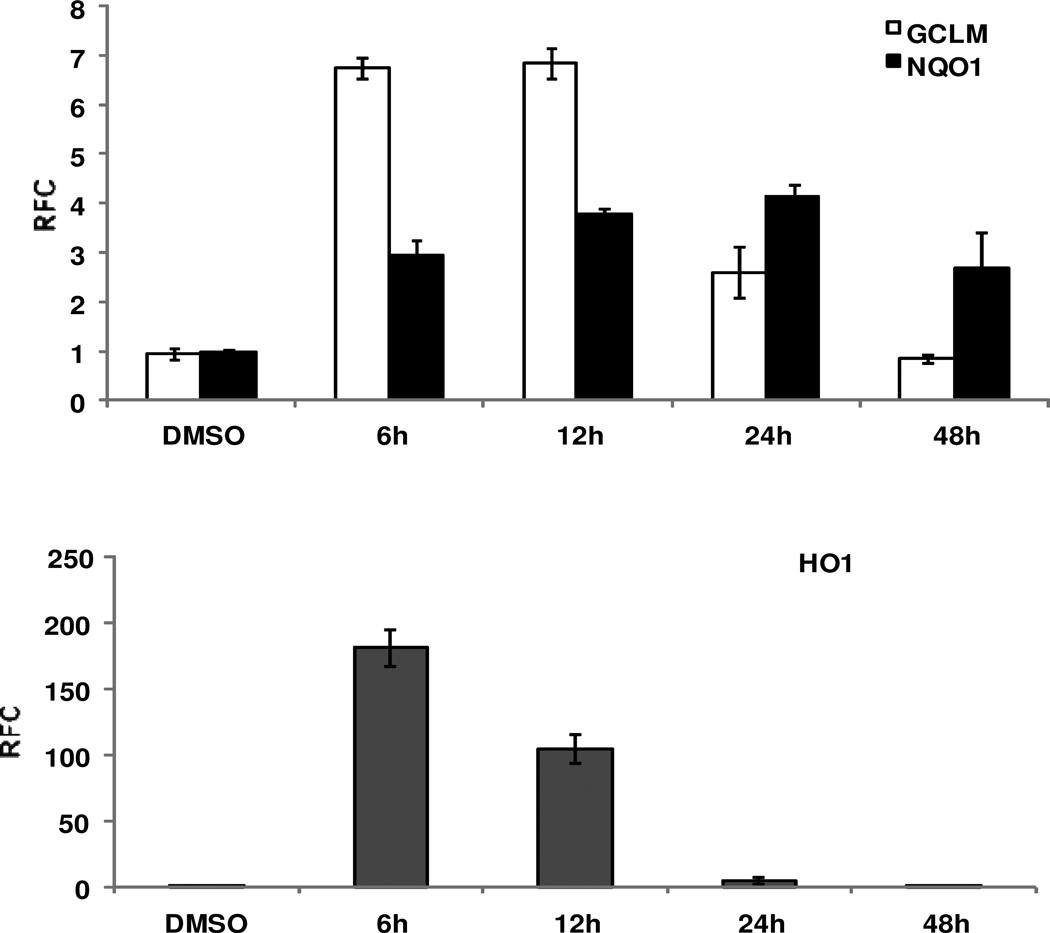
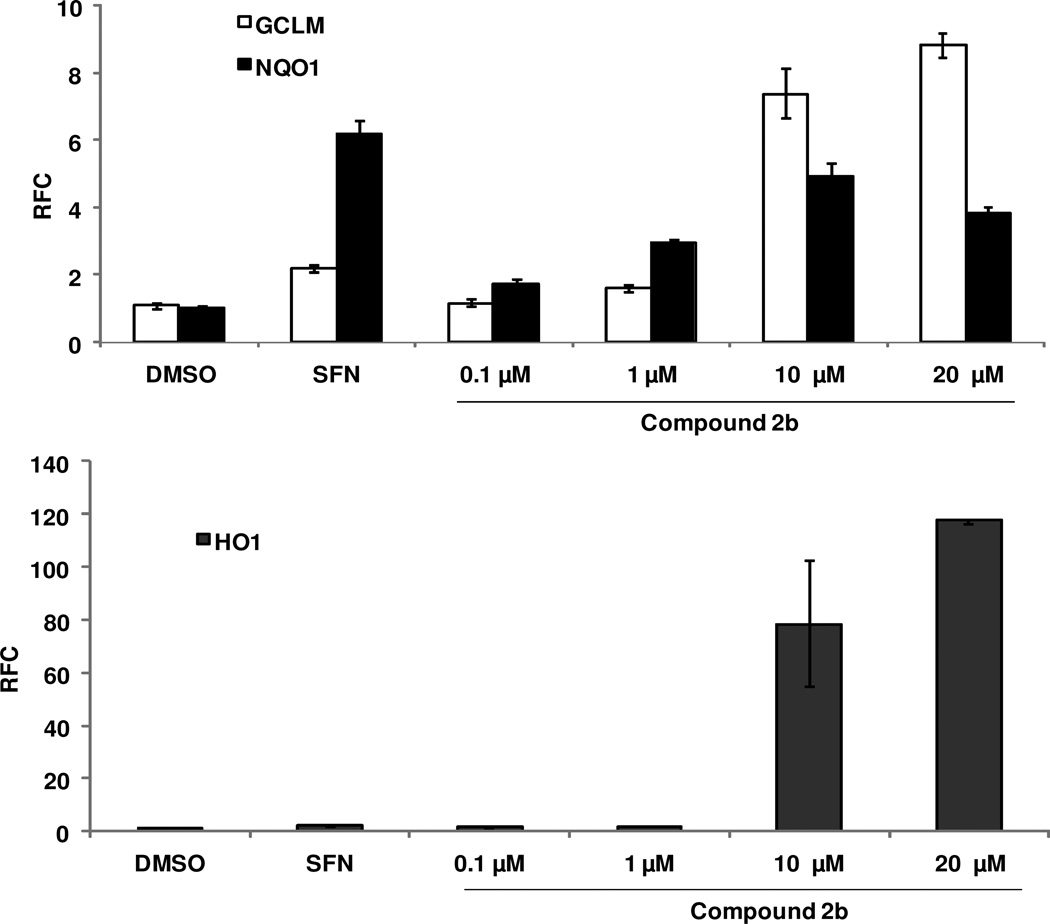
Next, we examined the concentration-dependent effect of 2b on the mRNA levels of Nrf2-driven antioxidant genes, GCLM, NQO1, and HO1. We measured the expression of these genes at 24 h after treatment with various concetrations (2.5, 5, 10, 20 µM) of 2b in Beas-2B cells. As shown in Figure 5, compound 2b significantly increased the Nrf2-regulated gene expression in a concentration-dependent manner. There was ~5- and 10-fold increase in the expression of GCLM and NQO1, respectively, at the highest concentration (20 µM) with no cytotoxicity. Interestingly, we found a dramatic concentration-dependent activation of Nrf2 genes. At 10 µM concentration of 2b, the expression of HO-1 was 6-fold higher compared to sulforaphane (Figure 4).
Figure 5. Time-dependent increase in Nrf2-regulated genes after treatment with compound 2b.
Human bronchial epithelial cells (Beas-2B) were treated with compound 2b (10 µM) at various time points. The expression of Nrf2-regulated genes GCLM, HO1, and NQO1 was analyzed in the tissues by qRT-PCR. β-actin was used for normalization. Data are representative of 3 independent experiments. Values shown are mean ± SD of triplicate wells (P ≤ 0.05).
Figure 4. Expression of Nrf2-regulated genes after treatment with compound 2b.
Human bronchial epithelial cells (Beas-2B) were treated with compound 2b at the indicated concentrations for 16–20 h. The expression of Nrf2-regulated genes GCLM, HO1, and NQO1 was analyzed in the tissues by qRT-PCR as a surrogate marker of Nrf2 activity. β-actin was used for normalization. Data are representative of 3 independent experiments. Values shown are mean ± SD of triplicate wells (P ≤ 0.05).
For the time-course studies, we measured the expression of antioxidant genes (GCLM, NQO1, and HO-1) at 6, 12, 24 and 48 h after treatment with 2b (10 µM) in Beas-2B cells. The time-course studies showed the highest induction of GCLM (~7-fold) and HO-1 (~150-fold) at 6 h after treatment with 2b (Figure 5). The expression of GCLM and HO-1 decreased at 6 h after treatment with 2b and was comparable to vehicle by 48 h. The expression of NQO1 was highest at 24 h and remained significantly elevated even at 48 h after treatment with compound 2b compared to vehicle. Taken together, these results suggest that 2b is a potent activator of Nrf2-regulated antioxidant defenses.
Activation of Nrf2 by compound 2b is independent of ROS generation
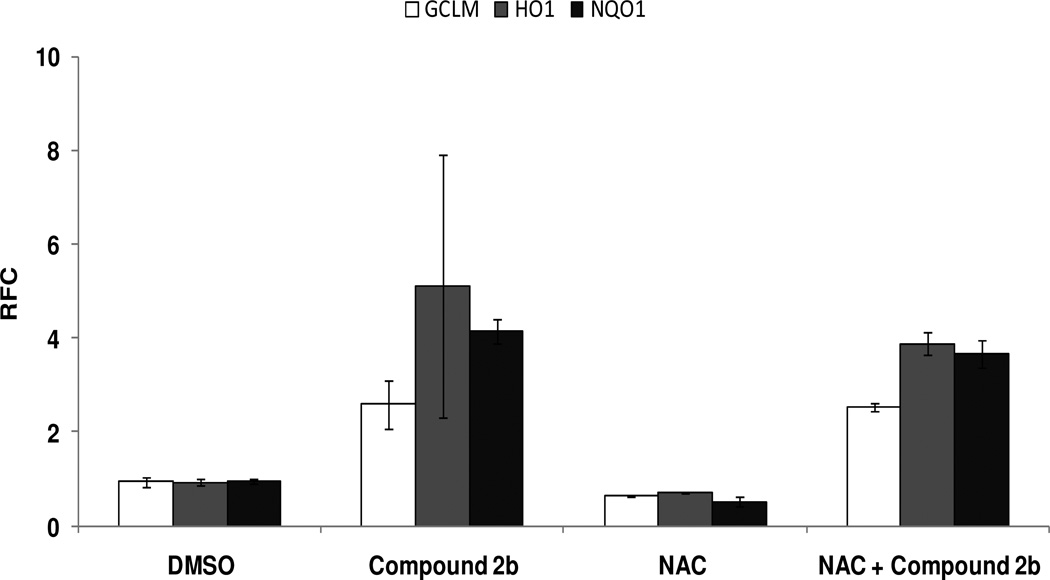
The activation of Nrf2 by various electrophiles and compounds that are Michael acceptor is attributed to changes in ROS production and or redox environment and or direct cysteine modification in Keap1.80, 81 We examined whether 2b activates Nrf2 by generating ROS or redox changes. Beas-2B cells were co-incubated with compound 2b and with or without N-acetyl-cysteine (NAC, 10 mM), and the expression of GCLM, NQO1, and HO1 was measured 24 h later. We found that 2b potentially increases the expression of Nr2-regulated antioxidant genes in the presence of NAC (Figure 6). NAC alone showed no induction of Nrf2-regulated genes. Taken together, these results suggest that the activation of Nrf2 by 2b is independent of ROS or redox changes. Further studies are required to determine whether compound 2b activates Nrf2 by direct thiol modification of Keap1.
Figure 6. Activation of Nrf2 genes by compound 2b is independent of ROS generation.
Human bronchial epithelial cells (Beas-2B) were treated with compound 2b (10 µM) in the presence of an antioxidant, N-acetyl cystiene (10 mM, NAC). Cells were harvested 24 h after the treatment, and the Nrf2-driven expression of NQO1, HO-1, and GCLM was quantified. β-actin was used for normalization. Data are representative of 3 independent experiments. Values shown are mean ± SD of triplicate wells (P ≤ 0.05).
CONCLUSIONS
In conclusion, we have identified a novel chalcone 2b as a potent activator of Nrf2 signaling pathway after screening a series of chalcone derivatives using cell-based and mouse models. Further studies are needed to evaluate and develop chalcone 2b as a potential drug for the treatment of inflammatory disorders.
EXPERIMENTAL SECTION
Chemistry
General methods
TLCs were run on pre-coated Merck silica gel 60F254 plates and observed under UV light. The products were isolated and purified by crystallization or using a Teledyne ISCO Rf Flash chromatography system with hexanes and ethyl acetate as eluents. The 1H (400 MHz), 13C (101 MHz), gCOSY, and gHSQC NMR spectra were taken on a Varian 400-MR spectrophotometer using TMS as an internal standard. Chemical shifts (δ) are expressed in ppm, coupling constants (J) are expressed in Hz, and splitting patterns are described as follows: s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet; dd = doublet of doublets; dt = doublet of triplets; td = triplet of doublets; ddd = doublet of doublet of doublets. For the verification of the product and purity analysis, the LC-MS was taken on an Agilent 1200 series system with an Agilent 6210 Time-Of-Flight (TOF) mass detector using Agilent Eclipse XDB-C-18 column (5 mm, 4.6 × 150 mm) using a flow rate of 0.9 mL/min and solvent system water (with 0.1% formic acid)/acetonitrile (ACN) (Gradient: 50% ACN @ 0 min, 80% ACN @ 7 min, 80% ACN @ 10 min and 50% ACN @ 15 min). All synthesized compounds were >95% pure, (exact purity is given in the subsequent sections with the characterization data). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and were used without further purification.
General procedure for synthesis of chalcones
In a 14-ml vial, the substituted acetophenone (1.25 mmol) and lithium hydroxide monohydrate (0.25 mmol) were dissolved in ethanol (5 ml) and the mixture was stirred at RT for 10 min followed by addition of substituted benzaldehyde (1.27 mmol). The reaction mixture was then stirred at RT and monitored by TLC using 25% ethyl acetate/hexanes as the solvent system. The reaction was quenched after 2 hrs by pouring into 50 ml of stirring ice cold water. If the product precipitated out after quenching with cold water, it was filtered off and crystallized with hot ethanol. In some examples, a sticky mass was observed in the aqueous solution after quenching. In those cases, the product was extracted by ethyl acetate (3 × 50 ml), dried over sodium sulfate, and concentrated under vacuum. The crude product was purified by flash chromatography using ethyl acetate/hexanes as the solvent system in increasing order of polarity.
(E)-2,3-diphenylprop-2-en-1-one (1a)
It was obtained as light yellow solid in 80% yield. 1H NMR (400 MHz, DMSO) δ 8.16 – 8.14 (m, 1H), 8.13 (t, J = 1.71, 1.71 Hz, 1H), 7.92 (d, J = 15.67 Hz, 1H), 7.89 – 7.85 (m, 2H), 7.74 (d, J = 15.68 Hz, 1H), 7.69 – 7.63 (m, 1H), 7.59 – 7.53 (m, 2H), 7.47 – 7.42 (m, 3H). 13C NMR (101 MHz, DMSO) δ 189.67, 144.48, 137.99, 135.08, 133.60, 131.09, 129.37, 129.34, 129.24, 128.96, 122.51. LC-MS (ESI-TOF): m/z 209.0963 ([C15H12O + H]+ calcd. 209.0961). Purity 100.00% (rt 7.39 min).
(E)-1-(2-methoxyphenyl)-3-phenylprop-2-en-1-one (1b)
It was obtained as yellow oil in 71% yield. 1H NMR (400 MHz, DMSO) δ 7.76 – 7.67 (m, 2H), 7.56 – 7.46 (m, 3H), 7.44 – 7.37 (m, 4H), 7.18 (d, J = 7.9 Hz, 1H), 7.05 (td, J = 7.5, 0.9 Hz, 1H), 3.85 (s, 3H). 13C NMR (101 MHz, DMSO) δ 192.60, 158.17, 142.97, 135.01, 133.53, 130.94, 129.98, 129.45, 129.23, 128.97, 127.41, 121.01, 112.79, 56.27. LC-MS (ESI-TOF): m/z 239.1072 ([C16H14O2 + H]+ calcd. 239.1067). Purity 98.02% (rt 7.21 min).
(E)-1-(3-methoxyphenyl)-3-phenylprop-2-en-1-one (1c)
It was obtained as yellow oil in 54% yield. 1H NMR (400 MHz, DMSO) δ 7.95 – 7.85 (m, 3H), 7.78 – 7.70 (m, 2H), 7.62 – 7.58 (m, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.47 – 7.42 (m, 3H), 7.23 (ddd, J = 8.2, 2.6, 0.8 Hz, 1H), 3.84 (s, 3H). 13C NMR (101 MHz, DMSO) δ 189.36, 159.99, 144.57, 139.42, 135.07, 131.12, 130.40, 129.42, 129.36, 122.51, 121.52, 119.69, 113.41, 55.83. LC-MS (ESI-TOF): m/z 239.1071 ([C16H14O2 + H]+ calcd. 239.1067). Purity 98.52% (rt 7.84 min).
(E)-1-(4-methoxyphenyl)-3-phenylprop-2-en-1-one (1d)
It was obtained as white solid in 76% yield. 1H NMR (400 MHz, DMSO) δ 8.16 (d, J = 9.0 Hz, 2H), 7.94 (d, J = 15.6 Hz, 1H), 7.90 – 7.83 (m, 2H), 7.69 (d, J = 15.6 Hz, 1H), 7.44 (dd, J = 5.1, 1.9 Hz, 3H), 7.07 (d, J = 9.0 Hz, 2H), 3.85 (s, 3H). 13C NMR (101 MHz, DMSO) δ 187.79, 163.68, 143.60, 135.24, 131.39, 130.88, 130.85, 129.33, 129.24, 122.43, 114.48, 56.03. LC-MS (ESI-TOF): m/z 239.1068 ([C16H14O2 + H]+ calcd. 239.1067). Purity 100.00% (rt 7.36 min).
(E)-3-phenyl-1-(2,4-dimethoxyphenyl)prop-2-en-1-one (1e)
It was obtained as yellow oil in 40% yield. 1H NMR (400 MHz, CDCl3) δ = 7.76 (d, J = 8.6 Hz, 1H), 7.68 (d, J = 15.8 Hz, 1H), 7.60 (dd, J = 7.3, 1.8 Hz, 2H), 7.52 (d, J = 15.8 Hz, 1H), 7.43 – 7.34 (m, 3H), 6.57 (dd, J = 8.6, 2.2 Hz, 1H), 6.50 (d, J = 2.1 Hz, 1H), 3.91 (s, 3H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 189.73, 164.44, 160.71, 141.62, 135.32, 132.51, 130.65, 129.43, 128.78, 127.52, 121.79, 106.46, 99.07, 56.42, 56.06. LC-MS (ESI-TOF): m/z 269.1171 ([C17H16O3 + H]+ calcd. 269.1172). Purity 96.00% (rt 7.25 min).
(E)-3-phenyl-1-(2,6-dimethoxyphenyl)prop-2-en-1-one (1f)
It was obtained as white solid in 60% yield. 1H NMR (400 MHz, DMSO) δ = 7.72 – 7.58 (m, 2H), 7.45 – 7.31 (m, 4H), 7.17 (d, J = 16.2 Hz, 1H), 6.97 (d, J = 16.2 Hz, 1H), 6.74 (d, J = 8.4 Hz, 2H), 3.70 (s, 6H). 13C NMR (101 MHz, DMSO) δ = 194.53, 157.29, 144.91, 134.61, 131.35, 131.11, 129.43, 129.04, 129.01, 118.25, 104.86, 56.24. LC-MS (ESI-TOF): m/z 269.1175 ([C17H16O3 + H]+ calcd. 269.1172). Purity 100.00% (rt 6.19 min).
(E)-3-phenyl-1-(2,5-dimethoxyphenyl)prop-2-en-1-one (1g)
It was obtained as yellow oil in 62% yield. 1H NMR (400 MHz, DMSO) δ = 7.76 – 7.67 (m, 2H), 7.50 (d, J = 16.0 Hz, 1H), 7.43 (d, J = 2.7 Hz, 3H), 7.40 (d, J = 12.2 Hz, 1H), 7.14 – 7.09 (m, 2H), 7.03 (dd, J = 2.6, 0.8 Hz, 1H), 3.80 (s, 3H), 3.73 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 192.17, 153.47, 152.36, 143.16, 135.00, 130.98, 129.67, 129.46, 128.99, 127.25, 119.02, 114.36, 114.33, 56.80, 56.00. LC-MS (ESI-TOF): m/z 269.1170 ([C17H16O3 + H]+ calcd. 269.1172). Purity 98.59% (rt 7.31 min).
(E)-3-phenyl-1-(3,4-dimethoxyphenyl)prop-2-en-1-one (1h)
It was obtained as yellow oil in 62% yield. 1H NMR (400 MHz, DMSO) δ 7.95 (d, J = 15.6 Hz, 1H), 7.93 – 7.85 (m, 3H), 7.70 (d, J = 15.6 Hz, 1H), 7.60 (d, J = 2.0 Hz, 1H), 7.44 (dd, J = 1.9, 5.1 Hz, 3H), 7.09 (d, J = 8.5 Hz, 1H), 3.86 (s, 3H), 3.84 (s, 3H). 13C NMR (101 MHz, DMSO) δ 187.77, 153.67, 149.23, 143.53, 135.25, 130.89, 130.87, 129.32, 129.27, 123.87, 122.36, 111.31, 111.11, 56.23, 56.02. LC-MS (ESI-TOF): m/z 269.1173 ([C17H16O3 + H]+ calcd. 269.1172). Purity 97.87% (rt 6.33 min).
(E)-3-phenyl-1-(3,5-dimethoxyphenyl)prop-2-en-1-one (1i)
It was obtained as yellow oil in 66% yield. 1H NMR (400 MHz, DMSO) δ 7.94 – 7.86 (m, 3H), 7.73 (d, J = 15.6 Hz, 1H), 7.44 (dd, J = 2.6, 3.8 Hz, 3H), 7.25 (d, J = 2.3 Hz, 2H), 6.78 (t, J = 2.3 Hz, 1H), 3.82 (s, 6H). 13C NMR (101 MHz, DMSO) δ 189.16, 161.14, 144.70, 140.05, 135.06, 131.12, 129.48, 129.33, 122.46, 106.71, 105.53, 56.01. LC-MS (ESI-TOF): m/z 269.1176 ([C17H16O3 + H]+ calcd. 269.1172). Purity 100.00% (rt 8.09 min).
(E)-3-phenyl-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (1j)
It was obtained as white solid in 68% yield. 1H NMR (400 MHz, DMSO) δ 7.99 – 7.85 (m, 3H), 7.73 (d, J = 15.5 Hz, 1H), 7.52 – 7.36 (m, 5H), 3.89 (s, 6H), 3.75 (s, 3H). 13C NMR (101 MHz, DMSO) δ 188.34, 153.37, 144.31, 142.44, 135.15, 133.39, 131.03, 129.42, 129.32, 122.35, 106.62, 60.64, 56.67. LC-MS (ESI-TOF): m/z 299.1284 ([C18H18O4 + H]+ calcd. 299.1278). Purity 100.00% (rt 6.97 min).
(E)-3-phenyl-1-(2,3,4-trimethoxyphenyl)prop-2-en-1-one (1k)
It was obtained as white solid in 66% yield. 1H NMR (400 MHz, DMSO) δ = 7.73 (dd, J = 6.8, 2.8 Hz, 2H), 7.54 (d, J = 15.9 Hz, 1H), 7.48 – 7.39 (m, 4H), 7.37 (d, J = 8.7 Hz, 1H), 6.93 (d, J = 8.8 Hz, 1H), 3.86 (s, 3H), 3.83 (s, 3H), 3.77 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 190.50, 157.16, 153.34, 142.86, 142.07, 135.08, 130.88, 129.47, 128.89, 126.98, 126.58, 125.60, 108.34, 62.16, 60.96, 56.54. LC-MS (ESI-TOF): m/z 299.1275 ([C18H18O4 + H]+ calcd. 299.1278). Purity 100.00% (rt 7.19 min).
(E)-3-phenyl-1-(2,4,6-trimethoxyphenyl)prop-2-en-1-one (1l)
It was obtained as yellow oil in 71% yield. 1H NMR (400 MHz, DMSO) δ = 7.64 (dd, J = 6.6, 3.1 Hz, 2H), 7.39 (dd, J = 5.1, 1.8 Hz, 3H), 7.19 (d, J = 16.1 Hz, 1H), 6.94 (d, J = 16.1 Hz, 1H), 6.30 (s, 2H), 3.82 (s, 3H), 3.70 (s, 6H). 13C NMR (101 MHz, DMSO) δ = 193.70, 162.41, 158.53, 143.97, 134.81, 130.90, 129.46, 129.40, 128.91, 111.45, 91.53, 56.26, 55.91. LC-MS (ESI-TOF): m/z 299.1276 ([C18H18O4 + H]+ calcd. 299.1278). Purity 100.00% (rt 6.23 min).
(E)-1-phenyl-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2a)
It was obtained as yellow solid in 64% yield. 1H NMR (400 MHz, DMSO) δ = 8.35 (d, J = 7.8 Hz, 1H), 8.25 – 8.15 (m, 2H), 8.05 (d, J = 15.3 Hz, 1H), 7.98 (dd, J = 15.5 Hz, 2.0 Hz, 1H), 7.89 – 7.76 (m, 2H), 7.76 – 7.65 (m, 2H), 7.61 (t, J = 7.7 Hz, 2H). 13C NMR (101 MHz, DMSO) δ = 189.33, 138.24 (d, J = 1.8 Hz), 137.47, 134.01, 133.42, 133.22 (d, J = 1.5 Hz), 130.99, 129.32, 129.26, 129.16, 127.96 (q, J = 29.2 Hz), 126.64, 126.62 (q, J = 6.0 Hz), 124.61 (q, J = 274.5 Hz). LC-MS (ESI-TOF): m/z 277.0833 ([C16H11F3O + H]+ calcd. 277.0835). Purity 100.00% (rt 6.97 min).
(E)-1-(2-methoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2b)
It was obtained as yellow oil in 72% yield. 1H NMR (400 MHz, CDCl3) δ = 7.90 – 7.82 (m, 1H), 7.71 (d, J = 7.8 Hz, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.55 (dd, J = 7.6 Hz, 1.8, 1H), 7.50 (t, J = 7.6 Hz, 1H), 7.45 – 7.36 (m, 2H), 7.22 (d, J = 15.7 Hz, 1H), 6.97 (td, J = 7.5 Hz, 0.8 Hz, 1H), 6.92 (d, J = 8.4 Hz, 1H), 3.82 (s, 4H). 13C NMR (101 MHz, DMSO) δ = 192.21, 158.40, 136.83 (d, J = 2.1 Hz), 134.05, 133.58, 133.29 (d, J = 1.6 Hz), 131.30, 130.82, 130.15, 128.79, 128.58, 127.81 (q, J = 29.2 Hz), 126.68 (q, J = 5.2 Hz), 124.55 (q, J = 274.5 Hz), 121.07, 112.76, 56.31. LC-MS (ESI-TOF): m/z 304.0940 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 96.40% (rt 8.69 min).
(E)-1-(3-methoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2c)
It was obtained as yellow solid in 26% yield. 1H NMR (400 MHz, DMSO) δ = 8.35 (d, J = 7.9 Hz, 1H), 8.06 – 7.94 (m, 2H), 7.88 – 7.76 (m, 3H), 7.72 – 7.63 (m, 2H), 7.52 (t, J = 7.9 Hz, 1H), 7.28 (ddd, J = 8.2 Hz, 2.7 Hz, 0.8 Hz, 1H), 3.87 (s, 3H). 1H NMR (400 MHz, CDCl3) δ = 8.13 (d, J = 15.6 Hz, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.60 (dd, J = 13.7 Hz, 7.1 Hz, 2H), 7.55 – 7.47 (m, 2H), 7.41 (dd, J = 15.9 Hz, 8.5 Hz, 2H), 7.15 (dd, J= 8.2 Hz, 1.9 Hz, 1H), 3.89 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 189.12, 160.04, 138.92, 138.33 (d, J = 2.2 Hz), 133.41, 133.21 (d, J = 1.7 Hz), 131.00, 130.47, 129.32, 127.96 (d, J = 29.2 Hz), 126.71, 126.62 (d, J = 6. 0 Hz), 124.62 (d, J = 273.5 Hz), 121.69, 120.04, 113.64, 55.86. LC-MS (ESI-TOF): m/z 304.0945 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 100.00% (rt 9.13 min).
(E)-1-(4-methoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2d)
It was obtained as yellow solid in 37% yield. 1H NMR (400 MHz, DMSO) δ = 8.34 (d, J = 7.9 Hz, 1H), 8.24 – 8.17 (m, 2H), 8.04 (d, J = 15.3 Hz, 1H), 7.95 (dd, J = 15.4 Hz, 2.2, 1H), 7.87 – 7.76 (m, 2H), 7.67 (t, J = 7.6 Hz, 1H), 7.16 – 7.07 (m, 2H), 3.89 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 187.42, 164.00, 137.46 (d, J = 2.2 Hz), 133.45 (d, J = 1.6 Hz), 133.38, 131.63, 130.78, 130.40, 129.21, 127.87 (d, J = 29.2 Hz), 126.67, 126.58 (d, J = 6.0 Hz), 124.64 (d, J = 274.5 Hz), 114.58, 56.08. LC-MS (ESI-TOF): m/z 304.0944 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 100.00% (rt 8.75 min).
(E)-1-(2,4-dimethoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2e)
It was obtained as yellow solid in 50% yield. 1H NMR (400 MHz, DMSO) δ = 8.07 (d, J = 7.6 Hz, 1H), 7.79 (dt, J = 14.8 Hz, 7.9 Hz, 3H), 7.69 – 7.60 (m, 3H), 6.71 (d, J = 2.3 Hz, 1H), 6.67 (dd, J = 8.6 Hz, 2.3 Hz, 1H), 3.92 (s, 3H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 189.18, 164.88, 161.01, 135.47 (d, J = 2.0 Hz), 133.64 (d, J = 1.3 Hz), 133.59, 132.74, 131.55, 130.55, 128.69, 127.74 (d, J = 29.2 Hz), 126.64 (d, J = 6.0 Hz), 124.62 (d, J = 274.5 Hz). 121.23, 106.69, 99.04, 56.50, 56.13. LC-MS (ESI-TOF): m/z 337.1050 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 100.00% (rt 8.71 min).
(E)-1-(2,6-dimethoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2f)
It was obtained as white solid in 67% yield. 1H NMR (400 MHz, DMSO) δ 8.07 (s, 1H), 8.01 (d, J = 7.9 Hz, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.61 (t, J = 7.8 Hz, 1H), 7.38 (t, J = 8.4 Hz, 1H), 7.30 (d, J = 16.3 Hz, 1H), 7.14 (d, J = 16.2 Hz, 1H), 6.74 (d, J = 8.5 Hz, 2H), 3.70 (s, 6H). 13C NMR (101 MHz, DMSO) δ 194.45, 157.36, 142.84, 135.92, 132.43, 131.45, 130.69, 130.41, 130.26 (q, J = 31.1 Hz), 127.17 (q, J = 3.6 Hz), 125.92 (q, J = 3.8 Hz), 124.38 (q, J = 272.5 Hz), 118.22, 104.90, 56.27. LC-MS (ESI-TOF): m/z 337.1045 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 100.00% (rt 7.68 min).
(E)-1-(2,5-dimethoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2g)
It was obtained as yellow solid in 80% yield. 1H NMR (400 MHz, DMSO) δ = 8.09 (d, J = 7.9 Hz, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.80 – 7.72 (m, 2H), 7.65 (t, J = 7.6 Hz, 1H), 7.51 (d, J = 15.7 Hz, 1H), 7.16 (d, J = 1.8 Hz, 2H), 7.09 (t, J = 1.8 Hz, 1H), 3.83 (s, 3H), 3.76 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 191.72, 153.50, 152.64, 136.95 (d, J = 2.1 Hz), 133.59, 133.28 (d, J = 1.7 Hz), 131.14, 130.86, 128.96, 128.79, 127.82 (q, J = 29.2 Hz), 126.69 (q, J = 16.6 Hz), 124.56 (d, J = 273.5 Hz), 119.72, 114.41, 114.33, 56.80, 56.04. LC-MS (ESI-TOF): m/z 337.1049 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 100.00% (rt 8.76 min).
(E)-1-(3,4-dimethoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2h)
It was obtained as yellow solid in 72% yield. 1H NMR (400 MHz, DMSO) δ = 8.33 (d, J = 7.8 Hz, 1H), 8.04 (d, J =15.3 Hz, 1H), 7.96 (dd, J = 6.2 Hz, 2.1 Hz, 1H), 7.94 (d, J= 2.0 Hz, 1H), 7.84 (d, J = 7.8 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.67 (t, J = 7.7 Hz, 1H), 7.63 (d, J = 2.0 Hz, 1H), 7.13 (d, J = 8.5 Hz, 1H), 3.89 (s, 3H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 187.44, 154.02, 149.31, 137.42 (d, J = 2.0 Hz), 133.49 (d, J = 1.0 Hz), 133.37, 130.76, 130.44, 129.26, 127.85 (q, J = 29.2 Hz), 126.66, 126.58 (q, J = 5.2 Hz), 124.65 (q, J = 273.5 Hz), 124.22, 111.40, 111.28, 56.28, 56.07. LC-MS (ESI-TOF): m/z 337.1048 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 100.00% (rt 7.77 min).
(E)-1-(3,5-dimethoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2i)
It was obtained as yellow oil in 72% yield. 1H NMR (400 MHz, DMSO) δ = 8.36 (d, J = 7.9 Hz, 1H), 7.98 (s, 2H), 7.85 (d, J = 7.8 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.68 (t, J = 7.6 Hz, 1H), 7.30 (d, J = 2.2, Hz 2H), 6.83 (t, J = 2.2 Hz, 1H), 3.85 (s, 6H). 1H NMR (400 MHz, CDCl3) δ = 8.12 (d, J = 13.9 Hz, 1H), 7.82 (d, J = 7.8 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.61 (t, J = 7.6 Hz, 1H), 7.51 (t, J = 7.7 Hz, 1H), 7.35 (d, J = 15.6 Hz, 1H), 7.14 (d, J = 2.2 Hz, 2H), 6.69 (t, J = 2.1 Hz, 1H), 3.86 (s, 7H). 13C NMR (101 MHz, DMSO) δ = 188.97, 161.20, 139.53, 138.45 (d, J = 2.2 Hz), 133.39, 133.18 (d, J = 1.6 Hz), 131.00, 129.40, 127.96 (q, J = 29.2 Hz), 126.67, 129.60 (q, J = 5.2 Hz), 124.62 (q, J = 273.5 Hz), 106.93, 105.88, 56.05. LC-MS (ESI-TOF): m/z 337.1047 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 100.00% (rt 9.37 min).
(E)-1-(3,4,5-trimethoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2j)
It was obtained as yellow solid in 80% yield. 1H NMR (400 MHz, DMSO) δ = 8.31 (d, J = 7.8 Hz, 1H), 8.02 (d, J = 15.2 Hz, 1H), 7.95 (dd, J = 15.4 Hz, 2.1 Hz, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.78 (d, J = 7.7 Hz, 1H), 7.66 (t, J = 7.6 Hz, 1H), 7.44 (s, 2H), 3.88 (s, 6H), 3.76 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 188.07, 153.41, 142.77, 138.14 (d, J=2.0), 133.37, 132.86, 130.91, 129.40, 127.77 (q, J = 29.2 Hz), 126.61, 126.61 (q, J = 5.0 Hz), 124.63 (q, J = 274.5 Hz), 106.86, 60.66, 56.68. LC-MS (ESI-TOF): m/z 367.1160 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 100.00% (rt 8.38 min).
(E)-1-(2,3,4-trimethoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2k)
It was obtained as yellow solid in 58% yield. 1H NMR (400 MHz, DMSO) δ = 8.10 (d, J = 7.7 Hz, 1H), 7.80 (dt, J = 22.4, 7.7 Hz, 3H), 7.66 (t, J = 7.6 Hz, 1H), 7.56 (d, J = 15.5 Hz, 1H), 7.46 (d, J = 8.8 Hz, 1H), 6.97 (d, J = 8.9 Hz, 1H), 3.89 (s, 3H), 3.86 (s, 3H), 3.80 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 189.74, 157.66, 153.66, 142.08, 136.44 (d, J = 2.1 Hz), 133.60, 133.46 (d, J = 2.0 Hz), 131.02, 130.73, 128.71, 127.78 (d, J = 29.2 Hz), 126.66 (d, J = 5.0 Hz), 125.95 (d, J = 7.0 Hz), 124.59 (d, J = 274.5 Hz), 108.48, 62.21, 60.98, 56.60. LC-MS (ESI-TOF): m/z 367.1152 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 96.17% (rt 8.70 min).
(E)-1-(2,4,6-trimethoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one (2l)
It was obtained as yellow solid in 72% yield. 1H NMR (400 MHz, DMSO) δ = 8.04 (d, J = 7.8 Hz, 1H), 7.77 (d, J = 7.7 Hz, 1H), 7.71 (t, J = 7.5 Hz, 1H), 7.60 (t, J = 7.6 Hz, 1H), 7.48 (dd, J = 15.9, 2.2 Hz, 1H), 7.01 (d, J = 15.8 Hz, 1H), 6.30 (s, 2H), 3.82 (s, 3H), 3.70 (s, 6H). 13C NMR (101 MHz, DMSO) δ = 193.37, 162.78, 162.78, 158.71, 138.33 (d, J = 2.2 Hz), 133.51, 133.07, 130.80, 128.83, 127.58 (q, J = 29.2 Hz), 126.61 (q, J = 6.0 Hz), 124.46 (q, J = 274.5 Hz), 110.73, 91.34, 56.23, 55.96. LC-MS (ESI-TOF): m/z 367.1157 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 96.17% (rt 7.58 min).
(E)-1-phenyl-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3a)
It was obtained as white solid in 68% yield. 1H NMR (400 MHz, DMSO) δ 8.36 (s, 1H), 8.26 – 8.18 (m, 3H), 8.15 (d, J = 15.7 Hz, 1H), 7.89 – 7.78 (m, 2H), 7.75 – 7.67 (m, 2H), 7.64 – 7.57 (m, 2H). 13C NMR (101 MHz, DMSO) δ 189.51, 142.61, 137.72, 136.28, 133.83, 133.36, 130.38, 130.26 (q, J = 28.1 Hz), 127.14 (q, J = 3.8 Hz), 125.62 (q, J = 3.7 Hz), 124.15 (q, J = 272.5 Hz), 124.38. LC-MS (ESI-TOF): m/z 277.0840 ([C16H11F3O + H]+ calcd. 277.0835). Purity 100.00% (rt 9.20 min).
(E)-1-(2-methoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3b)
It was obtained as yellow oil in 45% yield. 1H NMR (400 MHz, DMSO) δ 8.57 (t, J = 1.9 Hz, 1H), 8.29 – 8.19 (m, 2H), 7.73 (t, J = 8.0 Hz, 1H), 7.69 – 7.61 (m, 2H), 7.61 – 7.57 (m, 1H), 7.57 – 7.52 (m, 1H), 7.22 (d, J = 7.9 Hz, 1H), 7.08 (td, J = 7.5, 0.9 Hz, 1H), 3.89 (s, 3H). LC-MS (ESI-TOF): m/z 304.0941 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 96.00% (rt 8.88 min).
(E)-1-(3-methoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3c)
It was obtained as white solid in 21% yield. 1H NMR (400 MHz, DMSO) δ 8.35 (s, 1H), 8.21 (d, J = 7.8 Hz, 1H), 8.11 (d, J = 15.7 Hz, 1H), 7.87 – 7.77 (m, 3H), 7.70 (t, J = 7.78 Hz, 1H), 7.66 (dd, J = 1.6, 2.5 Hz, 1H), 7.52 (t, J = 7.94 Hz, 1H), 7.27 (ddd, J = 0.8, 2.6, 8.2 Hz, 1H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO) δ 189.28, 160.03, 142.68, 139.18, 136.27, 133.32, 130.41, 130.36, 130.25 (q, J = 31.2 Hz), 127.15 (q, J = 3.7 Hz), 125.72 (q, J = 3.7 Hz), 124.50 (q, J = 272.5 Hz), 124.46, 121.70, 119.76, 113.68, 55.85. LC-MS (ESI-TOF): m/z 304.0945 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 100.00% (rt 9.35 min).
(E)-1-(4-methoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3d)
It was obtained as white solid in 59% yield. 1H NMR (400 MHz, DMSO) δ 8.33 (s, 1H), 8.24 – 8.20 (m, 2H), 8.18 (d, J = 7.8 Hz, 1H), 8.13 (d, J = 15.7 Hz, 1H), 7.82 – 7.74 (m, 2H), 7.69 (t, J = 7.8 Hz, 1H), 7.14 – 7.07 (m, 2H), 3.88 (s, 3H). 13C NMR (101 MHz, DMSO) δ 187.69, 163.85, 141.74, 136.45, 133.23, 131.57, 130.67, 130.34, 130.24 (q, J = 32.7 Hz), 126.93 (q, J = 3.8 Hz), 125.49 (q, J = 3.7 Hz), 124.52 (q, J = 272.5 Hz), 124.45, 114.49, 56.05. LC-MS (ESI-TOF): m/z 304.0951 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 100.00% (rt 8.99 min).
(E)-1-(2,4-dimethoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3e)
It was obtained as white solid in 52% yield. 1H NMR (400 MHz, DMSO) δ 8.11 – 8.03 (m, 2H), 7.77 (d, J = 7.8 Hz, 1H), 7.72 – 7.58 (m, 4H), 6.70 (d, J = 2.3 Hz, 1H), 6.66 (dd, J = 2.3, 8.6 Hz, 1H), 3.90 (s, 3H), 3.86 (s, 3H). 13C NMR (101 MHz, d2o) δ 189.57, 164.52, 160.77, 139.58, 136.52, 132.49, 131.97, 130.39, 130.18 (q, J = 31.2 Hz), 129.38, 126.69 (q, J = 3.7 Hz), 125.52 (q, J = 3.8 Hz, H), 124.37 (q, J = 272.5 Hz), 121.55, 106.44, 99.03, 56.38, 56.02. LC-MS (ESI-TOF): m/z 337.1044 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 100.00% (rt 8.93 min).
(E)-1-(2,6-dimethoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3f)
It was obtained as light yellow solid in 72% yield. 1H NMR (400 MHz, DMSO) δ = 8.07 (d, J = 7.8 Hz, 1H), 7.76 (d, J = 7.8, 1H), 7.71 (dd, J = 11.4 Hz, 4.0 Hz, 1H), 7.61 (t, J = 7.6 Hz, 1H), 7.45 (d, J = 2.1 Hz, 1H), 7.40 (dd, J = 11.1 Hz, 5.7 Hz, 1H), 7.02 (d, J = 15.9 Hz, 1H), 6.75 (d, J = 8.4 Hz, 2H), 3.70 (s, 6H). 13C NMR (101 MHz, DMSO) δ = 194.52, 157.33, 139.62 (d, J = 2.2 Hz), 133.52, 132.81 (d, J = 1.6 Hz), 132.57, 131.77, 131.02, 128.90, 127.59 (q, J = 30.2 Hz), 126.64 (q, J = 5.7 Hz), 124.38 (q, J = 274.5 Hz), 117.44, 104.69, 56.22. LC-MS (ESI-TOF): m/z 337.1050 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 98.65% (rt 7.83 min).
(E)-1-(2,5-dimethoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3g)
It was obtained as yellow oil in 71% yield. 1H NMR (400 MHz, DMSO) δ 8.12 (s, 1H), 8.08 (d, J = 7.8 Hz, 1H), 7.79 (d, J = 7.8 Hz, 1H), 7.67 (t, J = 7.8 Hz, 1H), 7.62 (d, J = 16.1 Hz, 1H), 7.55 (dd, J = 0.9, 16.0 Hz, 1H), 7.18 – 7.12 (m, 2H), 7.09 – 7.03 (m, 1H), 3.82 (d, J = 0.8 Hz, 3H), 3.76 (d, J = 0.9 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 192.15, 153.49, 152.47, 141.17, 136.27, 132.29, 130.48, 130.27 (q, J = 31.2 Hz), 129.50, 129.14, 127.06 (q, J = 3.7 Hz), 125.83 (q, J = 3.3 Hz), 124.42 (d, J = 272.5 Hz), 119.20, 114.42, 114.37, 56.84, 56.04. LC-MS (ESI-TOF): m/z 337.1048 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 96.60% (rt 8.96 min).
(E)-1-(3,4-dimethoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3h)
It was obtained as white solid in 64% yield. 1H NMR (400 MHz, d2o) δ 8.11 (dd, J = 11.7, 8.7 Hz, 3H), 7.98 – 7.91 (m, 1H), 7.79 (dd, J = 19.6, 12.0 Hz, 3H), 7.63 (d, J = 1.9 Hz, 1H), 7.13 (d, J = 8.5 Hz, 1H), 3.89 (s, 3H), 3.87 (s, 3H). 13C NMR (101 MHz, d2o) δ 187.59, 153.86, 149.24, 141.43, 139.26 (d, J = 1.3 Hz), 130.59, 130.23 (d, J = = 31.2 Hz), 129.74, 126.02 (q, J = 3.7 Hz), 125.06, 124.45 (d, J = 272.5 Hz), 124.08, 111.29, 111.13, 56.21, 56.00. LC-MS (ESI-TOF): m/z 337.1041 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 98.47% (rt 8.03 min).
(E)-1-(3,5-dimethoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3i)
It was obtained as light yellow solid in 72% yield. 1H NMR (400 MHz, DMSO) δ 8.34 (s, 1H), 8.22 (d, J = 7.8 Hz, 1H), 8.08 (d, J = 15.7 Hz, 1H), 7.88 – 7.77 (m, 2H), 7.70 (t, J = 7.8 Hz, 1H), 7.32 (d, J = 2.3 Hz, 2H), 6.83 (t, J = 2.2 Hz, 1H), 3.85 (s, 6H). 13C NMR (101 MHz, DMSO) δ 189.08, 161.18, 142.83, 139.77, 136.24, 133.33, 130.33, 130.24 (q, J = 32.2 Hz), 127.17 (q, J = 3.6 Hz), 125.85 (q, J = 3.7 Hz), 124.50 (q, J = 273.5 Hz), 124.36, 106.98, 105.52, 56.03. LC-MS (ESI-TOF): m/z 337.1049 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 100.00% (rt 9.55 min).
(E)-1-(3,4,5-trimethoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3j)
It was obtained as white solid in 60% yield. 1H NMR (400 MHz, DMSO) δ 8.28 – 8.19 (m, 2H), 8.06 (d, J = 15.7 Hz, 1H), 7.86 – 7.75 (m, 2H), 7.69 (t, J = 7.8 Hz, 1H), 7.44 (s, 2H), 3.89 (s, 6H), 3.76 (s, 3H). 13C NMR (101 MHz, DMSO) δ 188.34, 153.39, 142.69, 142.48, 136.33, 133.16, 132.95, 130.33, 130.24 (q, J = 32.2 Hz), 127.13 (q, J = 3.7 Hz), 126.02 (q, J = 3.7 Hz), 124.49 (q, J = 272.5 Hz), 124.34, 106.90, 60.66, 56.75. LC-MS (ESI-TOF): m/z 367.1155 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 96.17% (rt 8.61 min).
(E)-1-(2,3,4-trimethoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3k)
It was obtained as yellow oil in 46% yield. 1H NMR (400 MHz, DMSO) δ 8.13 (s, 1H), 8.09 (d, J = 7.8 Hz, 1H), 7.78 (d, J = 7.7 Hz, 1H), 7.71 – 7.56 (m, 3H), 7.42 (d, J = 8.7 Hz, 1H), 6.96 (d, J = 8.9 Hz, 1H), 3.89 (s, 3H), 3.85 (s, 3H), 3.80 (s, 3H). 13C NMR (101 MHz, DMSO) δ 190.46, 157.30, 153.41, 142.11, 140.87, 136.36, 132.23, 130.49, 130.27 (q, J = 31.2 Hz), 128.92, 126.96 (q, J = 3.7 Hz), 126.42, 125.69, 125.66 (q, J = 5.0 Hz), 124.43 (q, J = 272.5 Hz), 108.33, 62.14, 60.97, 56.57. LC-MS (ESI-TOF): m/z 367.1157 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 97.99% (rt 8.83 min).
(E)-1-(2,4,6-trimethoxyphenyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-one (3l)
It was obtained as light yellow solid in 69% yield. 1H NMR (400 MHz, DMSO) δ 8.05 (s, 1H), 8.00 (d, J = 7.8 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.61 (t, J = 7.8 Hz, 1H), 7.32 (d, J = 16.2 Hz, 1H), 7.11 (d, J = 16.2 Hz, 1H), 6.30 (s, 2H), 3.82 (s, 3H), 3.70 (s, 6H). 13C NMR (101 MHz, DMSO) δ 193.58, 162.54, 158.63, 141.94, 136.11, 132.29, 130.39, 130.24 (q, J = 30.2 Hz), 127.00 (d, J = 4.0 Hz), 125.81 (d, J = 4.0 Hz), 124.40 (q, J = 272.5 Hz), 111.35, 91.55, 56.27, 55.93. LC-MS (ESI-TOF): m/z 367.1154 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 100.00% (rt 7.86 min).
(E)-1-phenyl-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4a)
It was obtained as white solid in 59% yield. 1H NMR (400 MHz, DMSO) δ 8.20 – 8.11 (m, 3H), 8.08 (d, J = 15.7 Hz, 2H), 7.83 – 7.75 (m, 3H), 7.72 – 7.65 (m, 1H), 7.62 – 7.54 (m, 2H). 13C NMR (101 MHz, DMSO) δ 189.53, 142.43, 139.13 (d, J = 1.3 Hz), 137.68, 133.87, 130.49 (q, J = 32.2 Hz), 129.91, 129.30, 129.11, 126.14 (q, J = 3.8 Hz), 125.13, 124.44 (q, J = 272.5 Hz). LC-MS (ESI-TOF): m/z 277.0834 ([C16H11F3O + H]+ calcd. 277.0835). Purity 100.00% (rt 9.35 min).
(E)-1-(2-methoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4b)
It was obtained as yellow oil in 79% yield. 1H NMR (400 MHz, DMSO) δ 7.97 (d, J = 8.1 Hz, 2H), 7.79 (d, J = 8.2 Hz, 2H), 7.72 (d, J = 8.2 Hz, 1H), 7.61 – 7.48 (m, 6H), 7.22 (d, J = 8.0 Hz, 1H), 7.08 (td, J = 7.5, 0.9 Hz, 1H), 3.88 (s, 3H), 3.86 (s, 2H). LC-MS (ESI-TOF): m/z 304.0948 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 94.00% (rt 9.09 min).
(E)-1-(3-methoxyphenyl)-4-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4c)
It was obtained as white solid in 61% yield. 1H NMR (400 MHz, DMSO) δ 8.14 (d, J = 8.1 Hz, 2H), 8.07 (d, J = 15.7 Hz, 1H), 7.85 – 7.80 (m, 3H), 7.79 (d, J = 4.3 Hz, 1H), 7.67 – 7.62 (m, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.27 (dd, J = 8.2, 2.6 Hz, 1H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO) δ 189.29, 160.04, 142.49, 139.13, 13.50 (d, J = 31.2 Hz), 130.45, 129.95, 126.12 (q, J = 3.7 Hz), 124.5 (d, J = 272.46 Hz), 125.20, 121.65, 119.90, 113.58, 55.86. LC-MS (ESI-TOF): m/z 304.0943 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 100.00% (rt 9.52 min).
(E)-1-(4-methoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4d)
It was obtained as white solid in 56% yield. 1H NMR (400 MHz, DMSO) δ 8.23 – 8.01 (m, 5H), 7.76 (dd, J = 21.4, 11.6 Hz, 3H), 7.08 (d, J = 8.2 Hz, 2H), 3.86 (s, 3H). 13C NMR (101 MHz, DMSO) δ 187.70, 163.89, 141.58, 139.30, 131.55, 130.60, 130.32 (d, J = 32.2 Hz), 129.78, 126.11 (dd, J = 3.8 Hz), 125.18, 124.5 (d, J = 272.5 Hz), 114.55, 56.06. LC-MS (ESI-TOF): m/z 304.0940 ([C17H13 F3O2 + H]+ calcd. 307.0940). Purity 100.00% (rt 9.13 min).
(E)-1-(2,4-dimethoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4e)
It was obtained as off white solid in 40% yield. 1H NMR (400 MHz, DMSO) δ 7.92 (d, J = 7.4 Hz, 2H), 7.76 (d, J = 7.5 Hz, 2H), 7.70 – 7.50 (m, 3H), 6.74 – 6.57 (m, 2H), 3.89 (s, 3H), 3.84 (s, 3H). 13C NMR (101 MHz, DMSO) δ 189.45, 164.74, 160.94, 139.41, 132.65, 130.12) q, J = 32.2 Hz), 130.10, 129.33, 126.21 (dd, J = 3.5 Hz), 124.50 (d, J = 272.5 Hz), 121.48, 106.62, 99.07, 56.48, 56.10. LC-MS (ESI-TOF): m/z 337.1046 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 96.35% (rt 9.13 min).
(E)-1-(2,6-dimethoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4f)
It was obtained as light yellow solid in 64% yield. 1H NMR (400 MHz, DMSO) δ 7.92 (d, J = 8.3 Hz, 2H), 7.75 (d, J = 8.2 Hz, 2H), 7.41 (t, J = 8.4 Hz, 1H), 7.29 (d, J = 16.2 Hz, 1H), 7.13 (d, J = 16.2 Hz, 1H), 6.77 (d, J = 8.5 Hz, 2H), 3.73 (s, 6H). 13C NMR (101 MHz, DMSO) δ 194.25, 157.40, 142.53, 138.76 (d, J = 1.4 Hz), 131.59, 131.29, 130.55 (q, J = 31.2 Hz), 126.15 (q, J = 3.8 Hz), 124.42 (q, J = 272.5 Hz), 118.12, 104.93, 56.29. LC-MS (ESI-TOF): m/z 337.1047 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 100.00% (rt 7.97 min).
(E)-1-(2,5-dimethoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4g)
It was obtained as yellow oil in 45% yield. 1H NMR (400 MHz, DMSO) δ 7.95 (d, J = 8.1 Hz, 2H), 7.77 (d, J = 8.2 Hz, 2H), 7.59 (d, J = 16.0 Hz, 1H), 7.54 (d, J = 16.0 Hz, 1H), 7.17 – 7.12 (m, 2H), 7.08 – 7.04 (m, 1H), 3.82 (s, 3H), 3.74 (s, 3H). 1H NMR (400 MHz, CDCl3) δ 7.67 (dd, J = 17.3, 8.7 Hz, 5H), 7.51 (d, J = 15.9 Hz, 1H), 7.23 (d, J = 3.1 Hz, 1H), 7.06 (dd, J = 9.0, 3.2 Hz, 1H), 6.96 (d, J = 9.0 Hz, 1H), 3.88 (s, 3H), 3.82 (s, 3H). 13C NMR (101 MHz, DMSO) δ 191.85, 153.50, 152.62, 140.78, 139.13 (d, J = 1.4 Hz), 130.37 (d, J = 32.2 Hz), 129.72, 129.54, 129.28, 126.22 (q, J = 3.7 Hz), 124.46 (d, J = 272.5 Hz), 119.51, 114.44, 114.37, 56.83, 56.01. LC-MS (ESI-TOF): m/z 337.1048 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 96.42% (rt 9.16 min).
(E)-1-(3,4-dimethoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4h)
It was obtained as white solid in 64% yield. 1H NMR (400 MHz, DMSO) δ 8.31 (s, 1H), 8.20 (d, J = 7.8 Hz, 1H), 8.12 (d, J = 15.6 Hz, 1H), 7.99 (dd, J = 2.0, 8.5 Hz, 1H), 7.83 – 7.75 (m, 2H), 7.70 (t, J = 7.8 Hz, 1H), 7.62 (d, J = 2.0 Hz, 1H), 7.12 (d, J = 8.5 Hz, 1H), 3.89 (s, 3H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO) δ 187.70, 153.90, 149.31, 141.68, 136.46, 133.10, 130.72, 130.33, 130.24 (d, J = 32.2 Hz), 126.93 (q, J = 3.6 Hz), 125.64 (q, J = 3.6 Hz), 124.52 (d, J = 272.4 Hz), 124.39, 124.24, 111.30, 111.21, 56.27, 56.09. LC-MS (ESI-TOF): m/z 337.1042 ([C18H15 F3O3 + H]+ calcd. 337.1046). Purity 95.94% (rt 8.19 min).
(E)-1-(3,4,5-trimethoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4i)
It was obtained as white solid in 77% yield. 1H NMR (400 MHz, DMSO) δ 8.11 (d, J = 8.2 Hz, 2H), 8.06 (d, J = 15.6 Hz, 1H), 7.83 – 7.74 (m, 3H), 7.43 (s, 2H), 3.89 (s, 6H), 3.76 (s, 3H). 13C NMR (101 MHz, DMSO) δ 188.24, 153.40, 142.69, 142.27, 139.19 (d, J = 1.3 Hz), 133.08, 130.44 (q, J = 31.2 Hz), 129.95, 126.08 (q, J = 3.7 Hz), 125.04, 124.50 (q, J = 271.4 Hz), 106.80, 60.65, 56.70. LC-MS (ESI-TOF): m/z 367.1158 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 96.17% (rt 8.78 min).
(E)-1-(2,3,4-methoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4j)
It was obtained as white solid in 63% yield. 1H NMR (400 MHz, DMSO) δ 7.95 (d, J = 6.8 Hz, 2H), 7.77 (d, J = 7.0 Hz, 2H), 7.58 (s, 2H), 7.40 (d, J = 8.3 Hz, 1H), 6.94 (d, J = 8.6 Hz, 1H), 3.86 (s, 3H), 3.83 (s, 3H), 3.77 (s, 3H). 1H NMR (400 MHz, CDCl3) δ 7.68 (dd, J = 23.7, 8.2 Hz, 5H), 7.59 (d, J = 15.9 Hz, 1H), 7.53 (d, J = 8.8 Hz, 1H), 6.78 (d, J = 8.8 Hz, 1H), 3.94 (s, 4H), 3.92 (s, 6H). 13C NMR (101 MHz, DMSO) δ 190.22, 157.48, 153.53, 142.08, 140.54, 139.21, 130.30 (q, J = 32.2 Hz), 126.26, 126.23, 125.79, 124.48 (q, J = 271.5 Hz), 108.41, 62.17, 60.97, 56.58. LC-MS (ESI-TOF): m/z 367.1158 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 100.00% (rt 9.05 min).
(E)-1-(2,4,6-methoxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (4k)
It was obtained as yellow oil in 86% yield. 1H NMR (400 MHz, DMSO) δ 7.91 (d, J = 8.2 Hz, 2H), 7.75 (d, J = 8.3 Hz, 2H), 7.31 (d, J = 16.2 Hz, 1H), 7.11 (d, J = 16.2 Hz, 1H), 6.32 (s, 2H), 3.84 (s, 3H), 3.73 (s, 6H). 13C NMR (101 MHz, DMSO) δ 193.27, 162.66, 158.73, 141.53, 138.97 (d, J = 1.4 Hz), 130.33 (q, J = 32.2 Hz), 126.14 (q, J = 3.8 Hz), 124.45 (q, J = 271.4 Hz), 111.28, 91.57, 91.40, 56.29, 55.94. LC-MS (ESI-TOF): m/z 367.1152 ([C19H17 F3O4 + H]+ calcd. 367.1152). Purity 96.54% (rt 8.03 min).
(E)-1-phenyl-3-(2-nitrophenyl)prop-2-en-1-one (5a)
It was obtained as off white solid in 46% yield. 1H NMR (400 MHz, DMSO) δ = 8.24 – 8.20 (m, 1H), 8.20 – 8.15 (m, 2H), 8.11 (dd, J = 8.1, 1.2 Hz, 1H), 8.00 (d, J = 15.5 Hz, 1H), 7.91 (d, J = 15.5 Hz, 1H), 7.88 – 7.81 (m, 1H), 7.76 – 7.68 (m, 2H), 7.60 (t, J = 7.6 Hz, 2H). 13C NMR (101 MHz, DMSO) δ = 189.52, 149.24, 139.03, 137.48, 134.22, 133.98, 131.53, 130.19, 129.98, 129.33, 129.18, 126.83, 125.16. LC-MS (ESI-TOF): m/z 254.0819 ([C15H11NO3 + H]+ calcd. 254.0812). Purity 100.00% (rt 6.68 min).
(E)-1-(2-methoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5b)
It was obtained as light yellow solid in 49% yield. 1H NMR (400 MHz, DMSO) δ = 8.09 (dd, J = 8.2, 1.2 Hz, 1H), 7.99 (dd, J = 7.9, 1.2 Hz, 1H), 7.84 – 7.76 (m, 2H), 7.73 – 7.66 (m, 1H), 7.61 – 7.57 (m, 1H), 7.55 (dd, J = 7.7, 1.7 Hz, 1H), 7.42 (d, J = 15.7 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 7.08 (td, J = 7.5, 0.9 Hz, 1H), 3.90 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 192.08, 158.46, 149.07, 137.47, 134.36, 134.07, 131.33, 130.24, 130.22, 129.62, 128.56, 125.20, 121.06, 112.82, 56.32. LC-MS (ESI-TOF): m/z 284.0916 ([C16H13NO4 + H]+ calcd. 284.0917). Purity 97.28% (rt 6.52 min).
(E)-1-(3-methoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5c)
It was obtained as off white solid in 21% yield. 1H NMR (400 MHz, DMSO) δ = 8.18 (d, J = 7.7 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.97 (d, J = 15.5 Hz, 1H), 7.83 (dd, J = 17.6, 11.4 Hz, 2H), 7.75 (d, J = 7.5 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.61 (s, 1H), 7.50 (t, J = 7.9 Hz, 1H), 7.25 (d, J = 6.4 Hz, 1H), 3.84 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 189.36, 160.02, 149.23, 139.13, 138.91, 134.21, 131.53, 130.50, 130.16, 130.02, 126.91, 125.14, 121.70, 120.06, 113.60, 55.87. LC-MS (ESI-TOF): m/z 284.0918 ([C16H13NO4 + H]+ calcd. 284.0917). Purity 100.00% (rt 6.91 min).
(E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5d)
It was obtained as off white solid in 32% yield. 1H NMR (400 MHz, DMSO) δ = 8.17 (t, J = 7.9 Hz, 3H), 8.07 (d, J = 8.1 Hz, 1H), 7.97 – 7.85 (m, 2H), 7.81 (t, J = 7.6 Hz, 1H), 7.68 (t, J = 7.7 Hz, 1H), 7.09 (d, J = 8.4 Hz, 2H), 3.86 (s, 3H). 1H NMR (400 MHz, CDCl3) δ = 8.15 – 8.00 (m, 3H), 7.74 (d, J = 7.6 Hz, 1H), 7.68 (t, J= 7.6 Hz, 1H), 7.56 (t, J = 7.1 Hz, 1H), 7.32 (d, J = 15.6 Hz, 1H), 7.00 (d, J = 8.9 Hz, 2H), 3.90 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 187.58, 163.98, 149.23, 138.00, 134.15, 131.63, 131.36, 130.38, 130.26, 129.91, 126.89, 125.09, 114.59, 56.08. LC-MS (ESI-TOF): m/z 284.0919 ([C16H13NO4 + H]+ calcd. 284.0917). Purity 100.00% (rt 6.55 min).
(E)-1-(2,4-dimethoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5e)
It was obtained as yellow solid in 57% yield. 1H NMR (400 MHz, DMSO) δ = 8.06 (dd, J = 8.2 Hz, 1.2 Hz, 1H), 7.94 (d, J = 7.7 Hz, 1H), 7.82 – 7.73 (m, 2H), 7.69 – 7.61 (m, 2H), 7.51 (d, J = 15.6 Hz, 1H), 6.68 (d, J = 2.2 Hz, 1H), 6.64 (dd, J = 8.6, 2.3 Hz, 1H), 3.90 (s, 4H), 3.84 (s, 4H). 13C NMR (101 MHz, DMSO) δ = 189.17, 164.87, 161.02, 149.09, 136.03, 134.33, 132.76, 131.63, 131.12, 130.48, 129.55, 125.14, 121.20, 106.68, 99.03, 56.48, 56.13. LC-MS (ESI-TOF): m/z 314.1027 ([C17H15NO5 + H]+ calcd. 314.1023). Purity 97.57% (rt 6.62 min).
(E)-1-(2,6-dimethoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5f)
It was obtained as white solid in 82% yield. 1H NMR (400 MHz, DMSO) δ = 8.06 (dd, J = 8.1, 1.2 Hz, 1H), 7.98 (dd, J = 7.8, 1.3 Hz, 1H), 7.78 (ddd, J = 7.8, 1.2, 0.6 Hz, 1H), 7.70 – 7.64 (m, 1H), 7.52 (d, J = 16.0 Hz, 1H), 7.40 (t, J = 8.4 Hz, 1H), 6.96 (d, J = 16.0 Hz, 1H), 6.77 (d, J = 8.4 Hz, 2H), 3.75 (s, 7H). 13C NMR (101 MHz, DMSO) δ = 194.37, 157.44, 148.75, 140.06, 134.33, 132.66, 131.72, 131.52, 129.86, 129.66, 125.21, 117.59, 104.80, 56.26. LC-MS (ESI-TOF): m/z 314.1024 ([C17H15NO5 + H]+ calcd. 314.1023). Purity 100.00% (rt 5.74 min).
(E)-1-(2,5-dimethoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5g)
It was obtained as yellow solid in 49% yield. 1H NMR (400 MHz, d2o) δ = 8.09 (dd, J = 8.1, 1.1 Hz, 1H), 7.97 (d, J = 7.7 Hz, 1H), 7.85 – 7.76 (m, 2H), 7.73 – 7.65 (m, 1H), 7.41 (d, J = 15.8 Hz, 1H), 7.16 (d, J = 1.8 Hz, 2H), 7.09 (t, J = 1.8 Hz, 1H), 3.84 (s, 3H), 3.76 (s, 3H). 13C NMR (101 MHz, d2o) δ = 191.59, 153.41, 152.61, 137.66, 134.31, 131.31, 131.11, 130.19, 129.56, 128.86, 125.14, 119.67, 114.33, 114.32, 56.74, 55.96. LC-MS (ESI-TOF): m/z 314.1029 ([C17H15NO5 + H]+ calcd. 314.1023). Purity 100.00% (rt 6.63 min).
(E)-1-(3,4-dimethoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5h)
It was obtained as yellow solid in 17% yield. 1H NMR (400 MHz, DMSO) δ = 8.20 (d, J = 7.8 Hz, 1H), 8.10 (dd, J = 8.1, 1.0 Hz, 1H), 7.99 – 7.88 (m, 3H), 7.84 (t, J = 7.6 Hz, 1H), 7.74 – 7.67 (m, 1H), 7.62 (d, J = 2.0 Hz, 1H), 7.13 (d, J = 8.5 Hz, 1H), 3.89 (s, 3H), 3.87 (s, 3H). 1H NMR (400 MHz, CDCl3) δ = 8.06– 8.12 (m, 2H), 7.74 (d, J = 7.7 Hz, 1H), 7.72 – 7.65 (m, 2H), 7.62 (d, J = 1.7 Hz, 1H), 7.56 (t, J = 7.2 Hz, 1H), 7.31 (d, J = 15.7 Hz, 1H), 6.95 (d, J = 8.4 Hz, 1H), 3.99 (s, 3H), 3.98 (s, 3H). 13C NMR (101 MHz, d2o) δ = 187.54, 153.92, 149.23, 149.15, 137.91, 134.06, 131.25, 130.35, 130.26, 129.89, 126.80, 125.02, 124.14, 111.33, 111.21, 56.22, 56.00. LC-MS (ESI-TOF): m/z 314.1027 ([C17H15NO5 + H]+ calcd. 314.1023). Purity 100.00% (rt 5.63 min).
(E)-1-(3,5-dimethoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5i)
It was obtained as off white solid in 57% yield. 1H NMR (400 MHz, DMSO) δ = 8.21 (dd, J = 7.8, 1.3 Hz, 1H), 8.10 (dd, J = 8.1, 1.2 Hz, 1H), 7.98 (d, J = 15.5 Hz, 1H), 7.89 – 7.80 (m, 2H), 7.74 – 7.68 (m, 1H), 7.28 (d, J = 2.3 Hz, 2H), 6.82 (t, J = 2.3 Hz, 1H), 3.85 (s, 6H). 13C NMR (101 MHz, DMSO) δ = 189.21, 161.19, 149.24, 139.53, 139.25, 134.18, 131.52, 130.15, 130.09, 126.89, 125.13, 106.91, 105.89, 56.05. LC-MS (ESI-TOF): m/z 314.1024 ([C17H15NO5 + H]+ calcd. 314.1023). Purity 100.00% (rt 7.21 min).
(E)-1-(3,4,5-trimethoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5j)
It was obtained as off white solid in 31% yield. 1H NMR (400 MHz, DMSO) δ = 8.13 (dd, J = 27.6, 8.0 Hz, 2H), 7.97 (d, J = 15.4 Hz, 1H), 7.92 – 7.79 (m, 2H), 7.70 (t, J = 7.7 Hz, 1H), 7.42 (s, 2H), 3.88 (s, 6H), 3.76 (s, 3H). 13C NMR (101 MHz, DMSO) δ = 188.38, 153.39, 149.18, 142.64, 139.01, 134.20, 132.84, 131.45, 130.35, 130.12, 126.80, 125.16, 106.80, 60.66, 56.65. LC-MS (ESI-TOF): m/z 344.1130 ([C18H17NO6 + H]+ calcd. 344.1129). Purity 99.00% (rt 6.25 min).
(E)-1-(2,3,4-trimethoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5k)
It was obtained as light white solid in 32% yield. 1H NMR (400 MHz, DMSO) δ = 8.07 (d, J = 8.0 Hz, 1H), 7.97 (d, J = 7.6 Hz, 1H), 7.85 – 7.75 (m, 2H), 7.67 (t, J = 7.7 Hz, 1H), 7.43 (dd, J = 12.2 Hz, 3.2 Hz, 2H), 6.95 (d, J = 8.8 Hz, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 3.77 (s, 3H). LC-MS (ESI-TOF): m/z 344.1127 ([C18H17NO6 + H]+ calcd. 344.1129). Purity 100.00% (rt 6.52 min).
(E)-1-(2,4,6-trimethoxyphenyl)-3-(2-nitrophenyl)prop-2-en-1-one (5l)
It was obtained as yellow solid in 61% yield. 1H NMR (400 MHz, DMSO) δ = 8.03 (dd, J = 8.1 Hz, 1.0, 1H), 7.93 (d, J = 7.7 Hz, 1H), 7.75 (t, J = 7.6 Hz, 1H), 7.64 (t, J = 7.8 Hz, 1H), 7.53 (d, J = 15.9 Hz, 1H), 6.92 (d, J = 15.9 Hz, 1H), 6.30 (s, 2H), 3.82 (s, 3H), 3.72 (s, 6H). 13C NMR (101 MHz, DMSO) δ = 193.25, 162.76, 158.80, 148.78, 138.79, 134.30, 133.17, 131.33, 130.06, 129.61, 125.18, 110.82, 91.42, 56.26, 55.94. LC-MS (ESI-TOF): m/z 344.1141 ([C18H17NO6 + H]+ calcd. 344.1129). Purity 100.00% (rt 5.77 min).
Biology
Cell culture and treatment
Human bronchial epithelial (Beas-2B) cells were cultured in DMEM:F12 (pH 7.4) supplemented with 10% (v/v) FBS, 100 mg/l gentamicin and genetisin. Beas-2B cells were grown in 48-well plates for 24 h and then treated with a series of chalcone derivatives dissolved in DMSO for various time points. The concentration of DMSO did not exceed 0.1%. RNA was isolated and gene expression was measured after 16 h.
Cell viability assay
The cytotoxicity of chalcone derivatives was analyzed by using trypan blue exclusion test and was further confirmed by colorimetric methylthiazolydiphenyl-tetrazolium bromide (MTT) assay as described82. Briefly, Beas-2B cells were treated with chalcone analogs or DMSO alone (0.1%, as vehicle) for 24 h. Four hours before the end of incubation, the medium was removed and 100 µl of MTT (5 mg/ml in serum free medium) was added to each well. The MTT was removed after 4 h, cells were washed with PBS, and 100 µl DMSO was added to each well to dissolve the water-insoluble MTT-formazan crystals. The absorbance was recorded at 570 nm in a plate reader (Molecular Devices, Sunnyvale, CA).
Generation of stable transfectants
Beas-2B cells overexpressing ARE luciferase reporter plasmid were obtained by transfecting Beas-2B cells with 3 µg of NQO1-ARE reporter plasmid and 0.3 µg of pUB6 empty vector (Invitrogen, Carlsbad, CA). Stable transfectants were selected using blasticidin at a concentration of 6 µg/ml. Stable clones were expanded and screened for the expression of ARE luciferase.83
ARE reporter assay
Beas-2B cells stably expressing NQO1-ARE luciferase were seeded onto a 96-well plate at a density of 10,000 cells/well for 16 h before incubation with test compounds. Next day, cells were treated with the indicated concentrations of compound 2b. Cells were also treated with DMSO, which was used as the solvent. The reporter activity was measured after 16 h exposure using the luciferase assay kit (Promega, Madison, WI). The level of increase in luciferase activity reflects the degree of Nrf2 activity.83
Mice in vivo study
All experiments in mice were performed in accordance with the standards established by the US Animal Welfare Acts, set forth in NIH guidelines and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee. C57BL/6 mice (male, 7 weeks) were maintained on AIN 76A diet (Harlan Tekland, Madison, WI) and water ad libitum and housed at a temperature (range, 20–23°C) under 12 h light/dark cycles. The mice were treated with chalcone analogs (50 mg/kg body weight) or vehicle or sulforaphane as a positive control by gavage. After 24 h treatment, the small intestines were harvested and stored at −80°C until analysis.
RNA extraction and gene expression analysis
Total RNA was extracted from cells/tissue using Qiagen RNeasy kit (Qiagen Corporation, Valencia, CA) and reverse transcription was performed by using random hexamers and MultiScribe reverse transcriptase according to the manufacturer’s recommendations (Applied Biosystems, Foster City, CA, USA). Quantitative real-time RT-PCR analyses of Nrf2, NQO1, HO1, and GCLM were performed by using Assay-on-Demand primers and probe sets from Applied Biosystems. Assays were performed using the ABI 7000 Taqman system (Applied Biosystems, Foster City, CA). β-actin was used for normalization.
Statistics
The values are represented as mean ± SE and analyzed by student’s t-test. Differences were considered significant at P ≤ 0.05.
Figure 1. Structure activity relationship of chalcone derivatives.
ACKNOWLEDGEMENTS
VK, MH, and SVM thank the National Cancer Institute (NCI) Developmental Therapeutics Program. This project has been funded in whole or in part with funds from the NCI, National Institutes of Health, Grant HSN261200800001E. This work was also supported by National Institutes of Health Grant HL081205 (SB), National Heart, Lung, and Blood Institute Specialized Centers of Clinically Oriented Research Grant P50HL084945, the Flight Attendant Medical Research Institute (SB and RKT), and National Institute on Environmental Health Sciences Grants P50ES015903, P01 ES018176, and ES03819 (SB). VSP and SKS would like to thank the University of Delhi and the Department of Scientific and Industrial Research (DSIR, New Delhi) for financial support.
ABBREVIATION USED
- Nrf2
Nuclear factor-erythroid 2 p45-related factor 2
- b-ZIP
basic-leucine zipper
- Keap 1
Kelch-like ECH-associated protein 1
- ARE
antioxidant response element
- HO-1
heme oxygenase-1
- NQO1
NAD (P)H:quinone oxidoreductase 1
- GCLM
glutamate-cysteine ligase modifier subunit
REFERENCES
- 1.Kensler TW, Wakabayash N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacool. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 2.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamato M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 5.Misra V, Lee H, Singh A, Huang K, Thimmulappa RK, Mitzner W, Biswal S, Tankersley CG. Global expression profiles from C57BL/6J and DBA/2J mouse lungs to determine aging-related genes. Physiol. Genomics. 2007;31:429–440. doi: 10.1152/physiolgenomics.00060.2007. [DOI] [PubMed] [Google Scholar]
- 6.Surh YJ, Kundu JK, Na HK. Nrf2 as a Master Redox Switch in Turning on the Cellular Signaling Involved in the Induction of Cytoprotective Genes by Some Chemopreventive Phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 7.Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, Gabrielson E, Feinstein E, Biswal S. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niture SK, Jain AK, Jaiswal AK. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J. Cell Sci. 2009;122:4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Cheng SE, Lee IT, Lin CC, Kou YR, Yang CM. Cigarette smoke particle-phase extract induces HO-1 expression in human tracheal smooth muscle cells: role of the c-Src/NADPH oxidase/MAPK/Nrf2 signaling pathway. Free Radical Biol. Med. 2010;48:1410–1422. doi: 10.1016/j.freeradbiomed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H: quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem. J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chartoumpekis D, Ziros PG, Psyrogiannis A, Kyriazopoulou V, Papavassiliou AG, Habeos IG. Simvastatin lowers reactive oxygen species level by Nrf2 activation via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 2010;396:463–466. doi: 10.1016/j.bbrc.2010.04.117. [DOI] [PubMed] [Google Scholar]
- 12.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 13.Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. In: Higgins PJ, editor. Transcription Factors: Methods and Protocols. Vol. 647. Springer; 2010. pp. 37–74. [DOI] [PubMed] [Google Scholar]
- 14.Wan JX, Diaz-Sanchez D. Antioxidant enzyme induction: A new protective approach against the adverse effects of diesel exhaust particles. Inhal. Toxicol. 2007;19:177–182. doi: 10.1080/08958370701496145. [DOI] [PubMed] [Google Scholar]
- 15.Singh A, Rangasamy T, Thimmulappa RK, Lee H, Osburn WO, Brigelius-Flohe R, Kensler TW, Yamamoto M, Biswal S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am. J. Respir. Cell Mol. Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin HJ, Zhou HY, Dai AH, Huang HF, Lin JH, Frankl HD, Lee ER, Haile RW. Glutathione transferase GSTT1, broccoli, and prevalence of colorectal adenomas. Pharmacogenetics. 2002;12:175–179. doi: 10.1097/00008571-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Kirsch M, De Groot H. NAD(P)H a directly operating antioxidant? FASEB J. 2001;15:1569–1574. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 20.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu R, Lei W, Mandlekar S, Weber MJ, Der CJ, Wu J, Kong ANT. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J. Biol. Chem. 1999;274:27545–27552. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- 22.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 23.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell. Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway - Identification of novel gene clusters for cell survival. J. Biol. Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 25.Kwak M-K, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, Yamamoto M, Kensler TW. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat. Res. 2001:305–315. doi: 10.1016/s0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 26.Balogun E, Hoque M, Gong PF, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcurnin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Go ML, Wu X, Liu XL. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005;12:483–499. doi: 10.2174/0929867053363153. [DOI] [PubMed] [Google Scholar]
- 28.Dimmock JR, Elias DW, Beazely MA, Kandepu NM. Bioactivities of chalcones. Curr. Med. Chem. 1999;6:1125–1149. [PubMed] [Google Scholar]
- 29.Xia Y, Yang ZY, Xia P, Bastow KF, Nakanishi Y, Lee KH. Antitumor agents. Part 202: Novel 2 '-amino chalcones: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2000;10:699–701. doi: 10.1016/s0960-894x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 30.Bois F, Beney C, Boumendjel A, Mariotte AM, Conseil G, Di Pietro A. Halogenated chalcones with high-affinity binding to P-glycoprotein: Potential modulators of multidrug resistance. J. Med. Chem. 1998;41:4161–4164. doi: 10.1021/jm9810194. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Wilairat P, Go ML. Antimalarial alkoxylated and hydroxylated chalones: Structure-activity relationship analysis. J. Med. Chem. 2001;44:4443–4452. doi: 10.1021/jm0101747. [DOI] [PubMed] [Google Scholar]
- 32.Dominguez JN, Charris JE, Lobo G, de Dominguez NG, Moreno MM, Riggione F, Sanchez E, Olson J, Rosenthal PJ. Synthesis of quinolinyl chalcones and evaluation of their antimalarial activity. Eur. J. Med. Chem. 2001;36:555–560. doi: 10.1016/s0223-5234(01)01245-4. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh HK, Lee TH, Wang JP, Wang JJ, Lin CN. Synthesis and anti-inflammatory effect of chalcones and related compounds. Pharm. Res. 1998;15:39–46. doi: 10.1023/a:1011940401754. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh HK, Tsao LT, Wang JP, Lin CN. Synthesis and anti-inflammatory effect of chalcones. J. Pharm. Pharmacol. 2000;52:163–171. doi: 10.1211/0022357001773814. [DOI] [PubMed] [Google Scholar]
- 35.Herencia F, Ferrandiz ML, Ubeda A, Dominguez JN, Charris JE, Lobo GM, Alcaraz MJ. Synthesis and anti-inflammatory activity of chalcone derivatives. Bioorg. Med. Chem. Lett. 1998;8:1169–1174. doi: 10.1016/s0960-894x(98)00179-6. [DOI] [PubMed] [Google Scholar]
- 36.Lin YM, Zhou YS, Flavin MT, Zhou LM, Nie WG, Chen FC. Chalcones and flavonoids as anti-tuberculosis agents. Biorg. Med. Chem. 2002;10:2795–2802. doi: 10.1016/s0968-0896(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 37.Rojas J, Paya M, Dominguez JN, Ferrandiz ML. The synthesis and effect of fluorinated chalcone derivatives on nitric oxide production. Bioorg. Med. Chem. Lett. 2002;12:1951–1954. doi: 10.1016/s0960-894x(02)00317-7. [DOI] [PubMed] [Google Scholar]
- 38.Herencia F, Ferrandiz ML, Ubeda A, Guillen I, Dominguez JN, Charris JE, Lobo GM, Alcaraz MJ. 4-dimethylamino-3 ',4 '-dimethoxychalcone downregulates iNOS expression and exerts anti-inflammatory effects. Free Radical Biol. Med. 2001;30:43–50. doi: 10.1016/s0891-5849(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 39.Ducki S, Forrest R, Hadfield JA, Kendall A, Lawrence NJ, McGown AT, Rennison D. Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorg. Med. Chem. Lett. 1998;8:1051–1056. doi: 10.1016/s0960-894x(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 40.Maria K, Dimitra HL, Maria G. Synthesis and Anti-Inflammatory Activity of Chalcones and Related Mannich Bases. Med. Chem. 2008;4:586–596. doi: 10.2174/157340608786242070. [DOI] [PubMed] [Google Scholar]
- 41.Viana GSB, Bandeira MAM, Matos FJA. Analgesic and antiinflammatory effects of chalcones isolated from Myracrodruon urundeuva Allemao. Phytomedicine. 2003;10:189–195. doi: 10.1078/094471103321659924. [DOI] [PubMed] [Google Scholar]
- 42.Cheng JH, Hung CF, Yang SC, Wang JP, Won SJ, Lin CN. Synthesis and cytotoxic, anti-inflammatory, and anti-oxidant activities of 2 ',5 '-dialkoxylchalcones as cancer chemopreventive agents. Biorg. Med. Chem. 2008;16:7270–7276. doi: 10.1016/j.bmc.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 43.Heidari MR, Foroumadi A, Amirabadi A, Samzadeh-Kermani A, Azimzadeh BS, Eskandarizadeh A. Evaluation of Anti-inflammatory and Analgesic Activity of a Novel Rigid 3, 4-Dihydroxy Chalcone in Mice. In: Diederich M, editor. Natural Compounds and Their Role in Apoptotic Cell Signaling Pathways. Wiley-Blackwell; 2009. pp. 399–406. [DOI] [PubMed] [Google Scholar]
- 44.Cheenpracha S, Karalai C, Ponglimanont C, Subhadhirasakul S, Tewtrakul S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Biorg. Med. Chem. 2006;14:1710–1714. doi: 10.1016/j.bmc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Svetaz L, Tapia A, Lopez SN, Furlan RLE, Petenatti E, Pioli R, Schmeda-Hirschmann G, Zacchino SA. Antifungal chalcones and new caffeic acid esters from Zuccagnia punctata acting against soybean infecting fungi. J. Agric. Food. Chem. 2004;52:3297–3300. doi: 10.1021/jf035213x. [DOI] [PubMed] [Google Scholar]
- 46.Saavedra MJ, Borges A, Dias C, Aires A, Bennett RN, Rosa ES, Simoes M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010;6:174–183. doi: 10.2174/1573406411006030174. [DOI] [PubMed] [Google Scholar]
- 47.Aponte JC, Verastegui M, Malaga E, Zimic M, Quiliano M, Vaisberg AJ, Gilman RH, Hammond GB. Synthesis, cytotoxicity, and anti-Trypanosoma cruzi activity of new chalcones. J. Med. Chem. 2008;51:6230–6234. doi: 10.1021/jm800812k. [DOI] [PubMed] [Google Scholar]
- 48.Lahtchev KL, Batovska DI, Parushev SP, Ubiyvovk VM, Sibirny AA. Antifungal activity of chalcones: A mechanistic study using various yeast strains. Eur. J. Med. Chem. 2008;43:2220–2228. doi: 10.1016/j.ejmech.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Batovska DI, Todorova IT. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010;5:1–29. doi: 10.2174/157488410790410579. [DOI] [PubMed] [Google Scholar]
- 50.Torigoe T, Arisawa M, Itoh S, Fujiu M, Maruyama HB. Anti-mutagenic chalcones - antagonizing the mutagenicity of benzo (a) pyrene on salmonella-typhimurium. Biochem. Biophys. Res. Commun. 1983;112:833–842. doi: 10.1016/0006-291x(83)91693-5. [DOI] [PubMed] [Google Scholar]
- 51.Lee SA, Ryu HW, Kim YM, Choi S, Lee MJ, Kwak TK, Kim HJ, Cho M, Park KH, Lee JW. Blockade of Four-Transmembrane L6 Family Member 5 (TM4SF5)-Mediated Tumorigenicity in Hepatocytes by a Synthetic Chalcone Derivative. Hepatology. 2009;49:1316–1325. doi: 10.1002/hep.22777. [DOI] [PubMed] [Google Scholar]
- 52.Shibata S. Anti-tumerigenic chaclones. Stem Cells. 1994;12:44–52. doi: 10.1002/stem.5530120109. [DOI] [PubMed] [Google Scholar]
- 53.Devincenzo R, Scambia G, Panici PB, Ranelletti FO, Bonanno G, Ercoli A, Dellemonache F, Ferrari F, Piantelli M, Mancuso S. Effect of synthetic and naturally-occurring chalcones on ovarian cancer cell-growth-structure-activity-relationships. Anti-Cancer Drug Des. 1995;10:481–490. [PubMed] [Google Scholar]
- 54.Wei BL, Teng CH, Wang JP, Won SJ, Lin CN. Synthetic 2 ',5 '-dimethoxychalcones as G(2)/M arrest-mediated apoptosis-inducing agents and inhibitors of nitric oxide production in rat macrophages. Eur. J. Med. Chem. 2007;42:660–668. doi: 10.1016/j.ejmech.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi T, Takasuka N, Iigo M, Baba M, Nishino H, Tsuda H, Okuyama T. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E-2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004;95:448–453. doi: 10.1111/j.1349-7006.2004.tb03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JH, Jung HS, Giang PM, Jin X, Lee S, Son PT, Lee D, Hong YS, Lee K, Lee JJ. Blockade of nuclear factor-kappa B signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J. Pharmacol. Exp. Ther. 2006;316:271–278. doi: 10.1124/jpet.105.092486. [DOI] [PubMed] [Google Scholar]
- 57.Ban HS, Suzuki K, Lim SS, Jung SH, Lee S, Ji J, Lee HS, Lee YS, Shin KH, Ohuchi K. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by 2 '-hydroxychalcone derivatives in RAW 264.7 cells. Biochem. Pharmacol. 2004;67:1549–1557. doi: 10.1016/j.bcp.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Liu YC, Hsieh CW, Wu CC, Wung BS. Chalcone inhibits the activation of NF-kappa B and STAT3 in endothelial cells via endogenous electrophile. Life Sci. 2007;80:1420–1430. doi: 10.1016/j.lfs.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 59.Foresti R, Hoque M, Monti D, Green CJ, Motterlini R. Differential activation of heme oxygenase-1 by chalcones and rosolic acid in endothelial cells. J. Pharmacol. Exp. Ther. 2005;312:686–693. doi: 10.1124/jpet.104.074153. [DOI] [PubMed] [Google Scholar]
- 60.Wu CC, Hsieh CW, Lai PH, Lin JB, Liu YC, Wung BS. Upregulation of endothelial heme oxygenase-1 expression through the activation of the JNK pathway by sublethal concentrations of acrolein. Toxicol. Appl. Pharmacol. 2006;214:244–252. doi: 10.1016/j.taap.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 61.Shibata T, Yamada T, Ishii T, Kumazawa S, Nakamura H, Masutani H, Yodoi J, Uchida K. Thioredoxin as a molecular target of cyclopentenone prostaglandins. J. Biol. Chem. 2003;278:26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- 62.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 63.Micheli F, Degiorgis F, Feriani A, Paio A, Pozzan A, Zarantonello P, Seneci P. A combinatorial approach to [1,5]benzothiazepine derivatives as potential antibacterial agents. J. Comb. Chem. 2001;3:224–228. doi: 10.1021/cc0000949. [DOI] [PubMed] [Google Scholar]
- 64.Bu XY, Zhao LY, Li YL. A facile synthesis of 6-C-prenylflavanones. Synthesis-Stuttgart. 1997:1246–1248. [Google Scholar]
- 65.Sinisterra JV, Garciaraso A, Cabello JA, Marinas JM. An improved procedure for the Claisen-Schmidt reaction. Synthesis-Stuttgart. 1984:502–504. [Google Scholar]
- 66.Alcantara AR, Marinas JM, Sinisterra JV. Synthesis of 2'-hydroxychalcones and related-compounds in interfacial solid-liquid conditions. Tetrahedron Lett. 1987;28:1515–1518. [Google Scholar]
- 67.Climent MJ, Corma A, Iborra S, Velty A. Activated hydrotalcites as catalysts for the synthesis of chalcones of pharmaceutical interest. J. Catal. 2004;221:474–482. [Google Scholar]
- 68.Daskiewicz JB, Comte G, Barron D, Di Pietro A, Thomasson F. Organolithium mediated synthesis of prenylchalcones as potential inhibitors of chemoresistance. Tetrahedron Lett. 1999;40:7095–7098. [Google Scholar]
- 69.Sebti S, Solhy A, Smahi A, Kossir A, Oumimoun H. Dramatic activity enhancement of natural phosphate catalyst by lithium nitrate. An efficient synthesis of chalcones. Catal. Commun. 2002;3:335–339. [Google Scholar]
- 70.Calloway NO, Green LD. Reactions in the presence of metallic halides I beta-unsaturated ketone formation as a side reaction in Friedel-Crafts acylations. J. Am. Chem. Soc. 1937;59:809–811. [Google Scholar]
- 71.Breslow DS, Hauser CR. Condensations. XI. Condensations of certain active hydrogen compounds effected by boron trifluoride and aluminum chloride. J. Am. Chem. Soc. 1940;62:2385–2388. [Google Scholar]
- 72.Szell T, Sohar I. New nitrochalcones. Can. J. Chem. 1969;47:1254–1258. [Google Scholar]
- 73.Irie K, Watanabe K. Aldol condensations with metal(II) complex catalysts. Bull. Chem. Soc. Jpn. 1980;53:1366–1371. [Google Scholar]
- 74.Nakano T, Irifune S, Umano S, Inada A, Ishii Y, Ogawa M. Cross-condensation reactions of cycloalkanones with aldehydes and primary alcohols under the influence of zirconocene complexes. J. Org. Chem. 1987;52:2239–2244. [Google Scholar]
- 75.Iranpoor N, Kazemi F. RuCl3 catalyses aldol condensations of aldehydes and ketones. Tetrahedron. 1998;54:9475–9480. [Google Scholar]
- 76.Eddarir S, Cotelle N, Bakkour Y, Rolando C. An efficient synthesis of chalcones based on the Suzuki reaction. Tetrahedron Lett. 2003;44:5359–5363. [Google Scholar]
- 77.Bhagat S, Sharma R, Sawant DM, Sharma L, Chakraborti AK. LiOH center dot H2O as a novel dual activation catalyst for highly efficient and easy synthesis of 1,3-diaryl-2-propenones by Claisen-Schmidt condensation under mild conditions. J. Mol. Catal. A-Chem. 2006;244:20–24. [Google Scholar]
- 78.Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, Taguchi K, Yamamoto M, Kensler TW. Genetic or pharmacologic amplification of Nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol. Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bloom D, Dhakshinamoorthy S, Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of Nrf2 decreases its capacity to upregulate antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2002;21:2191–2200. doi: 10.1038/sj.onc.1205288. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci U S A. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar S, Singh BK, Pandey AK, Kumar A, Sharma SK, Raj HG, Prasad AK, Van der Eycken E, Parmar VS, Ghosh B. A chromone analog inhibits TNF-alpha induced expression of cell adhesion molecules on human endothelial cells via blocking NF-kappaB activation. Bioorg. Med. Chem. 2007;15:2952–2962. doi: 10.1016/j.bmc.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 83.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLOS Med. 2006;3:1865–1876. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]