Abstract
Introduction
Bleeding is the major complication associated with warfarin therapy. Some antidepressants are also associated with increased bleeding risk. Warfarin and antidepressants are used frequently in combination, but it is unclear whether concomitant use increases the risk of bleeding beyond that with warfarin alone. The primary goal of this study was to determine whether use of warfarin and an antidepressant increases the risk for bleeding outcomes compared to warfarin use alone. The secondary goal was to characterize the risk of bleeding in warfarin-treated patients taking one specific class of antidepressant, selective serotonin reuptake inhibitors (SSRIs).
Materials and Methods
This was a retrospective, single-center, study of warfarin-treated patients prescribed (n=46) and not prescribed (n=54) an antidepressant. Medical records over six months were reviewed for INR values, medical history, bleeding type and incidence, and hospitalization due to bleeding. Patients were included in the antidepressant group if taking concomitant warfarin and antidepressant therapy consistently for a period of 6 months and in the control group if not taking an antidepressant with warfarin.
Results
Use of any antidepressant with warfarin was not associated with the incidence of any bleeding or major bleeding during the 6-month period. However, use of an SSRI with warfarin was associated with an increase in any bleeding event (OR 2.6, 95% CI, 1.01–6.4 p=0.04). Use of a SSRI remained a significant predictor of bleeding after accounting for other factors associated with bleeding risk.
Conclusion
This data suggest it is important to clarify the interaction between warfarin and SSRIs in regard to bleeding risk given the high frequency of their concomitant use.
Keywords: Anticoagulation, Antidepressant, SSRI, Bleeding, Warfarin
Introduction
Warfarin is commonly prescribed for the primary and secondary prevention of thromboembolism. Bleeding is the major complication associated with anticoagulant therapy, with warfarin increasing the risk for major bleeding by 0.3 to 0.5% per year and the risk for intracranial hemorrhage by 0.2% per year compared to controls.1 As a vitamin K antagonist, warfarin prevents thrombus formation through its inhibitory effects on the activation of clotting factors II, VII, IX, X. Warfarin is highly protein bound and metabolized through the liver, primarily by the cytochrome (CYP) P450 2C9, 1A2, 3A4, and 2C19 enzymes.2 Because of its narrow therapeutic index, warfarin requires close monitoring as supratherapeutic anticoagulation increases the risk for bleeding, and subtherapeutic anticoagulation increases risk for thrombosis. Alterations in diet, consumption of alcohol, and use of medications that interfere with warfarin metabolism can increase the risk for bleeding with warfarin.
Antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly prescribed to treat major depression, anxiety, and other psychiatric disorders in patients taking warfarin. Tricyclic antidepressants (TCAs) are also commonly prescribed to treat major depressive disorder as well as for management of peripheral neuropathies. Data suggest that various antidepressant agents may increase bleeding risk.3–12 In particular, case reports and case-control studies demonstrate an increased risk of bleeding, including upper gastrointestinal bleeding, with SSRIs and the SNRI venlafaxine.4, 6–8, 11–13 There are several potential mechanisms by which SSRIs and SNRIs may increase bleeding. These include direct platelet effects, such as the antagonism of serotonin transporters, thereby impairing platelet aggregation;14 depletion of platelet serotonin levels;3, 5, 11, 15, 16 and reduction in platelet count.17 Concomitant use of warfarin with an SSRI or SNRI could potentially increase the risk for bleeding beyond that of either agent alone due to the distinct pharmacodynamic effects of each drug class on different aspects of clotting physiology. As a class, TCAs do not appear to increase bleeding risk when used alone; however, they could potentially increase bleeding risk with warfarin through their inhibitory effects on warfarin metabolism.4, 18
The authors hypothesized that use of antidepressant drug therapy is associated with an increased risk for bleeding with warfarin. The objective of this study was to determine whether concomitant use of warfarin and any antidepressant increases the risk for bleeding compared to warfarin use alone. Secondly, the risk of bleeding with SSRI utilization in the context of warfarin therapy was characterized.
Materials and Methods
PATIENTS
Adult outpatients (≥18 years of age) who were treated with warfarin for ≥6 months and followed in the pharmacist-managed Antithrombosis Clinic at University of Illinois Medical Center at Chicago were considered for inclusion in this study. Warfarin-treated patients are seen in clinic at least once every 4 weeks for assessment of the international normalized ratio (INR) and warfarin adjustment as necessary. Pharmacists in this Antithrombosis Clinic systematically assess and document alterations in diet, consumption of alcohol, and use of medications that interfere with warfarin metabolism; monitor laboratory values; perform physical assessment for bleeding events; and adjust warfarin therapy as needed at each clinic visit. Patients in the study were included in one of two groups: 1) an antidepressant-warfarin group (ADP-WARF) and 2) a warfarin only group (WARF). Those in the ADP-WARF group were anticoagulated with warfarin and on concurrent daily scheduled antidepressant therapy (with an SSRI, SNRI, trazodone, TCA, mirtazapine, or buproprion) for 6 months during the data collection period of January 1, 2007 through November 1, 2009. These patients were matched by age and race to patients in the WARF group who had no history of antidepressant use during a 6-month data collection period. This study was approved by the Institutional Review Board at the University of Illinois at Chicago.
DATA COLLECTION
The following data were collected for each subject on the baseline date, defined as six months prior to the most recent Antithrombosis Clinic visit between the dates of January 1, 2007 and November 1, 2009: demographic information; past medical history, including indication for warfarin; alcohol, tobacco, and illicit drug use; mean weekly warfarin dose; concomitant medications; and INR. A baseline date of six months prior to the most recent visit was set to ensure consistency in the electronic medical record. Bleeding risk was assessed by calculating the Beyth’s Outpatient Bleeding Risk Index19 and Kuijer’s Bleeding Risk Score20 (see Appendix). Beyth’s Outpatient Bleeding Risk Index provides a prediction of major bleeding based on age, a history of gastrointestinal bleeding, history of stroke, recent myocardial infarction, low hematocrit, low creatinine, or a diagnosis of diabetes mellitus. Kuijer’s Bleeding Risk score provides an estimate of both minor and major bleeding by considering age, sex, and known malignancy. The primary outcome in this study was the occurrence of any bleeding during a six month assessment period. Therefore, Kuijer’s Bleeding Risk score was used to compare differences in bleeding risk between the two groups. Beyth’s index was additionally used to compare risk for major bleeding and to identify additional clinical variables related to bleeding outcomes in these patients. The following additional data were collected from the medical record during the 6 months between the baseline date and most recent clinic visit for both groups: bleeding events; interventions for bleeding; INR values; any hospitalizations secondary to bleeding; changes in medication; patient-reported changes in diet or alcohol consumption; and any laboratory assessment of hemoglobin, hematocrit, platelet count, or heme occult stool. All INR values were determined by point-of-care assay (ProTime™ Microcoagulation System, Edison, New Jersey) in the Antithrombosis Clinic or from a venous blood draw in the clinical laboratory for those values requiring confirmation. Other laboratory measurements were performed in the clinical laboratory at our institution.
DATA ANALYSIS
The authors’ primary hypothesis was that use of antidepressant drug therapy would be associated with an increased risk for bleeding with warfarin. This hypothesis was based on previous data illustrating the potential for both pharmacokinetic and pharmacodynamic interactions between antidepressants and warfarin.18, 21–24 Therefore the primary analysis was powered to compare bleeding outcomes between patients taking any antidepressant with warfarin (ADP-WARF) (n=46) and warfarin alone (WARF) (n=54) groups. Previous data also illustrate the relationship between the SSRI class of antidepressants and an increased risk for bleeding, which is associated with the disruption of platelet aggregation due to peripheral serotonin transporter antagonism.3, 4, 6, 7, 9, 11, 12, 14, 15 Therefore a secondary exploratory analysis was completed to compare the incidence of bleeding in those patients taking an SSRI with warfarin therapy (n=25) with those who were not taking an SSRI with warfarin (n=75). Patients taking more than one antidepressant, one of which was an SSRI were included in the SSRI group. Major bleeding was defined as a bleeding event that required an intervention. Interventions included holding ≥1 dose of warfarin, administering phytonadione, blood transfusion, emergency department visit, or hospitalization. Minor bleeding was defined as a bleeding event that did not require an intervention. Minor bleeding events included hemorrhoidal bleeding, genitourinary bleeding, gum bleeding, otorrhagia, prolonged bleeding from a skin breakage, blood in the sputum, or epistaxis. Notation of a hematoma in the medical record was included as a minor bleeding event unless it required an intervention, as described above, in which case it was categorized as a major event. Bruises were not considered as minor bleeding events. Any bleeding was defined as either a major bleeding or minor bleeding event. Hospitalization due to bleeding was categorized as any bleeding event resulting in an emergency department visit or hospital admission. An a priori sample size determination was completed with a the assumption of a baseline rate of any bleeding of approximately 32% in the warfarin-only group.20 Including ≥44 patients in each group was estimated to provide 80% power to detect a clinically significant 30 percentage point difference in the occurrence of any bleeding event between groups, assuming a two-tailed p-value of 0.05. Sample size was determined using SYSTAT 12 for Windows, SYSTAT Software, Inc. Chicago, IL. Data were compared between the ADP-WARF and WARF groups by χ2 analysis or Fisher’s Exact test as appropriate for nominal data and the Student’s unpaired t-test for continuous data, using SPSS 15 for Windows (Chicago, IL).
Clinical or laboratory variables associated with risk for any bleeding and major bleeding for the study population as a whole were determined by univariate logistic regression analysis. Factors tested included demographic characteristics, laboratory values, medical history, and SSRI use. Factors associated with bleeding risk in the univariate analysis (p<0.05) were subsequently entered into a multiple logistic regression analysis along with use of any antidepressant or SSRI use to identify adjusted odds ratios (ORs). Baseline (6 months prior to the most recent clinic visit) bleeding risk differences between the ADP-WARF and WARF groups were compared using the Kuijer’s Bleeding Risk to assess risk for overall bleeding and Beyth’s Outpatient Bleeding Risk Index as a second measure to assess risk for major bleeding. Components of the Beyth’s assessment found to be significantly associated with our bleeding outcomes (hematocrit and serum creatinine) were included in the prinary multiple regression analyses. Regression analyses investigating the relationship between SSRI utilization and bleeding outcomes additionally included variables that differed between those taking an SSRI and those who did, not such as history of GI bleeding, stroke, and sex.
Results
A total of 100 patients were included; 46 patients in the ADP-WARF group, and 54 in the WARF group. Twenty-five members (54%) of the ADP-WARF group were treated with an SSRI. Individual antidepressants used included: citalopram (n=2), escitalopram (n=6), fluoxetine (n=4), paroxetine (n=5), sertraline (n=8), duloxetine (n=1), mirtazapine (n=4), trazodone (n=6), amitriptyline (n=12), nortriptyline (n=4), imipramine (n=1), and bupropion (n=2). Some patients were on multiple ADPs: escitalopram and nortriptyline (n=1); bupropion, trazodone, and duloxetine (n=1); trazodone and imipramine (n=1); paroxetine and trazodone (n=2); nortriptyline and mirtazapine (n=1); escitalopram and trazodone (n=1); and amitriptyline and trazodone (n=1).
Patient characteristics are shown in Table 1. The groups were representative of the UIC patient population, which is predominantly African American and female. Female sex, history of stroke, and history of gastrointestinal bleeding were more common in the ADP-WARF group. There were no significant differences between treatment groups for any bleeding as measured by the Kuijer’s Bleeding Risk Index. However, patients in the ADP-WARF group had a higher risk for major bleeding as calculated by the Beyth’s Outpatient Bleeding Risk Index, with 26% of those in the ADP-WARF group at high risk compared to 6% in the WARF group (p<0.01). Use of other medications that increase bleeding risk, namely antiplatelet agents, and other characteristics were similar between groups. Patients in the two groups maintained an INR within therapeutic range (±0.2 INR units) over the 6-month data collection period 66±19% and 67±20% of the time for the ADP-WARF and WARF groups, respectively (p=0.76). Patients had an INR >4 at 4.7±6.3% and 5.0±8.2% of clinic visits for the ADP-WARF and WARF groups, respectively (p=0.87).
Table 1.
Baseline characteristicsA
| Characteristic | ADP-WARF (n=46) |
WARF (n=54) |
P value |
|---|---|---|---|
| Female sex | 39 (85) | 36 (67) | 0.04 |
| Age (years) | 59 ± 16 | 58 ± 16 | 0.85 |
| Race | |||
| African-American | 33 (72) | 35 (65) | 0.46 |
| Caucasian | 19 (28) | 19 (35) | |
| Past Medical History | |||
| Venous thromboembolism | 28 (61) | 30 (56) | 0.59 |
| Cerebrovascular event | 24 (52) | 14 (26) | <0.01 |
| Atrial fibrillation/flutter | 9 (20) | 13 (24) | 0.59 |
| Heart valve replacement | 3 (7) | 3 (6) | 1.00 |
| Peripheral vascular disease | 4 (9) | 6 (11) | 0.75 |
| Gastrointestinal bleed | 11 (24) | 2 (4) | <0.01 |
| Myocardial infarction | 1 (2) | 0 | 0.46 |
| Diabetes mellitus | 15 (33) | 11 (20) | 0.16 |
| Active malignancy | 6 (13) | 4 (7) | 0.51 |
| Beyth’s Bleeding Risk Index19 | |||
| Low | 6 (13) | 11 (20) | 0.015 |
| Intermediate | 28 (61) | 40 (74) | |
| High | 12 (26) | 3 (6) | |
| Kuijer’s Bleeding Risk | |||
| Low | 4 (9) | 8 (15) | 0.31 |
| Intermediate | 36 (78) | 43 (80) | |
| High | 6 (13) | 3 (6) | |
| Use of medications that increase bleeding riskB | 31 (67) | 31 (57) | 0.31 |
| Use of gastroprotective agentC | 10 (22) | 8 (15) | 0.37 |
| Serum creatinine >1.5 mg/dL | 12 (26) | 16 (30) | 0.69 |
| Hematocrit <30% | 11 (24) | 12 (22) | 0.84 |
| Platelets, thousand/µL | 213 ± 74 | 186 ± 52 | 0.04 |
| Target INR range | |||
| 2 to 3 | 40 (87) | 47 (87) | 0.99 |
| 2.5 to 3.5 | 6 (13) | 7 (13) | |
Mean ± SD or No. (%)
Baseline = 6 months prior to most recent clinic visit
aspirin, clopidogrel, dipyridamole, low molecular weight heparins
Proton Pump Inhibitors
ADP-WARF: antidepressant plus warfarin cohort; INR: international normalized ratio; WARF: no antidepressant
Use of any antidepressant was not significantly associated with risk for bleeding events. Twenty-two of 46 patients (48%) in the ADP-WARF group and 17/54 (32%) in the WARF group reported at least one bleeding event during the 6-month data collection period (OR=2.0, 95% CI 0.9–4.5, p=0.10). Six of 46 (13%) in the ADP-WARF group and 3/54 (6%) in the WARF group had a major bleeding event (OR=2.6, 95% CI 0.6–11, p=0.30). Five of 46 patients (11%) in the ADP-WARF group and 2/75 (2.7%) in the WARF group were hospitalized due to a major bleed (OR 6.5, 95% CI 0.7–57; p=0.09).
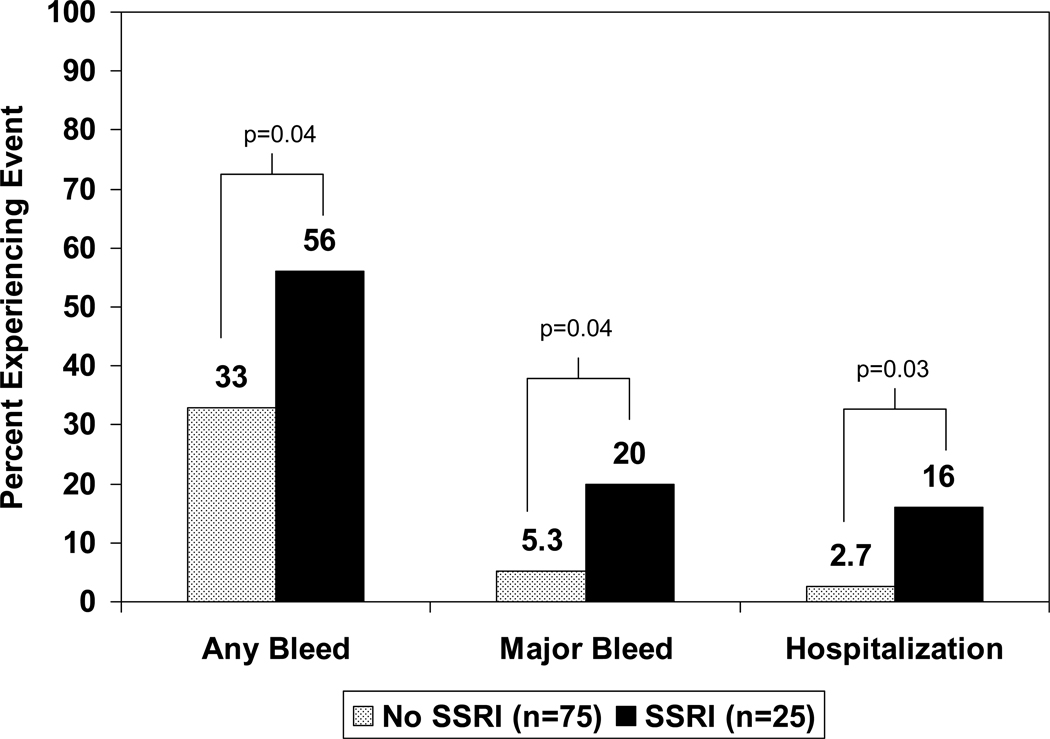
Antidepressant utilization was then stratified to compare those taking an SSRI with warfarin (n=25) with those not on an SSRI (n=75). Using this categorization, use of an SSRI was significantly associated with risk of any bleeding and major bleeding, compared to no SSRI use, as shown in Figure 1. Use of a SSRI increased the risk of any bleed (OR=2.6, 95% CI 1.01–6.4, p=0.04), major bleeding (OR=4.4, 95% CI 1.1–18, p=0.04), and hospitalization secondary to bleeding (OR=7.0, 95% CI 1.2–40, p=0.03) as compared to those not taking an SSRI.
Figure 1.
Bleeding incidence in warfarin-treated patients receiving an SSRI
The risk for major bleeding according to the Beyth’s Risk Index was higher in the WARF-ADP versus WARF group. Therefore the authors further examined whether use of any antidepressant or SSRI alone was associated with bleeding events after controlling for other bleeding risk factors. Factors examined for association with any bleeding and major bleeding were individual components of the Beyth’s Outpatient Bleeding Risk Index (age >65 years, history of stroke, history of gastrointestinal bleeding, recent myocardial infarction, hematocrit <30% at any point during the 6-month follow-up period, serum creatinine >1.5mg/dl at any point during the 6-month follow-up, and diabetes mellitus). Of these, only hematocrit <30% (p=0.003) and serum creatinine >1.5 mg/dL (p=0.005) were associated with risk for any bleeding by univariate analysis, while age >65 years (p=0.75), history of stroke (p=0.23), history of gastrointestinal bleeding (p=0.24), recent myocardial infarction (p=0.39), and diabetes (p=0.95) were not. After accounting for hematocrit and serum creatinine, use of any antidepressant was not a significant predictor of any bleeding or major bleeding. When medication utilization was stratified by use of an SSRI antidepressant, SSRI use remained a significant predictor of any bleeding (OR=4.0, 95% CI 1.5–10.9, p=0.014) and major bleeding (OR=9.4, 95% CI 1.4–62.5, p=0.02) after controlling for hematocrit <30% and serum creatinine >1.5 mg/dL. When additionally accounting for other factors that differed between patients taking and not taking an SSRI (sex, history of stroke, history of gastrointestinal bleed), SSRI use remained significantly associated with any bleeding (OR=4.2, 95% CI 1.3–13.0, p=0.014), but not major bleeding (OR=4.2, 95% CI 0.39–45.5, p=0.24).
Discussion
The primary finding from this study is that in outpatients receiving chronic warfarin therapy, use of any antidepressant did not significantly increase the risk for bleeding outcomes. However use of an SSRI increased the risk of any bleeding when compared to those not taking an SSRI. These findings remained unchanged after controlling for other clinical variables associated with bleeding outcomes (hematocrit and serum creatinine) as well as those that differed between patients taking and not taking an SSRI (e.g. sex, history of GI bleeding, or history of stroke). The findings of this study are consistent with that of Wallerstedt et al24 who also demonstrated an increased risk for hospital admission for bleeding in patients with atrial fibrillation who were taking concomitant SSRI (sertraline or citalopram) and warfarin. Similarly, Hauta-Aho et al23 reported that hospitalized patients taking an SSRI and warfarin for a 7-day period were at increased risk of any bleeding and upper gastrointestinal bleeding. In contrast, Kurdyak et al25 reported no increase in the incidence of hospitalization for bleeding during concomitant SSRI and warfarin therapy. However, their outcome of interest was limited to hospitalization for upper gastrointestinal bleeding rather than including other major bleeding events. Additionally, the authors noted that more patients on SSRIs were also prescribed gastroprotective agents, which may have negated any increase in gastrointestinal bleed risk in SSRI-treated patients. In a case-control study, Kharofa et al26 reported no increased risk of intracranial or subarachnoid hemorrhage with concomitant SSRI and warfarin use. However, the authors’ primary aim was to determine whether SSRI use was associated with hemorrhagic stroke, and their examination of SSRI with warfarin was a secondary analysis in an unspecified number of patients. Thus, it is questionable whether they had sufficient power to detect an increased bleeding risk with this combination.
There are several pharmacological and clinical reasons that may explain the increased risk of bleeding in patients taking both SSRIs and warfarin as opposed to warfarin alone. First, serotonin transporters, which regulate the reuptake of serotonin are present on platelets and are required for platelet aggregation. Serotonin reuptake inhibitors block serotonin transporter activity.9, 14 In particular, SSRIs with the highest affinities to serotonin transporters, such as paroxetine, sertraline and fluoxetine, increase the risk of upper gastrointestinal bleeding on their own, even after controlling for the prominent risk factors of age and previous bleeding.6 Other antidepressant agents that have lower affinity or no affinity for serotonin transporters do not clearly increase bleeding risk.11 This is perhaps one reason why a statistically significant increase in bleeding risk was not observed when all antidepressants were grouped together.
Secondly, SSRIs impair platelet aggregation secondary to depletion of platelet serotonin levels3,11,15,16 Ataoglu et al17, 27 demonstrated that subjects with depression had increased mean platelet volume correlating with enhanced platelet activity. Following exposure to escitalopram for 10 weeks, these subjects demonstrated significant reductions in mean platelet volume and in platelet count. While mean platelet volume has been shown to correspond with platelet reactivity, the mechanism of effect of reduced platelet count with SSRI has not been described. Similarly, depressed subjects treated with paroxetine have demonstrated normalization of increased platelet activation.27
Serotonin reuptake inhibitors may interfere with warfarin metabolism, thus increasing risk for supratherapeutic anticoagulation and bleeding. However, data in this regard still require clarification. The SSRIs fluoxetine and fluvoxamine inhibit CYP2C9-, 2C19- and 1A2-mediated warfarin metabolism.21, 28 However, only 4 (16%) of those on an SSRI were on fluoxetine in the study presented here, and none were taking fluvoxamine. All of the SSRIs associated with major bleeding events and listed in Table 3, with the exception of paroxetine, are also substrates for CYP2C19. Thus, they may compete with the less active R-enantiomer of warfarin for metabolism and potentially contribute to bleeding risk. As noted in Table 3, supratherapeutic INR values, greater than 3 or 3.5 as indicated, were observed in some patients experiencing a major bleed. However, the risk for bleeding significantly increases when the INR exceeds 4.29, 30 Four of the 6 patients (67%) in the ADP-WARF group had an INR<4 at the time of major bleeding suggesting that pharmacodynamic factors may play an important role in determining bleeding risk with warfarin and SSRIs.
Table 3.
Description of major bleeding events reported over 6-month data collection period
| Description of bleed |
INR at time of bleed |
Intervention | Antidepressant at Time of Bleed |
|---|---|---|---|
| ADP-WARF Cohort | |||
| Frank hematuria | 3.4 | Warfarin held | Nortriptyline |
| Melena with low hemoglobin and hypotension | 4.3 | Admitted to hospital, warfarin held, and received 2 units of packed red blood cells | Sertraline |
| Retroperitoneal hematoma following angiography | 2.9 | Prolonged time to hospital discharge, warfarin held, received 3 units of packed red blood cells | Escitalopram |
| Gastrointestinal bleed with low hemoglobin | 3.3 | Admitted to hospital and underwent sigmoidoscopy and colonoscopy | Sertraline |
| Low hemoglobin | 2.2 | Colonoscopy followed by tumor resection | Fluoxetine |
| Fall resulting in hematoma with low hemoglobin | 5.2 | Admitted to hospital and warfarin held | Paroxetine |
| WARF Cohort | |||
| Gastrointestinal bleeding with low hemoglobin | 1.9 | Admitted to hospital and received 3 units of packed red blood cells | |
| Chest tube bleed | 4.5 | Emergency room visit and warfarin held | |
| Hematoma | 6.2 | Received phytonadione 2.5 mg orally | |
If the findings of this study are confirmed, they have several implications. First, the relationship between SSRI exposure and bleeding outcomes during warfarin exposure needs to be confirmed. Secondly, these findings suggest that the benefit versus risk with SSRI therapy in warfarin-treated patients should be carefully evaluated. For those treated with concomitant SSRIs and warfarin, clinicians should be particularly vigilant in monitoring INR, blood counts, and signs and symptoms of bleeding and controlling other risk factors for bleeding. These findings should also encourage further research into the mechanism that may underlie the increased bleeding risk with these two classes of medications.
The results of this study should be interpreted in the context of its limitations. The retrospective nature of this study exposes it to patient recall bias, particularly when assessing bleeding events that may have been treated at outside institutions. Additionally, the sample size of this investigation was modest. Our a priori power calculation indicated that we were adequately powered to detect 30% differences in the occurrence of bleeding between the ADP-WARF and WARF groups. Significant differences were not observed using this categorization and level of clinical significance. Arguably, smaller differences in the occurrence of bleeding between groups may also be considered clinically significant and this study was not adequately powered to detect smaller effect sizes. The secondary analysis which further refined antidepressant exposure to SSRIs alone identified significant relationships to bleeding outcomes. It is possible that the outcomes observed when the sample was stratified by SSRI use represent type-I error. In the SSRI analysis, other clinical factors differed between those taking an SSRI and those who were not and were controlled for in our analyses. These factors included more females, a more patients reporting a GI bleed in the past, and more patients reporting a prior stroke in the SSRI group. It is well known that depression and antidepressant treatment are more common in women than in men,32, 33 that SSRIs are known to be associated with GI bleeding, 12, 34 and history of stroke notably increases the risk for depressed mood, which is commonly treated with SSRI antidepressants.35 However, our findings for any bleeding events over the course of treatment with an SSRI and warfarin were significant after controlling for these clinical factors.
Finally, medical records were used to acquire or verify much of the clinical information assessed in this study and thus, the data is subject to reporting error. Given these limitations, particularly the retrospective nature of the study, these results should be considered hypothesis generating and require confirmation.
Conclusions
The data presented herein suggest that use of an SSRI is associated with increased risk of bleeding in patients anticoagulated with warfarin and that patients requiring treatment with this medication combination may require more vigilant hematologic monitoring.
Table 2.
Minor bleeding events reported over 6-month data collection period
| Event | ADP-WARF (n=46) |
WARF (n=54) |
|---|---|---|
| Hemorrhoidal bleeding | 5 | 3 |
| Epistaxis | 9 | 5 |
| Prolonged bleeding time with cut | 2 | 3 |
| Genitourinary bleeding | 4 | 3 |
| Hematoma | 2 | 0 |
| Gum bleeding | 3 | 4 |
| Ear bleeding | 0 | 1 |
| Total number of minor bleeding events observed | 26 | 20 |
Acknowledgments
Sources of Support: This project was supported by the University of Illinois at Chicago (UIC) Center for Clinical Research Resources and Translational Science (CCTS), Award Number UL1RR029879 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Appendix
Calculation
Kuijer’s formula20: score = [1.63age] +[1.33sex] + [2.23 malignancy] Age >60, Gender, Malignancy, with ≥3 points high risk, 1–3 points intermediate risk, and 0 points low risk.
Beyth’s Outpatient Bleeding Risk Index19: 1 point for Age >65, History of stroke, History of GI bleeding, 1 or more following: recent MI; Hct <30%, SCr >1.5mg/dl, or DM] with 3–4 points high risk, 1–2 points intermediate risk, and 0 points low risk
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Faculty Disclosures: Dr. Bishop has grant support from Ortho-McNeil Janssen and an Honorarium from Eli Lilly and Company. Drs. Cochran, Cavallari, and Shapiro report no conflicts of interest to disclose.
Contributor Information
Kelly A. Cochran, University of Missouri-Kansas City School of Pharmacy.
Larisa H. Cavallari, Department of Pharmacy Practice, University of Illinois at Chicago College of Pharmacy.
Nancy L. Shapiro, Department of Pharmacy Practice, University of Illinois at Chicago College of Pharmacy.
Jeffrey R. Bishop, Department of Pharmacy Practice, University of Illinois at Chicago College of Pharmacy.
References
- 1.Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:257S–298S. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- 2.BMS. Coumadin Prescribing Information. Princeton, NJ, USA: Bristol-Meyers Squibb; 2010. [Google Scholar]
- 3.Dalton SO, Johansen C, Mellemkjaer L, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med. 2003;163:59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 4.de Abajo FJ, Rodriguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999;319:1106–1109. doi: 10.1136/bmj.319.7217.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meijer WE, Heerdink ER, Nolen WA, et al. Association of risk of abnormal bleeding with degree of serotonin reuptake inhibition by antidepressants. Arch Intern Med. 2004;164:2367–2370. doi: 10.1001/archinte.164.21.2367. [DOI] [PubMed] [Google Scholar]
- 6.Opatrny L, Delaney JA, Suissa S. Gastro-intestinal haemorrhage risks of selective serotonin receptor antagonist therapy: a new look. Br J Clin Pharmacol. 2008;66:76–81. doi: 10.1111/j.1365-2125.2008.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottervanger JP, Stricker BH, Huls J, et al. Bleeding attributed to the intake of paroxetine. Am J Psychiatry. 1994;151:781–782. doi: 10.1176/ajp.151.5.781. [DOI] [PubMed] [Google Scholar]
- 8.Sarma A, Horne MK., 3rd Venlafaxine-induced ecchymoses and impaired platelet aggregation. Eur J Haematol. 2006;77:533–537. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2919.x. [DOI] [PubMed] [Google Scholar]
- 9.Skop BP, Brown TM. Potential vascular and bleeding complications of treatment with selective serotonin reuptake inhibitors. Psychosomatics. 1996;37:12–16. doi: 10.1016/S0033-3182(96)71592-X. [DOI] [PubMed] [Google Scholar]
- 10.Tata LJ, Fortun PJ, Hubbard RB, et al. Does concurrent prescription of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs substantially increase the risk of upper gastrointestinal bleeding? Aliment Pharmacol Ther. 2005;22:175–181. doi: 10.1111/j.1365-2036.2005.02543.x. [DOI] [PubMed] [Google Scholar]
- 11.van Walraven C, Mamdani MM, Wells PS, et al. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ. 2001;323:655–658. doi: 10.1136/bmj.323.7314.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wessinger S, Kaplan M, Choi L, et al. Increased use of selective serotonin reuptake inhibitors in patients admitted with gastrointestinal haemorrhage: a multicentre retrospective analysis. Aliment Pharmacol Ther. 2006;23:937–944. doi: 10.1111/j.1365-2036.2006.02859.x. [DOI] [PubMed] [Google Scholar]
- 13.Andersohn F, Konzen C, Bronder E, et al. Citalopram-induced bleeding due to severe thrombocytopenia. Psychosomatics. 2009;50:297–298. doi: 10.1176/appi.psy.50.3.297. [DOI] [PubMed] [Google Scholar]
- 14.Abdelmalik N, Ruhe HG, Barwari K, et al. Effect of the selective serotonin reuptake inhibitor paroxetine on platelet function is modified by a SLC6A4 serotonin transporter polymorphism. J Thromb Haemost. 2008;6:2168–2174. doi: 10.1111/j.1538-7836.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- 15.Maurer-Spurej E, Pittendreigh C, Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost. 2004;91:119–128. doi: 10.1160/TH03-05-0330. [DOI] [PubMed] [Google Scholar]
- 16.Serebruany VL, Gurbel PA, O'Connor CM. Platelet inhibition by sertraline and N-desmethylsertraline: a possible missing link between depression, coronary events, and mortality benefits of selective serotonin reuptake inhibitors. Pharmacol Res. 2001;43:453–462. doi: 10.1006/phrs.2001.0817. [DOI] [PubMed] [Google Scholar]
- 17.Ataoglu A, Canan F. Mean platelet volume in patients with major depression: effect of escitalopram treatment. J Clin Psychopharmacol. 2009;29:368–371. doi: 10.1097/JCP.0b013e3181abdfd7. [DOI] [PubMed] [Google Scholar]
- 18.Schalekamp T, Klungel OH, Souverein PC, et al. Increased bleeding risk with concurrent use of selective serotonin reuptake inhibitors and coumarins. Arch Intern Med. 2008;168:180–185. doi: 10.1001/archinternmed.2007.32. [DOI] [PubMed] [Google Scholar]
- 19.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 20.Kuijer PM, Hutten BA, Prins MH, et al. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159:457–460. doi: 10.1001/archinte.159.5.457. [DOI] [PubMed] [Google Scholar]
- 21.Duncan D, Sayal K, McConnell H, et al. Antidepressant interactions with warfarin. Int Clin Psychopharmacol. 1998;13:87–94. doi: 10.1097/00004850-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Glueck CJ, Khalil Q, Winiarska M, et al. Interaction of duloxetine and warfarin causing severe elevation of international normalized ratio. Jama. 2006;295:1517–1518. doi: 10.1001/jama.295.13.1517. [DOI] [PubMed] [Google Scholar]
- 23.Hauta-Aho M, Tirkkonen T, Vahlberg T, et al. The effect of drug interactions on bleeding risk associated with warfarin therapy in hospitalized patients. Ann Med. 2009;41:619–628. doi: 10.1080/07853890903186168. [DOI] [PubMed] [Google Scholar]
- 24.Wallerstedt SM, Gleerup H, Sundstrom A, et al. Risk of clinically relevant bleeding in warfarin-treated patients--influence of SSRI treatment. Pharmacoepidemiol Drug Saf. 2009;18:412–416. doi: 10.1002/pds.1737. [DOI] [PubMed] [Google Scholar]
- 25.Kurdyak PA, Juurlink DN, Kopp A, et al. Antidepressants, warfarin, and the risk of hemorrhage. J Clin Psychopharmacol. 2005;25:561–564. doi: 10.1097/01.jcp.0000186869.67418.bc. [DOI] [PubMed] [Google Scholar]
- 26.Kharofa J, Sekar P, Haverbusch M, et al. Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke. 2007;38:3049–3051. doi: 10.1161/STROKEAHA.107.491472. [DOI] [PubMed] [Google Scholar]
- 27.Musselman DL, Marzec UM, Manatunga A, et al. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch Gen Psychiatry. 2000;57:875–882. doi: 10.1001/archpsyc.57.9.875. [DOI] [PubMed] [Google Scholar]
- 28.Kaminsky LS, de Morais SM, Faletto MB, et al. Correlation of human cytochrome P4502C substrate specificities with primary structure: warfarin as a probe. Mol Pharmacol. 1993;43:234–239. [PubMed] [Google Scholar]
- 29.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 30.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 31.Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;95:315–328. doi: 10.1016/0002-9343(93)90285-w. [DOI] [PubMed] [Google Scholar]
- 32.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 33.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 34.Targownik LE, Bolton JM, Metge CJ, et al. Selective serotonin reuptake inhibitors are associated with a modest increase in the risk of upper gastrointestinal bleeding. Am J Gastroenterol. 2009;104:1475–1482. doi: 10.1038/ajg.2009.128. [DOI] [PubMed] [Google Scholar]
- 35.Robinson RG, Spalletta G. Poststroke depression: a review. Can J Psychiatry. 55:341–349. doi: 10.1177/070674371005500602. [DOI] [PMC free article] [PubMed] [Google Scholar]