Abstract
Polyclonal antibodies have been generated by immunization of rabbits with a chemically synthesized C-terminal part of divercin V41 (DvnCt) conjugated to the carrier protein keyhole limpet hemocyanin (KLH). The sensitivity and reactivity of the DvnCt-KLH-generated antibodies were evaluated by enzyme-linked immunosorbent assay (ELISA) using supernatant from cultures of 13 representative lactic acid bacterium strains, and specificity was confirmed by Western blot analysis. Anti-DvnCt-KLH antibodies were able to recognize not only divercin V41 but also enterocin P and piscicocin V1b, two other members of the class IIa bacteriocins. Production and activity of DvnV41 were evaluated by ELISA and activity tests during the growth of Carnobacterium divergens V41 in MRS medium containing or not containing Tween 80. Divercin V41, enterocin P, and piscicocin V1b were therefore purified by a single-step immunoaffinity chromatography method using a Sepharose matrix CNBr-activated directed binding of anti-DvnCt-KLH polyclonal antibodies.
Many lactic acid bacteria (LAB) are known to secrete small, ribosomally synthesized antimicrobial peptides referred to as bacteriocins (20-22, 29, 32). Bacteriocins are of major interest to the food industry, as they can be used against pathogen and spoilage flora such as Brochothrix spp., Clostridium spp., Bacillus spp., and Staphylococcus spp. (11, 12, 20), and especially against food-borne Listeria monocytogenes, which is responsible for serious listeriosis outbreaks (15, 31). Four classes of bacteriocins have been defined on the basis of common characteristics, mainly structural ones (22). Various reports indicated that all class IIa bacteriocins have high-efficiency anti-Listeria activity. Among these classes, the class IIa bacteriocins contain the amino acid sequence YGNGV within their N-terminal regions (13, 22, 34) and are heat-stable small peptides (37 to 48 amino acids). Class IIa bacteriocins are promising candidates for industrial applications due to their high biological activity and their physicochemical properties (11, 14).
Increasing applications of class IIa bacteriocins as food preservatives could be facilitated by development and use of polyclonal antibodies generated against these antimicrobial peptides in sensitive and specific detection methods, such as immunoblotting and enzyme-linked immunosorbent assay (ELISA) (28).
Antibodies offer potential alternative methods of bacteriocin purification based on immunoaffinity strategies (37). Several reports describing generation of antibodies against class IIa bacteriocins were focused on pediocin (6, 7, 25, 26, 27, 28), while only one report dealt with enterocin A (27). Antibodies generated by immunization using the whole class IIa bacteriocin molecule either alone or conjugated to carriers (5, 7) have been scarcer than antibodies generated by using a chemically synthesized fragment derived from the C- or N-terminal region of the bacteriocin (25, 26, 27, 28). Our investigations are focused on divercin V41 as a model class IIa bacteriocin. It has been reported that divercin V41 is produced by Carnobacterium divergens V41 (33) and that the mature divercin V41 is a 43-amino-acid peptide with a molecular mass of 4,509 Da containing two disulfide bonds (30). The cleavage of divercin V41 by endoproteinase Asp-N releases an inactive hydrophilic N-terminal fragment and a hydrophobic C-terminal fragment active against Listeria monocytogenes (4). Recently, we demonstrated the role of divercin V41 in inhibition of Listeria monocytogenes in smoked salmon (35).
This paper describes the generation of polyclonal antibodies against a chemically synthesized C-terminal fragment of divercin V41. Once these antibodies were characterized, they were used to determine the production of divercin V41 during LAB growth in MRS medium containing or not containing Tween 80. In this work, we describe the first immunologically based method for the purification of class IIa bacteriocins. The technical approach developed here has been successfully used to purify divercin V41, enterocin P, and piscicocin V1b.
MATERIALS AND METHODS
Microorganisms, media, and bacteriocin assays.
The LAB strains used in this work are listed in Table 1. Except for Listeria, the microorganisms were propagated in MRS (De Man, Rogosa, Sharpe) medium (Biokar, Beauvais, France). For experimental needs, the MRST− medium (MRS without Tween 80) was prepared by assembling the individual components. Listeria was grown in Elliker broth (Biokar) for the agar diffusion test (ADT) or in brain heart infusion (BHI) broth (Biokar) for the microtiter plate assay (MPA). For specificity studies, microorganisms were propagated in MRST− at 30°C for 25 h. To carry out ADT and MPA, each culture was centrifuged at 10,000 × g at 4°C for 10 min and the resulting supernatant was heated at 100°C for 10 min and stored at −20°C until use. For immunoaffinity purification, C. divergens V41 and Enterococcus faecium P13 were grown in MRST− at 30°C for 25 h and Carnobacterium piscicola V1 was grown at 22°C for 48 h. The cultures were centrifuged at 10,000 × g at 4°C for 10 min, and the resulting supernatant was stored at 4°C.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Reference(s) or source |
|---|---|---|
| C. divergens | ||
| NCDO 2763 | Reference strain, not a bacteriocin producer | 19 |
| V41 | Divercin V41 producer | 33 |
| V41C9 | C. divergens V41 mutant; divV41− | 35 |
| C. piscicola V1 | Piscicocin V1a and V1b producer | 3, 33 |
| L. lactis IL1403 | Plasmid free, not a bacteriocin producer | 39 |
| L. lactis subsp. lactis ATCC 11454 | Nisin Z producer | 1 |
| P. acidilactici B5627 | Pediocin PA-1 producer | 2 |
| E. faecium | ||
| CTC492/T136 | Enterocin A and B producer | 10 |
| P13 | Enterocin P producer | 11 |
| Lactobacillus curvatus LTH11747 | Curvacin A producer | 38 |
| Lactobacillus sakei | ||
| MI401 | Bavaricin A producer | 23 |
| 706 | Sakacin A producer | 17 |
| Leuconostoc mesenteroides Y105 | Mesentericin Y105 producer | 16 |
| Listeria innocua F | Indicator organism | DSVa |
DSV, Direction des Services Vétérinaires, Nantes, France.
The ADT was performed as described by Pilet et al. (33). Briefly, 10-μl aliquots of twofold serial dilutions of supernatants were spotted on soft Elliker agar plates previously seeded with the indicator organism Listeria innocua F at 107 CFU/ml. The plates were incubated at 30°C for 16 h, allowing the growth of Listeria innocua F, and inhibition zones were detected by inspection. The titer (in arbitrary units per milliliter) was defined as the reciprocal of the lowest dilution that did not show inhibition.
The MPA was performed as described by Holo et al. (18). In this procedure, 50 μl of a twofold serial dilution (in BHI medium) of cell-free supernatant sample was added to 200 μl of a diluted (1:400 in BHI medium) fresh overnight culture of Listeria innocua F in the wells of a microtiter plate; growth inhibition was measured spectrophotometrically at 600 nm with a microtiter plate reader (Bio-Teck, Winooski, Vt.) after incubation at 30°C for 10 h. One bacteriocin unit (BU) was defined as the reciprocal of the dilution causing 50% growth inhibition (50% of the turbidity of the control culture without bacteriocin).
Preparation of immunoconjugates and immunization.
The amino acid sequence of the C-terminal fragment of divercin V41 (DvnCt) used for the generation of antibodies was NH2-DWGQASGCIGQTVVGGWLGGAIPGKC-COOH. Peptide DvnCt (residues 18 to 43 of divercin V41, 2,517.85 Da) was chemically synthesized and cyclized by formation of disulfide bond C8-C26. Cyclization was realized by slow oxidation of cysteine residues in Tris-dimethyl sulfoxide buffer. The purity of the peptide was monitored by reverse phase high-pressure liquid chromatography and was found to be higher than 95%; peptide identity was confirmed by mass spectrometry. Peptide dvnCt was then conjugated to keyhole limpet hemocyanin (KLH) by the glutaraldehyde method.
Two rabbits (New Zealand White) were immunized with DvnCt-KLH by the following scheme: (i) 500 μg of DvnCt-KLH in complete Freund's adjuvant (1:1) by intradermic injection on day 1, (ii) 500 μg in incomplete Freund's adjuvant (1:1) by subcutaneous injection at multiples sites on days 7 and 21, and (iii) 250 μg in incomplete Freund's adjuvant by subcutaneous injection on day 42. Rabbits were bled on days 35, 49, and 63. The conjugation, immunization, and bleeding processes were realized at Qbiogen Company.
ELISA.
Microtiter plates (Maxisorp; Nunc) were coated overnight at 37°C with 100 μl of culture supernatant diluted in 100 mM phosphate-buffered saline (PBS; pH 7.4). After this and each subsequent step, the coated microtiter wells were washed three times with PBS containing 0.05% (wt/vol) Tween 20 (PBS/T). Unoccupied sites in the wells were blocked by adding 250 μl of PBS/T containing 2% (wt/vol) freeze-dried low-fat milk (PBS/T/M) to each well and incubating at 37°C for 1 h. Each well was then filled with 100 μl of serum diluted 1:2,000 in PBS/T/M and incubated at 37°C for 90 min. Alkaline phosphate-conjugated goat anti-rabbit immunoglobulin G (IgG; A-8025; Sigma, St. Louis, Mo.) was diluted 1:3,000 in PBS/T/M; 100 μl was added to each well, and the plates were incubated at 37°C for 1 h. Bound antibodies were detected with 150 μl of p-nitrophenyl phosphate (N-2765; Sigma) per well at 1 mg/ml in 1 M Tris-HCl (pH 9.8). After incubation at 37°C for 30 min, the absorbance (405 nm) of each well was read with an automated ELISA reader. Samples were analyzed in duplicate, and each test was repeated twice.
For reactivity studies, plates were coated with culture supernatant dissolved in PBS at 2 mg of protein/ml as measured with the BCA protein assay reagent (kit 23225; Pierce, Rockford, Ill.). For the immunopurification sample, culture supernatants were initially diluted 1:10 in PBS. Flowthrough, wash, and eluate samples were used undiluted for coating plates.
Western blot analysis.
Proteins of cell-free supernatant were separated under reducing conditions, as described by Schagger and von Jagow (36); the gel system consisted of a 16.5% separating gel, a 10% spacer gel, and a stacking gel, each made with acrylamide-bisacrylamide at a 32:1 ratio. Each sample of supernatant was prepared by adding an equal volume of 2× loading buffer (250 mM Tris-HCl [pH 6.8], 2% [wt/vol] sodium dodecyl sulfate [SDS], 10% [vol/vol] glycerol, 10% [vol/vol] 2-mercaptoethanol, 0.01% [wt/vol] bromophenol blue). Thirty microliters was loaded into each well of the Tricine-SDS-polyacrylamide gel electrophoresis (PAGE) gel. After electrophoretic separation, half of the gel was stained with silver nitrate, as described by Blum et al. (8), and the proteins on the other half of the gel were transferred to nitrocellulose sheets (0.2-μm pore size; Sartorius) in Tris (25 mM)-SDS (0.1%, wt/vol)-glycine (192 mM)-ethanol (20%, vol/vol) buffer at 250 mA for 45 min. After transfer, nitrocellulose sheets were saturated with PBS containing 5% (wt/vol) freeze-dried low-fat milk for 1 h at room temperature. After three washes with PBS/T, the nitrocellulose sheets were incubated with anti-DvnCt-KLH serum diluted 1:50 in PBS for 1 h at room temperature. The nitrocellulose sheets were then washed three times with PBS/T and incubated for 1 h at room temperature with alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma; A-8025) diluted 1:3,000 in PBS. Finally, after three washes, the nitrocellulose sheets were equilibrated with 0.1 M Tris-HCl (pH 9.5) for 5 min, and substrate was added according to manufacturer recommendation (kit 170-6432; Bio-Rad, Hercules, Calif.). The reaction was stopped by adding 0.01 M acetic acid, and the sheets were then dried.
Immunoaffinity purification of the bacteriocins divercin V41, enterocin P, and piscicocin V1b.
Sera were purified by affinity chromatography using a protein A/G column (Pierce). Each purified IgG fraction was desalted and concentrated with 30-kDa-cutoff centrifugal concentrators (Millipore, Bedford, Mass.) and stored at −20°C. Purified antibodies were quantified by the method described by Lowry et al. (24) and the Rc Dc protein assay (Bio-Rad). The anti-DvnCt-KLH polyclonal antibodies were then coupled to 0.3 g of CNBr-activated Sepharose 4 Flow (Pharmacia, Amersham Biosciences) as recommended by the manufacturer. In this procedure, the matrix was washed 15 times with ice-cold 1 mM HCl and 5 times with coupling buffer (0.2 M NaHCO3, 0.5 M NaCl) and then 17 mg of anti-DvnCt-KLH antibodies dissolved in 0.5 ml of coupling buffer was added to 1 ml of matrix and incubated with gentle agitation at 4°C overnight. The remaining active groups were deactivated by five washes with 1 M ethanolamine and incubation at 4°C for 4 h. The matrix was then washed six times alternately between alkaline buffer (50 mM Tris, 1 M NaCl, pH 8) and acidic buffer (50 mM glycine, 1 M NaCl, pH 3.5). Finally, the matrix was washed with PBS, loaded in a column, and stored in 0.02% (wt/vol) sodium azide at 4°C. Coupling efficiency was determined by quantification of antibodies before and after coupling.
The performance of the immunocolumn was assessed by using 5 ml of culture supernatant. After application of each sample, the column was washed with 20 ml of PBS and bacteriocins were eluted by two 5-ml volumes of 6 M urea-0.1 M formic acid. All these steps were carried out at 6°C with 1-ml/min flow.
RESULTS AND DISCUSSION
Attempts to purify divercin V41 from C. divergens V41 as previously described (30) were unsuccessful since the amount of purified divercin V41 was not enough to be used as an immunogen. To overcome this situation, we have chemically synthesized DvnCt for use in the generation of polyclonal antibodies. The peptide of 26 amino acids generated was cyclized by the disulfide bond to have the same structural conformation of native bacteriocin, since this bond is necessary for bacteriocin activity (4). Production of antibodies against small peptides (such as the C-terminal part of divercin V41) requires enhancement of their immunogenicity by coupling these peptides to protein carriers. After formation of the disulfide bond, the peptide DvnCt was conjugated to KLH through the C-terminal cysteine group of the peptide, and the conjugate was used in the immunization of rabbits. In the present study, we have used KLH as the carrier protein because of its immunogenicity, and this strategy allowed an efficient detection of divercin V41 by the antipeptide antibodies generated.
After 63 days of the immunization process and four doses of the immunogen, the animals had apparent serum titers of 1:3,000 to 1:15,000 against the synthesized peptide DvnCt. The sensitivity of the anti-DvnCt-KLH antibodies for divercin V41 was then checked by ELISA (data not shown). Reactivity of anti-DvnCt-KLH antibodies against different antigens was tested. These antibodies displayed high recognition of KLH and purified divercin V41 (30), as well as of heated supernatant of a 25-h C. divergens V41 culture, whereas they did not show any recognition of samples in MRST− medium. The highest serum immunogen titers against C. divergens V41 culture supernatant were then used in this work.
Reactivity of anti-dvnCt-KLH antibodies with different bacteriocins.
The reactivity of polyclonal antibodies was tested against heated supernatants obtained from 25-h cultures of 13 representative LAB strains by ELISA (Table 2). Prior to ELISA, the presence or absence of bacteriocin activity in each supernatant was determined by the ADT using Listeria innocua F as the indicator organism. As expected, the anti-DvnCt-KLH antibodies showed a high reactivity with the supernatant from C. divergens V41 cultures, but no cross-reactivity was detected with supernatants from cultures of C. divergens V41C9 (divV41−), a mutant strain deficient in divercinV41 production (35). As noted previously (25), the use of mutant strains devoid of bacteriocin synthesis is useful in evaluating the specificity and immunoreactivity of polyclonal antipeptide antibodies, since the only difference between the wild-type and mutant strains is the presence or absence of bacteriocin activity in their supernatants.
TABLE 2.
Reactivity of anti-DvnCt-KLH serum polyclonal antibodies against culture supernatants of LAB as determined by ELISA
| Microorganism | Bacteriocin (class) | Cross- reactivity (%)a |
|---|---|---|
| P. acidilactici B5627 | Pediocin PA-1 (IIa) | 0.3 |
| C. divergens NCDO 2763 | 0 | |
| L. lactis subsp. lactis ATCC 11454 | Nisin Z (I) | 0 |
| L. lactis IL1403 | 0 | |
| C. divergens V41 | Divercin V41 (IIa) | 100 |
| C. divergens V41C9 | Not a divercin V41 producer | 0 |
| C. piscicola V1 | Piscicocin V1a (IIa) and V1b (IIa) | 34 |
| E. faecium P13 | Enterocin P (IIa) | 14.6 |
| E. faecium CTC492/T136 | Enterocin A (IIa) et B (II) | 0 |
| Lactobacillus curvatus LTH11743 | Curvacin A (IIa) | 0 |
| Lactobacillus sakei 706 | Sakacin A (IIa) | 0 |
| Leuconostoc mesenteroides Y105 | Mesentericin Y105 (IIa) | 0 |
| Lactobacillus sakei MI401 | Bavaricin A (IIa) | 0 |
Cross-reactivity is calculated as follows: (absorbance produced by a culture supernatant above the absorbance produced by MRST−/absorbance produced by the supernatant of C. divergens V41 above the absorbance produced by MRST) × 100.
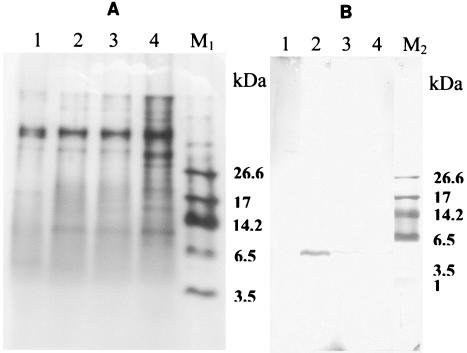
It is noteworthy that antibodies generated against divercin V41 were also able to cross-react with supernatants from C. piscicola V1 and E. faecium P13 cultures, with estimated percentages of cross-reactivity of 34 and 14.6%, respectively (Table 2). A very weak reaction with a specificity of 0.3% was obtained with supernatant from Pediococcus acidilactici B5627 (pediocin PA-1 producer) cultures, whereas no cross-reaction was observed with supernatants from Lactococcus lactis IL-1403 (not a bacteriocin producer), L. lactis subsp. lactis ATCC 11454 (nisin Z producer), E. faecium CTC492/T136 (enterocin A and B producer), Lactobacillus curvatus LTH11743 (curvacin A producer), Lactobacillus sakei 706 (sakacin A [curvacin A] producer), Leuconostoc mesenteroides Y105 (mesentericin Y105 producer), and Lactobacillus sakei MI401 (bavaricin A producer) cultures (Table 2). As expected, the anti-DvnCt-KLH antibodies did not cross-react with supernatant from C. divergens NCDO 2763, a reference strain that lacks bacteriocin activity. Furthermore, the cross-reactivity between the class IIa bacteriocins (pediocin, divercin V41, enterocin P, and piscicocin V1b) and anti-DvnCt-KLH-generated antibodies detected by ELISA was confirmed by Western analysis. As shown by silver staining of Tricine-SDS-PAGE gel (Fig. 1A), supernatants from 25-h cultures of P. acidilactici B5627 (lane 1), C. divergens V41 (lane 2), C. piscicola V1 (lane 3), and E. faecium P13 (lane 4) revealed the presence of many proteins, and the immunoblotting results (Fig. 1B) indicate that only one band per supernatant of each culture, divercin V41 (4,509 Da) (lane 2), piscicocin V1b (4,527 Da) (lane 3), and enterocin P (4,630 Da) (lane 4), was detected, while no band corresponding to piscicocin V1a (4,418 Da) (lane 3) was detected. Overall, these results demonstrate the specificity of generated antibodies. Despite a weak cross-reactivity observed in ELISA with supernatant from the P. acidilactici B5627 culture, the anti-DvnCt-KLH antibodies did not detect pediocin in the Western blot assay (Fig. 1B, lane 1).
FIG. 1.
Tricine SDS-PAGE and immunoblotting of culture supernatant from four class IIa LAB producer strains. (A) Silver-stained gel on which was loaded 25-h culture supernatants from P. acidilactici B5627 (lane 1), C. divergens V41 (lane 2), C. piscicola V1 (lane 3), and E. faecium P13 (lane 4). (B) Immunoblotting analysis of the gel. Lanes M1 (A) and M2 (B), stained Tricine SDS-PAGE gel and nitrocellulose, respectively, showing ultra-low-range molecular mass standards (M3546; Sigma).
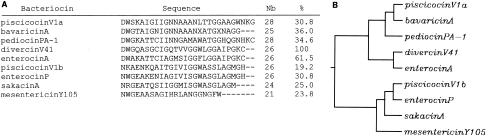
Previously, Martinez et al. (27) synthesized polyclonal antibodies against the C-terminal fragment of enterocin A, a class IIa bacteriocin produced by E. faecium T136. The analysis revealed that the antibodies were very specific since no cross-reactivity between the antipeptide polyclonal antibodies and class IIa bacteriocins other than enterocin A (e.g., sakacin P, sakacin A, enterocin P, and pediocin PA-1) was observed. According to these authors, the absence of cross-reaction is due to the high sequence diversity present in the C-terminal regions of class IIa bacteriocins tested. Homologies between C-terminal protein sequences of class IIa bacteriocins used in ELISA have been analyzed by multiple sequence alignment with the CLUSTALW program (Fig. 2A), and the resulting dendrogram showed that the C-terminal regions of the bacteriocins displaying cross-reactivity in ELISA (i.e., enterocin P and piscicocin V1b) are phylogenetically distant from that of divercin V41 (Fig. 2B). In contrast, the C-terminal region of enterocin A revealed high sequence similarity to that of divercin V41 (61.5%), whereas the DvnCt-KLH-generated antibodies did not react with supernatants derived from enterocin A producer E. faecium CTC492/T136. This difference in interaction between the antibodies and the bacteriocin could be ascribed to the structural conformation adopted by enterocin A, which might limit access of the antibodies to the epitope. Previous studies established that polyclonal antibodies generated against the C-terminal region of sakacin P and pediocin PA-1, class IIa bacteriocins produced by Lactobacillus sakei LTH 673 and P. acidilactici 347, were able to recognize pediocin PA-1 but not sakacin P, despite the 100% similarity between the sequences chosen for antibody production (27). These authors explained this reactivity by the preferential structure adopted by this part of the sakacin P molecule.
FIG. 2.
C-terminal sequence homologies of class IIa bacteriocins used in ELISA. Multiple alignment (A) and the generated dendrogram (B) were obtained by the CLUSTALW program (GenomeNet CLUSTALW of the Kyoto Center) for piscicocin V1a (piscicolin 126; gi|1346723), bavaricin A (gi|2493155), pediocin PA-1 (gi|548575), divercin V41 (gi|3849841), enterocin A (gi|7023945), piscicocin V1b (carnobacteriocin BM1; gi|584889), enterocin P (gi|2612870), sakacin A (curvacin A; gi|1363367), and mesentericin Y105 (gi|1709151). The accession number of each bacteriocin is in parentheses. The numbers of C-terminal amino acids aligned (Nb) and the percentages of homology between divercin V41 and other bacteriocins are shown.
Production measurement and bacteriocin activity of divercin V41 during growth in MRS and MRST−.
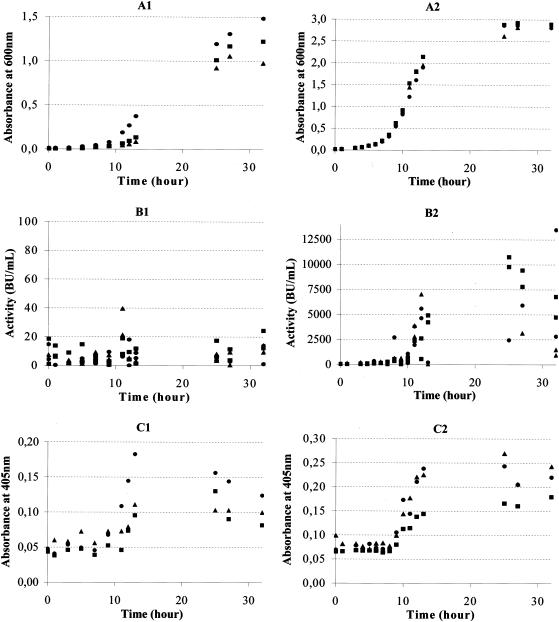
The growth of C. divergens V41 in both MRS and MRST− was monitored by measuring the absorbance at 600 nm. The growth experiments showed that the lag phase and logarithmic phase were reached two times more rapidly in MRST− (5 and 2.5 h, respectively) (Fig. 3A2) than in MRS (10 and 5 h, respectively) (Fig. 3A1) and that the optical density (OD) at the stationary phase was at least twofold higher when cells were grown in MRST− than when they were grown in MRS. Thus, the growth of C. divergens V41 in MRST− was more efficient than growth in MRS (Fig. 3A1 and A2). This is the first evidence of a correlation between cell growth and the presence of Tween 80 in the culture medium of C. divergens V41. As reported in Materials and Methods, bacteriocin activity was determined by MPA during growth of C. divergens V41 in MRS (Fig. 3B1) and MRST− (Fig. 3B2). The bacteriocin activity during the growth in MRS is negligible (<50 BU/ml) in comparison to that observed when C. divergens V41 is grown in MRST− (up to 11 × 103 BU/ml). The production of divercin V41 by C. divergens V41 grown in MRS (Fig. 3C1) and MRST− (Fig. 3C2) was measured by ELISA. Remarkably, the quantity of bacteriocin per unit of OD at 600 nm between 10 and 32 h of growth is twofold higher in MRST− than in MRS (data not shown); this difference could not be attributed to the difference in cell number (Fig. 3A1 and A2).
FIG. 3.
Growth, divercin V41 activity, and ELISA detection during growth of C. divergens V41 in MRS medium containing Tween 80 (MRS) or not containing Tween 80 (MRST−). Shown are growth experiments for C. divergens V41 in MRS (A1, B1, and C1) and MRST− (A2, B2, and C2). The growth was determined by measuring absorbance at 600 nm (A1 and A2). (B1 and B2) Bacteriocin activity obtained by MPA during growth of C. divergens V41 in MRS or in MRST−. (C1 and C2) Divercin V41 measurement by ELISA during growth of C. divergens V41 in MRS or in MRST−. The results were obtained from three independent cultures (▪, culture 1; •, culture 2; ▴, culture 3) of C. divergens V41 during 32 h.
In MRST−, bacteriocin activity started after 5 h of logarithmic growth and increased until the beginning of the stationary phase before starting to taper off. Production of divercin V41 in MRST− started earlier, just at the beginning of logarithmic phase (Fig. 3C2), while in MRS this production was delayed for 3 h (Fig. 3C1). The quantification of divercin V41 was carried out by ELISA during growth of C. divergens V41 in MRST−; the results indicated that production of divercin V41 started 8 h after the propagation of the strain, and the MPA enabled detection of an increase in bacteriocin activity at 11 h after inoculation. In MRS, the amount of divercin V41 seemed to increase during the logarithmic phase and decrease during the stationary phase, whereas in MRST− the quantity of divercin V41 seemed to increase until it reached a stable level. The increase in bacteriocin activity and in bacteriocin production observed during the C. divergens V41 logarithmic phase is in good agreement with previous observations made by Worobo et al. (40) and Quadri et al. (35). The observed decrease in bacteriocin activity in MRST− is not due to degradation of divercin V41, as its level appeared to be quite stable during stationary phase (Fig. 3B2 and C2). We speculate that the decrease of bacteriocin activity when cells were grown in MRS could be attributed to divercin V41 degradation or to a conformational modification of the epitope of this antimicrobial peptide (Fig. 3B1). This hypothesis is supported by our ELISA results (Fig. 3B2), which indicate a decrease in bacteriocin quantity during stationary phase. It should be noted that anti-DvnCt-KLH antibodies enabled, for the first time, the detection and the measurement of divercin V41 production during cell growth by ELISA. This approach could be extended to study production of other class IIa bacteriocins.
Immunoaffinity chromatography purification of divercin V41, enterocin P, and piscicocin V1b.
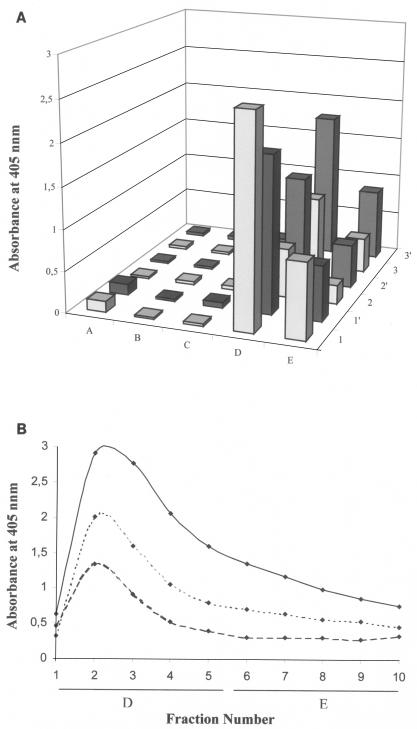
The supernatants of C. divergens V41, C. piscicola V1, and E. faecium P13 were loaded onto columns containing 1 ml of matrix coupled to 11 mg of anti-DvnCt-KLH polyclonal antibodies. Only trace amounts of bacteriocins were detected in flowthrough and wash fractions. The bacteriocins were largely retained by the columns and recovered in the first elution step (73.8, 72.1, and 67.3%, respectively), and the remainder was recovered in the second elution step (Fig. 4A). The bacteriocin activity of the elution fraction was evaluated by ADT, which revealed smaller levels of activity (21.5 × 103, 1.5 × 103, and 1.75 × 103 arbitrary units [AU]) than those detected in culture supernatants from C. divergens V41, C. piscicola V1, and E. faecium P13 (192.0 × 103, 16.0 × 103, and 4.0 × 103 AU, respectively). Elution fractions were collected in 1-ml aliquots, and the quantity and activity present in each aliquot were measured by ELISA and ADT. The purification of three antimicrobial peptides revealed the maximum of bacteriocin quantity (Fig. 4B) and activity (data not shown) in the second aliquot. The results of ELISA (Fig. 4A and B) confirmed the efficiency of the immunocolumn developed in this study for purification of divercin V41, piscicocin V1b, and enterocin P using polyclonal antibodies generated against divercin V41 despite a weak cross-reactivity to piscicocin V1b and enterocin P. The weak activity of the three bacteriocins detected in the elution fraction was due to glycine-hydrochloride buffer, which can affect their activities necessitating the use a soft elution buffer. Development of rapid purification methods for class IIa bacteriocins is of major interest, and to our knowledge this is the first report in which three bacteriocins of class IIa (divercin V41, enterocin P, and piscicocin V1b) have been purified by an immunological method.
FIG. 4.
(A) Results of ELISA for bacteriocin detection in supernatant diluted 1:10 in PBS (row A), flowthrough (row B), wash fluid (row C), and first (row D) and last (row E) 5-ml elution fractions during the immunoaffinity chromatography purification of divercin V41, piscicocin V1b, and enterocin P from culture supernatant of C. divergens V41 (rows 1 and 1′), C. piscicola V1 (rows 2 and 2′), and E. faecium P13 (rows 3 and 3′), respectively. (B) Bacteriocin in 1-ml aliquots was quantified during elution of divercin V41 (solid line), piscicocin V1b (dashed line), and enterocin P (dotted line). Note that each supernatant was immunopurified twice and that each sample was tested twice.
In this study, antibodies against divercin V41 were obtained by immunization of rabbits with a synthetic peptide designed from the C-terminal amino acid sequence of divercin V41. These anti-DvnCt antibodies recognized divercin V41, piscicocin V1b, and enterocin P, and immunopurification of these three bacteriocins was achieved
Anti-DvnCt antibodies showed the absence of correlation between quantity and activity of divercin V41 when C. divergens V41 was grown in MRS medium containing or not containing Tween 80. Furthermore, these antibodies could be used for immunolocalization of divercin V41 in bacterial strains and in foods in which this bacteriocin has been naturally produced or added (1, 9), and they may be applied to ELISA of divercin V41 in foods or used as a tool to study bacteriocin production and activity. Taken together all these results led us to conclude that these antibodies could be used for the rapid identification and isolation of strains producing divercin V41, enterocin P, or piscicocin V1b from many sources (2, 13).
Acknowledgments
C.R. is a recipient of Ph.D. scholar fellowship from Région des Pays de la Loire. This work was supported by the program of fundamental research in microbiology and infectious and parasitic diseases 2000 to 2002 (Ministère de la Jeunesse, de l'Education Nationale et de la Recherche) and by VANAM II (Région des Pays de la Loire).
We thank F. K. Vogensen, I. Nes, J. M. Berjeaud, and L. Cintas for providing strains used in this work. We are indebted to Fernando Sesma and S. Branda for critical reading and English improvement of the manuscript.
REFERENCES
- 1.Benech, R. O., E. E. Kheadr, C. Lacroix, and I. Fliss. 2002. Antibacterial activities of nisin Z encapsulated in liposomes or produced in situ by mixed culture during cheddar cheese ripening. Appl. Environ. Microbiol. 68:5607-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennik, M. H. J., E. J. Smid, and L. G. M. Gorris. 1997. Vegetable-associated Pediococcus parvulus produces pediocin PA-1. Appl. Environ. Microbiol. 63:2074-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhugaloo-Vial, P., X. Dousset, A. Metivier, O. Sorokine, P. Anglade, P. Boyaval, and D. Marion. 1996. Purification and amino acid sequences of piscicocins V1a and V1b, two class IIa bacteriocins secreted by Carnobacterium piscicola V1 that display significantly different levels of specific inhibitory activity. Appl. Environ. Microbiol. 62:4410-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhugaloo-Vial, P., J. P. Douliez, D. Moll, X. Dousset, P. Boyaval, and D. Marion. 1999. Delineation of key amino acid side chains and peptide domains for antimicrobial properties of divercin V41, a pediocin-like bacteriocin secreted by Carnobacterium divergens V41. Appl. Environ. Microbiol. 65:2895-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhunia, A. K., M. C. Johnson, B. Ray, and E. L. Belden. 1990. Antigenic property of pediocin AcH produced by Pediococcus acidilactici H. J. Appl. Bacteriol. 69:211-215. [DOI] [PubMed] [Google Scholar]
- 6.Bhunia, A. K., and M. G. Johnson. 1992. Monoclonal antibody-colony immunoblot method specific for isolation of Pediococcus acidilactici from foods and correlation with pediocin (bacteriocin) production. Appl. Environ. Microbiol. 58:2315-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhunia, A. K. 1994. Monoclonal antibody-based enzyme immunoassay for pediocins of Pediococcus acidilactici. Appl. Environ. Microbiol. 60:2692-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 9.Bouksaim, M., C. Lacroix, R. Bazin, and R. E. Simard. 1999. Production and utilization of polyclonal antibodies against nisin in an ELISA and for immuno-location of nisin in producing and sensitive bacterial strains. J. Appl. Microbiol. 87:500-510. [DOI] [PubMed] [Google Scholar]
- 10.Casaus, P., T. Nilsen, L. M. Cintas, I. F. Nes, P. E. Hernandez, and H. Holo. 1997. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology 143:2287-2294. [DOI] [PubMed] [Google Scholar]
- 11.Cintas, L. M., P. Casaus, L. S. Håvarstein, P. E. Hernández, and I. F. Nes. 1997. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eijsink, V. G., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ennahar, S., D. Aoude-Werner, O. Sorokine, A. Van Dorsselaer, F. Bringel, J.-C. Hubert, and C. Hasselmann. 1996. Production of pediocin AcH by Lactobacillus plantarum WHE 92 isolated from cheese. Appl. Environ. Microbiol. 62:4381-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ennahar, S., K. Sonomoto, and A. Ishizaki. 1999. Class IIa bacteriocins from lactic acid bacteria: antibacterial activity and food preservation. J. Biosci. Bioeng. 87:705-716. [DOI] [PubMed] [Google Scholar]
- 15.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hechard, Y., B. Derijard, F. Letellier, and Y. Cenatiempo. 1992. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J. Gen. Microbiol. 138:2725-2731. [DOI] [PubMed] [Google Scholar]
- 17.Holck, A., L. Axelsson, S. E. Birkeland, T. Aukrust, and H. Blom. 1992. Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb706. J. Gen. Microbiol. 138:2715-2720. [DOI] [PubMed] [Google Scholar]
- 18.Holo, H., O. Nilssen, and I. F. Nes. 1991. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J. Bacteriol. 173:3879-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopzapfel, W. H., and E. S. Gerber. 1983. Lactobacillus divergens sp. nov., a new heterofermentative Lactobacillus species producing L(+) lactate. Syst. Appl. Microbiol. 4:522-534. [DOI] [PubMed] [Google Scholar]
- 20.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack, R. W., J. Wan, J. Gordon, K. Harmark, B. E. Davidson, A. J. Hillier, R. E. Wettenhall, M. W. Hickey, and M. J. Coventry. 1996. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl. Environ. Microbiol. 62:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 23.Larsen, A. G., F. K. Vogensen, and J. Josephsen. 1993. Antimicrobial activity of lactic acid bacteria isolated from sourdoughs: purification and characterization of bavaricin A, a bacteriocin produced by Lactobacillus bavaricus MI401. J. Appl. Bacteriol. 75:113-122. [DOI] [PubMed] [Google Scholar]
- 24.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 25.Martinez, J. M., M. I. Martinez, A. M. Suarez, C. Herranz, P. Casaus, L. M. Cintas, J. M. Rodriguez, and P. E. Hernandez. 1998. Generation of polyclonal antibodies of predetermined specificity against pediocin PA-1. Appl. Environ. Microbiol. 64:4536-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez, J. M., M. I. Martinez, C. Herranz, A. Suarez, M. F. Fernandez, L. M. Cintas, J. M. Rodriguez, and P. E. Hernandez. 1999. Antibodies to a synthetic 1-9-N-terminal amino acid fragment of mature pediocin PA-1: sensitivity and specificity for pediocin PA-1 and cross-reactivity against class IIa bacteriocins. Microbiology 145:2777-2787. [DOI] [PubMed] [Google Scholar]
- 27.Martinez, J. M., J. Kok, J. W. Sanders, and P. E. Hernandez. 2000. Heterologous coproduction of enterocin A and pediocin PA-1 by Lactococcus lactis: detection by specific peptide-directed antibodies. Appl. Environ. Microbiol. 66:3543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez, M. I., J. M. Rodriguez, A. Suarez, J. M. Martinez, J. I. Azcona, and P. E. Hernandez. 1997. Generation of polyclonal antibodies against a chemically synthesized N-terminal fragment of the bacteriocin pediocin PA-1. Lett. Appl. Microbiol. 24:488-492. [DOI] [PubMed] [Google Scholar]
- 29.Metivier, A., M. F. Pilet, X. Dousset, O. Sorokine, P. Anglade, M. Zagorec, J. C. Piard, D. Marion, Y. Cenatiempo, and C. Fremaux. 1998. Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergens V41: primary structure and genomic organization. Microbiology 144:2837-2844. [DOI] [PubMed] [Google Scholar]
- 30.Metivier, A., P. Boyaval, F. Duffes, X. Dousset, J. P. Compoint, and D. Marion. 2000. Triton X-114 phase partitioning for the isolation of a pediocin-like bacteriocin from Carnobacterium divergens. Lett. Appl. Microbiol. 30:42-46. [DOI] [PubMed] [Google Scholar]
- 31.Muriana, P. M. 1996. Bacteriocins for control of Listeria spp. in food. J. Food Prot. 56(Suppl.):54-63. [DOI] [PubMed] [Google Scholar]
- 32.Nes, I. F., D. B. Diep, L. S. Havarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 33.Pilet, M. F., X. Dousset, R. Barré, G. Novel, M. Desmazeaud, and J. C. Piard. 1995. Evidence for two bacteriocins produced by Carnobacterium piscicola and Carnobacterium divergens isolated from fish and active against Listeria monocytogenes. J. Food Prot. 58:256-262. [DOI] [PubMed] [Google Scholar]
- 34.Quadri, L. E., M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 269:12204-12211. [PubMed] [Google Scholar]
- 35.Richard, C., A. Brillet, M. F. Pilet, H. Prévost, and D. Drider. 2003. Evidence on inhibition of Listeria monocytogenes by divercin V41 action. Lett. Appl. Microbiol. 36:288-292. [DOI] [PubMed] [Google Scholar]
- 36.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 37.Suarez, A. M., J. I. Azcona, J. M. Rodriguez, B. Sanz, and P. E. Hernandez. 1997. One-step purification of nisin A by immunoaffinity chromatography. Appl. Environ. Microbiol. 63:4990-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tichaczek, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocins curavacin A from Lactobacillus curvatus LTH 1174 and sakacin P from L. sakei LTH673. Syst. Appl. Microbiol. 15:460-468. [Google Scholar]
- 39.Venema, K., M. H. Dost, P. A. Beun, A. J. Haandrikman, G. Venema, and J. Kok. 1996. The genes for secretion and maturation of lactococcins are located on the chromosome of Lactococcus lactis IL-1403. Appl. Environ. Microbiol. 62:1689-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worobo, R. W., T. Henkel, M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1994. Characteristics and genetic determinant of a hydrophobic peptide bacteriocin, carnobacteriocin A, produced by Carnobacterium piscicola LV17A. Microbiology 140:517-526. [DOI] [PubMed] [Google Scholar]