DndE is one of five essential proteins that are required for the DNA phosphorothioation process. However, its exact biochemical role in sulfur modification of DNA remains unclear. In this study, the DndE protein homologue from Salmonella enterica serovar Cerro 87 was overexpressed, purified and crystallized.
Keywords: DndE, DNA phosphorothioation, DNA sulfur modification, Salmonella enterica
Abstract
The phenomenon of DNA phosphorothioation (DNA sulfur modification) is widespread among prokaryotes and may serve as a mechanism to restrict gene transfer among bacteria. DndE is one of five essential proteins that are required for the DNA phosphorothioation process. However, its exact biochemical role in sulfur modification of DNA remains unclear. In this study, the DndE protein homologue from Salmonella enterica serovar Cerro 87 was overexpressed, purified and crystallized. The crystals of the DndE protein diffracted to 2.7 Å resolution and belonged to space group P3121. These results will facilitate detailed structural analysis of DndE and further elucidation of its biochemical function.
1. Introduction
Phosphorothioation (sulfur modification) of Streptomyces lividans 1326 genomic DNA causes the DNA to be degraded during electrophoresis (the DNA degradation phenotype; Dnd; Zhou et al., 1988 ▶, 2005 ▶). In the DNA phosphorothioation process a nonbridging O atom of the phosphate group in the DNA backbone is replaced by a sulfur. It has recently been found that exogenous sulfur-modified DNA can be cleaved in certain bacteria that harbour the type IV restriction endonuclease ScoMcrA, thus restricting gene transfer among bacteria (Liu et al., 2010 ▶).
Five proteins encoded by the dnd gene cluster are essential for the DNA sulfur-modification procedure (Zhou et al., 2005 ▶). Functional homologues of these five dnd genes have been found in various bacteria, including Salmonella enterica serovar Cerro 87 (Ou et al., 2009 ▶; Xu et al., 2010 ▶; Wang et al., 2011 ▶). Of these five proteins, DndA, DndC, DndD and DndE are required for DNA phosphorothioation to occur, while mutation of the dndB gene aggravates the Dnd phenotype (Liang et al., 2007 ▶; Xu et al., 2009 ▶), implying that DndB may function in determining the specificity of the phosphorothioation sites. DndA was characterized as a pyridoxal phosphate-containing cysteine desulfurase that specifically catalyzes the formation of l-alanine and elemental sulfur using l-cysteine as the substrate. DndC was found to contain a 4Fe–4S cluster and to have obvious ATP pyrophosphatase activity (You et al., 2007 ▶). A DndD homologue from Pseudomonas fluorescens Pf0-1, SpfD, has ATPase activity and presumably provides the energy required for stabilization of DNA secondary structure during the DNA phosphorothioation process (Yao et al., 2009 ▶). In contrast, the exact biochemical function of DndE remains unclear.
To understand the biochemical function of DndE, it would be valuable to determine its three-dimensional crystal structure. In this study, we overexpressed, purified and crystallized the DndE homologue from S. enterica serovar Cerro 87 (hereafter referred to as DndE; Ou et al., 2009 ▶; Xu et al., 2010 ▶). In addition, diffraction data were collected from DndE crystals and processed to 2.7 Å resolution. These results will provide a basis for the ultimate structure determination of DndE, which should shed light on the biochemical role of this essential protein in the DNA phosphorothioation process.
2. Materials and methods
2.1. Cloning and protein expression
DNA sequences encoding S. enterica serovar Cerro 87 DndE (a full-length construct with 117 residues in total and a construct consisting of residues 1–112) were amplified by PCR using the following primers: 5′-GAGACGGGGCATATGATGCTCCCGAATCGAATG-3′ (forward; NdeI restriction site indicated in bold), 5′-GCGTCAGCGGCCGCCTATATATTCTTTGAAAAATC-3′ (reverse; NotI restriction site indicated in bold) and 5′-TTCTTACTCGAGTCATCTTTTAAGCTTCG-3′ (reverse; XhoI restriction site indicated in bold). The full-length DndE insert was cloned into the NdeI and NotI sites of the expression vector pET28a (Novagen) and the DndE(1–112) insert was cloned into the NdeI and XhoI sites of pET28a using standard molecular-cloning procedures. Successfully cloned plasmids were verified by sequencing. Both DndE protein constructs were expressed as N-terminally His-tagged proteins in E. coli strain BL21 (DE3). When the OD600 reached 0.6–0.8, the bacterial culture was induced with 0.2 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and grown for a further 8 h at 298 K (or overnight at 293 K). The bacterial cells were then harvested by centrifugation at 2 500g for 20 min.
2.2. Protein purification
The cell pellets were homogenized in buffer A (25 mM Tris–HCl pH 8.0, 300 mM NaCl, 20 mM imidazole), which was supplemented with 1 µg ml−1 aprotinin, 1 µg ml−1 leupeptin, 30 µg ml−1 lysozyme and 0.05 mM phenylmethylsulfonyl fluoride (PMSF), and lysed by sonication. Cell debris was removed by centrifugation at 15 000g for 30 min at 277 K. The supernatant was passed through a nickel-affinity column (GE Healthcare) pre-equilibrated with 50 column volumes of buffer A. The column was then washed with a further 50 column volumes of buffer A and five column volumes of buffer B (25 mM Tris–HCl pH 8.0, 300 mM NaCl, 100 mM imidazole). The target protein was eluted with buffer C (25 mM Tris–HCl pH 8.0, 300 mM NaCl, 500 mM imidazole) and was further purified by gel-filtration chromatography using a Superdex 200 10/300 GL column (GE Healthcare) pre-equilibrated with 10 mM Tris–HCl pH 8.0, 100 mM NaCl, 2 mM dithiothreitol (DTT). The purity of the protein was verified by SDS–PAGE and Coomassie Blue staining. The purified protein from Superdex 200 gel-filtration chromatography was concentrated to 20 mg ml−1, cooled in liquid nitrogen and stored at 193 K until use.
2.3. Crystallization
Crystallization trials for full-length DndE and DndE(1–112) were performed at 287 K using the hanging-drop vapour-diffusion method in 48-well plates. Typically, 1 µl reservoir solution was mixed with 1 µl protein solution and equilibrated against 80 µl reservoir solution. Initial crystallization screening trials were performed using Crystal Screen, Index, PEG/Ion and SaltRx kits from Hampton Research. After two weeks, small crystals of DndE(1–112) were obtained from condition No. 1 of the SaltRx kit, which consists of 1.8 M sodium acetate pH 7.0 and 0.1 M bis-tris propane pH 7.0. Over 20 different buffers were screened as possible replacements for the 0.1 M bis-tris propane pH 7.0 found in the initial hit; longer and thicker crystals were obtained by changing the buffer component to 0.1 M sodium cacodylate pH 6.0. After further optimization, diffracting crystals were obtained with 1.8 M sodium acetate pH 7.0 and 0.1 M sodium cacodylate pH 5.8, still using the hanging-drop vapour-diffusion method in 48-well plates at 287 K.
2.4. Data collection
Prior to data collection, DndE(1–112) protein crystals were flash-cooled in liquid nitrogen and tested on an in-house X-ray generator at Shanghai Institute of Organic Chemistry. The crystals were cryoprotected by transferring them in cryoloops into a cryoprotectant, which consisted of reservoir solution supplemented with 30%(v/v) glycerol, replacing the water in the crystallant with cryoprotectant. A complete diffraction data set was collected on beamline BL17U1 at Shanghai Synchrotron Radiation Facility (People’s Republic of China). The data set was collected at a wavelength of 0.9792 Å and was processed to 2.7 Å resolution. Intensity data were integrated and scaled using the HKL-2000 software (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
Recombinant full-length DndE was overexpressed as an N-terminally His-tagged protein, purified by Ni2+-affinity chromatography and gel-filtration chromatography and subjected to extensive crystallization screening. Despite considerable effort, only clusters of needle-shaped crystals were obtained (not shown) which were not suitable for further X-ray diffraction analysis. A careful examination of the alignment of 32 DndE homologues (http://mml.sjtu.edu.cn/dndDB/; Ou et al., 2009 ▶) showed that the last five residues of DndE are not as conserved as the other parts of the protein. Therefore, a construct of DndE (residues 1–112) was produced in which the last five residues of DndE were omitted. The DndE(1–112) protein was then overexpressed and purified using the same procedure as used for full-length DndE (Fig. 1 ▶). Crystallization screening and further optimization of the crystallization conditions yielded rod-shaped crystals (Fig. 2 ▶) with a thickness that was much improved compared with the crystals of full-length DndE protein.
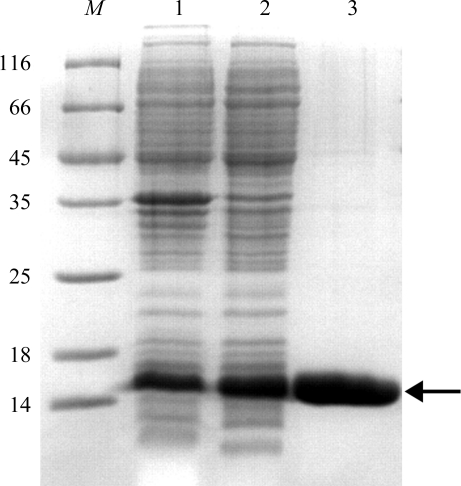
Figure 1.
Purification of the DndE(1–112) protein. Protein samples were analyzed using 15% SDS–PAGE followed by Coomassie Blue staining. Lane M, protein molecular-weight marker (labelled in kDa); lane 1, whole cell lysate after sonication; lane 2, supernatant after centrifugation; lane 3, finally purified protein from gel-filtration chromatography. The arrow indicates the band corresponding to DndE(1–112) protein.
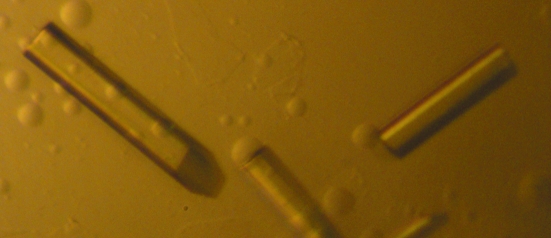
Figure 2.
Crystals of DndE(1–112) protein. The maximum dimensions of the crystals are about 0.25 × 0.03 × 0.03 mm.
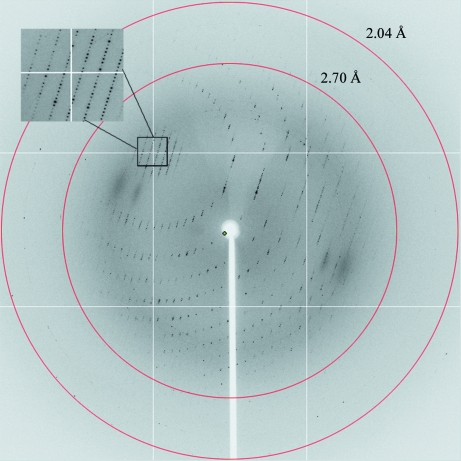
Crystals of the DndE(1–112) protein diffracted to 2.7 Å resolution (Fig. 3 ▶) and belonged to the trigonal space group P3121, with unit-cell parameters a = 72.2, b = 72.2, c = 260.4 Å. Diffraction data were collected and processed (Table 1 ▶) with a final R merge value of 8.2% (32.6% for the highest resolution shell). The data completeness, data multiplicity and average I/σ(I) value of the collected data set were 100.0%, 11.6 and 29.3, respectively (100.0%, 11.6 and 7.9, respectively, for the highest resolution shell).
Figure 3.
A typical X-ray diffraction pattern of the DndE(1–112) protein crystal. The crystal diffraction data set was processed to 2.7 Å resolution using the HKL-2000 software (Otwinowski & Minor, 1997 ▶).
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution shell.
| Beamline | BL17U1 |
| Wavelength (Å) | 0.9792 |
| Crystal-to-detector distance (mm) | 300 |
| Oscillation range per frame (°) | 1 |
| Images taken | 360 |
| Resolution (Å) | 50.0–2.7 (2.8–2.7) |
| Space group | P3121 |
| Mosaicity (°) | 0.349 |
| Unit-cell parameters (Å) | a = b = 72.2, c = 260.4 |
| No. of molecules per asymmetric unit | 6 |
| Matthews coefficient (Å3 Da−1) | 2.48 |
| Solvent content (%) | 50.4 |
| No. of observed reflections | 482075 |
| No. of unique reflections | 41581 |
| Completeness (%) | 100.0 (100.0) |
| Multiplicity | 11.6 (11.6) |
| Average I/σ(I) | 29.3 (7.9) |
| Rmerge† (%) | 8.2 (32.6) |
R
merge = 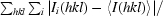
 , where I
i(hkl) is the intensity of observation i of reflection hkl.
, where I
i(hkl) is the intensity of observation i of reflection hkl.
Based on consideration of the Matthews coefficient, the most probable number of DndE(1–112) molecules in the asymmetric unit is six. In this case the Matthews coefficient is 2.48 Å3 Da−1, corresponding to a solvent content of 50.4%. Crystallization of selenomethionine-derivatized DndE(1–112) protein is in progress, which will be used for multi-wavelength anomalous diffraction (MAD) data collection. Structural determination of DndE will provide new insights into the biochemical role of this essential protein in the DNA phosphorothioation process.
Acknowledgments
We thank Dr Jiahai Zhou (Shanghai Institute of Organic Chemistry) for assistance in the screening for diffracting crystals. We also thank Dr Jianhua He, Dr Sheng Huang and the other members of the staff of beamline BL17U1 at Shanghai Synchrotron Radiation Facility (China) for assistance during data collection. This work was supported by grants from the National Natural Science Foundation of China (grant Nos. 30900225, 30821005 and 90919021), the Innovation Program of Shanghai Municipal Education Commission (grant No. 09ZZ23), the ‘Dawn’ Program of Shanghai Education Commission (grant No. 08SG11), the Shanghai Pujiang Program (grant No. 09PJ1405500), the Key Project of the Chinese Ministry of Education (grant No. 109060), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, the National Comprehensive Technology Platforms for Innovative Drug R&D (2009ZX09301-007) and the State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry (Shanghai, China).
References
- Liang, J., Wang, Z., He, X., Li, J., Zhou, X. & Deng, Z. (2007). Nucleic Acids Res. 35, 2944–2954. [DOI] [PMC free article] [PubMed]
- Liu, G., Ou, H.-Y., Wang, T., Li, L., Tan, H., Zhou, X., Rajakumar, K., Deng, Z. & He, X. (2010). PLoS Genet. 6, e1001253. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Ou, H.-Y., He, X., Shao, Y., Tai, C., Rajakumar, K. & Deng, Z. (2009). PLoS One, 4, e5132. [DOI] [PMC free article] [PubMed]
- Wang, L., Chen, S., Vergin, K. L., Giovannoni, S. J., Chan, S. W., DeMott, M. S., Taghizadeh, K., Cordero, O. X., Cutler, M., Timberlake, S., Alm, E. J., Polz, M. F., Pinhassi, J., Deng, Z. & Dedon, P. C. (2011). Proc. Natl Acad. Sci. USA, 108, 2963–2968. [DOI] [PMC free article] [PubMed]
- Xu, T., Liang, J., Chen, S., Wang, L., He, X., You, D., Wang, Z., Li, A., Xu, Z., Zhou, X. & Deng, Z. (2009). BMC Microbiol. 9, 41. [DOI] [PMC free article] [PubMed]
- Xu, T., Yao, F., Zhou, X., Deng, Z. & You, D. (2010). Nucleic Acids Res. 38, 7133–7141. [DOI] [PMC free article] [PubMed]
- Yao, F., Xu, T., Zhou, X., Deng, Z. & You, D. (2009). FEBS Lett. 583, 729–733. [DOI] [PubMed]
- You, D., Wang, L., Yao, F., Zhou, X. & Deng, Z. (2007). Biochemistry, 46, 6126–6133. [DOI] [PubMed]
- Zhou, X., Deng, Z., Firmin, J. L., Hopwood, D. A. & Kieser, T. (1988). Nucleic Acids Res. 16, 4341–4352. [DOI] [PMC free article] [PubMed]
- Zhou, X., He, X., Liang, J., Li, A., Xu, T., Kieser, T., Helmann, J. D. & Deng, Z. (2005). Mol. Microbiol. 57, 1428–1438. [DOI] [PubMed]