Abstract
φ-0303 is a temperate bacteriophage isolated from Lactobacillus helveticus CNRZ 303 strain after mitomycin C induction. In this work, the gene coding for a lytic protein of this bacteriophage was cloned using a library of φ-0303 in Escherichia coli DH5α. The lytic activity was detected by its expression, using whole cells of the sensitive strain L. helveticus CNRZ 892 as the substrate. The lysin gene was within a 4.1-kb DNA fragment of φ-0303 containing six open reading frames (ORFs) and two truncated ORFs. No sequence homology with holin genes was found within the cloned fragment. An integrase-encoding gene was also present in the fragment, but it was transcribed in a direction opposite that of the lysin gene. The lysin-encoding lys gene was verified by PCR amplification from the total phage DNA and subcloned. The lys gene is a 1,122-bp sequence encoding a protein of 373 amino acids (Mur-LH), whose product had a deduced molecular mass of 40,207 Da. Comparisons with sequences in sequence databases showed homology with numerous endolysins of other bacteriophages. Mur-LH was expressed in E. coli BL21, and by renaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis with L. helveticus CNRZ 892 as the substrate, the recombinant protein showed an apparent molecular mass of 40 kDa. The N-terminal sequence of the protein confirmed the start codon. Hydrolysis of cell walls of L. helveticus CNRZ 303 by the endolysin and biochemical analysis of the residues produced demonstrated that Mur-LH has N-acetylmuramidase activity. Last, the endolysin exhibited a broad spectrum of lytic activity, as it was active on different species, mainly thermophilic lactobacilli but also lactococci, pediococci, Bacillus subtilis, Brevibacterium linens, and Enterococcus faecium.
The phenomenon of lysis in fermentation industries, especially the dairy industry, is very significant. Two mechanisms can be involved: either an autolysin, which is a bacterial enzyme capable of degrading cell wall peptidoglycan under specific conditions, or a bacteriophage (virulent or temperate). In the latter case, the last step of lysis involves two proteins: a holin, which is a transmembrane protein forming pores in the cytoplasmic membrane, and a lysin (endolysin), which hydrolyzes the peptidoglycan of the cell wall, reaching its substrate as a result of the pores formed. Some genes encoding phage endolysins have been characterized in lactic acid bacteria (LAB) (for a pre-1996 review, see reference 26). The available data concern the endolysins of lactococcal bacteriophages φ-vML3 (31, 32), φ-c2 (40), φ-LC3 (2), φ-Tuc2009 (1), POO1 (14), and φ10MC, a temperate bacteriophage of Oenococcus oeni (11). Regarding the bacteriophages of lactobacilli, the endolysins of phage LL-H of Lactobacillus delbrueckii (38), phages φ-gle (16) and SC921 (41) of Lactobacillus plantarum, phage mv1 of Lactobacillus bulgaricus (3), phage PL-1 of Lactobacillus casei (17), and phage φadh of Lactobacillus gasseri (13) have been studied. The biochemical activity has been clearly demonstrated for only a few of these enzymes (17, 38); sequence homologies suggest activity for the others. The endolysins of LAB have been shown to be organized into two domains: the N-terminal domain, which is responsible for the catalytic activity of the protein, and the C-terminal domain, which is responsible for the binding to the substrate.
Lactobacillus helveticus is a species commonly used as starter for the production of Swiss-type cheeses. Up to now, no molecular work has been done to elucidate the working of the endolysin-encoding bacteriophage genes. The φ-0303 phage is a temperate phage of L. helveticus CNRZ 303 that can be induced after mitomycin C treatment of the strain or during the manufacture of Swiss-type cheese (9). It has an isometric head and a tail with a contractile sheath and belongs to Bradley group A (4) or to the Myoviridae family of the International Committee on Taxonomy of Viruses (24). It also belongs to the most important group of phages described in L. helveticus: the short-tailed group (30). Its DNA is 39.9 kb long (10). In this work, we describe the cloning of the φ-0303 phage lysin gene and its nucleotide sequence, as well as the spectrum of activity and specificity of the recombinant protein.
MATERIALS AND METHODS
Phages and bacterial strains.
The φ-0303 phage was obtained after mitomycin C induction of the L. helveticus CNRZ 303 strain. Induction, amplification, and purification methods have been previously described (10). Several Lactobacillus strains were propagated in MRS broth (Difco, Sparks, Md.). The Lactobacillus strains were L. helveticus, L. acidophilus, L. delbrueckii subsp. bulgaricus, L. delbrueckii subsp. lactis, L. casei, L. paracasei subsp. paracasei, L. fermentum, and L. brevis strains. L. brevis, L. casei, and L. paracasei were grown at 37 or 30°C. Pediococcus acidilactici and Leuconostoc lactis strains were propagated at 37 and 25°C, respectively, in MRS broth. Streptococcus thermophilus and Lactococcus lactis strains were propagated in M17 broth (Difco) at 43 and 30°C, respectively. Propionibacterium freudenreichii and Propionibacterium jensenii strains were grown in YEL broth (23) at 30°C. Enterococcus faecalis, Enterococcus faecium, and Salmonella enterica subsp. enterica serovar Abortusovis were propagated in Trypticase soy broth (TSB) (Difco) at 37°C. Pseudomonas fluorescens, Bacillus subtilis, Brevibacterium linens, and Listeria innocua were propagated in TSB at 30°C, under aerobic conditions with shaking (250 rpm). Clostridium sporogenes was propagated in RCM broth (Difco) at 37°C under anaerobic conditions. Escherichia coli strains DH5α, BL21, and CIP 61.11 were grown in Luria-Bertani (LB) medium, broth, or agar at 37°C under aerobic conditions with shaking (250 rpm), as previously described (28). Ampicillin and kanamycin (Eurobio, Les Ulis, France) were added to the LB medium when specified, at a final concentration of 50 μg/ml. For the selection of recombinant plasmids of E. coli, isopropyl-β-d-thiogalactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were added when specified at concentrations of 0.5 mM and 80 μg/ml, respectively.
DNA manipulation.
All DNA manipulations were performed by standard methods (28). Restriction enzymes, T4 DNA ligase (Promega, Madison, Wis.), and calf intestinal alkaline phosphatase (Boehringer, Mannheim, Germany) were used according to the supplier's recommendations.
Identification of a phage endolysin-encoding gene.
A total EcoRI digestion of the φ-0303 phage DNA was performed. With T4 DNA ligase, the resulting fragments were cloned into the EcoRI-digested pOB vector (15) treated with alkaline phosphatase to prevent self-ligation. The resulting plasmids were transformed into competent cells of E. coli DH5α, prepared by the RbCl method (28), and selected on LB medium containing ampicillin. All clones were screened for the presence of lytic activity. The clones were grown in duplicate on LB agar with ampicillin and on LB agar with ampicillin containing a concentrated suspension of autoclaved L. helveticus CNRZ 892 cells (opaque plates) (see below). After incubation at 37°C overnight, the opaque plates were treated with chloroform vapor (30 min at room temperature), which permeabilized the clones (releasing the intracellular material). After a subsequent 2-h incubation at 37°C, one lysin-producing clone was identified by a zone of clearing around the colony. The corresponding clone on the non-chloroform-treated plate was then grown on LB medium until the optical density at 600 nm (OD600) reached 0.6. The plasmid in the clone was prepared using a QIAprep Spin Plasmid Miniprep kit (Qiagen, Hilden, Germany), and the insert was characterized by EcoRI digestion and sequenced (fragment of 4.1 kb sequenced by MWG Biotech, Ebersberg, Germany).
Subcloning of the endolysin-encoding region.
Using the 4.1-kb phage DNA fragment sequence obtained above, two primers were specifically designed to amplify the lysin gene region, which included cut sites for restriction enzymes EcoRI and BamHI, respectively (underlined sequences): 5′-GCGGATCCGGAAGTGGCCACAGGTATG-3′ and 5′-GCGAATTCGACAGTGTTGCACAAATG-3′. These primers were used in a PCR with total φ-0303 phage DNA as the template. The 1.5-kb product was sequenced on both strands by MWG Biotech. The sequence was identical to that obtained with the cloned 4.1-kb fragment. The 1.5-kb amplified fragment was subcloned into the EcoRI and BamHI sites of the pET-24d(+) vector (Novagen, Nottingham, United Kingdom). The recombinant plasmid was named pET-(lys). The presence of the insert in this vector was verified by digestion of pET-(lys) with EcoRI and BamHI.
Preparation of a crude endolysin extract.
E. coli BL21 containing the recombinant plasmid pET-(lys) was grown in LB medium containing ampicillin until the OD600 reached 0.6. Two milliliters of this culture was diluted to 200 ml with fresh LB medium and grown with shaking at 37°C until the OD600 reached 1. The culture was harvested (8,000 × g, 10 min, 4°C), and the cells were washed in sterile water and resuspended in 5 ml of sterile water at 4°C. The activity of this suspension was tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and zymograms. For activity assays in petri dishes (see below), the suspension was sonicated (1 min, 4°C) and centrifuged (8,000 × g, 10 min, 4°C), and the supernatant was stored at −20°C until use (crude endolysin extract). Crude E. coli BL21/pET extracts were prepared under the same conditions.
Preparation of substrates (concentrated cell suspension) to test endolysin lytic spectrum.
The cells of different species were grown on the appropriate growth medium, as previously described, until an OD600 of 1 was achieved. The culture was then centrifuged (8,000 × g, 4°C, 10 min), and the cells were washed three times with sterile water at 4°C (resuspension and centrifugation as described above). The pellet was resuspended in water (1% [vol/vol] of initial culture volume) and homogenized by vortexing in the presence of sterile glass beads. The suspension was autoclaved (115°C, 15 min) after removal of glass beads and stored at 4°C until use.
SDS-PAGE and zymogram (renaturing SDS-PAGE).
SDS-PAGE was performed by the method of Laemmli (18), with a Mini Protean II cell unit system (Bio-Rad Laboratories, Inc., Hercules, Calif.). All gels contained 12% (wt/vol) acrylamide and were stained with Coomassie brilliant blue R-250. Zymograms were performed by the method of Leclerc and Asselin (19). Samples were mixed 1:1 (vol/vol) with sample buffer (62.5 mM Tris-HCl [pH 6.8] containing 25% [vol/vol] glycerol, 2% [wt/vol] SDS, and 10 mg of dithiothreitol per ml) and boiled for 2 min. Samples were loaded on a 12% (wt/vol) polyacrylamide separating gel (pH 8.8) containing 200 μl of concentrated cell suspension (as described above) of L. helveticus CNRZ 892. After electrophoresis (1 h at a constant voltage of 180 V and at room temperature), the gels were soaked for 30 min in distilled water at room temperature. They were then transferred into a 50 mM Tris-HCl buffer (pH 7.4) containing 1% (vol/vol) Triton X-100 and gently shaken for 12 h at 37°C to renature the enzymes. Lytic activities appeared as translucent bands on the opaque background. In order to determine the apparent molecular mass, prestained protein standards (Pharmacia) were used.
Determination of the lytic spectrum of the protein produced.
A lytic assay to determine the lytic spectrum of the protein produced was developed. LB agar plates containing autoclaved concentrated cell suspensions (2% vol/vol) (see above) were prepared. The presence of a cell suspension in the medium caused the agar to appear opaque. A well with a diameter of 0.5cm was cut into the agar, and 50 μl of the crude endolysin extract (preparation described earlier) thawed at 4°C was added into the well. After incubation (24 h at 37°C), a clear halo around the well indicated lysis. The crude E. coli BL21/pET extract was prepared, tested in the same manner, and used as a control.
Determination of the enzymatic specificity of the endolysin. (i) Preparation of cell walls of L. helveticus CNRZ 303 by mechanical disruption.
The cells from 1 liter of a culture of L. helveticus CNRZ 303 (OD600 of 1) were harvested by centrifugation (8,000 × g, 15 min, 4°C). The pellet was resuspended in distilled water at 4°C (10% [vol/vol] of the initial culture volume), and this suspension was broken in a pilot homogenizer (Standsted Fluid Power, Stansted, United Kingdom) at a pressure of 100 MPa, as previously described (27). After mechanical disruption, the suspension was centrifuged (30,000 × g, 20 min, 4°C). The pellet containing the cell walls was washed three times with sterile water at 4°C and resuspended in a 25 mM Tris-HCl buffer (pH 8) to a final OD600 of 1 (a concentration of 1.9 mg [dry weight] per ml resulted in an OD600 of 1). The cell wall suspension was then maintained at −20°C until use.
(ii) Lysis of the cell walls by the endolysin.
In order to inactivate the endogenous bacterial endolysins, the cell walls were thawed at 4°C and heated for 1 h at 100°C. After the cell walls were allowed to cool, the crude endolysin extract thawed at 4°C was added (1:15 [vol/vol]). The mixture was incubated at 40°C, and the decrease in absorbance (600 nm) was measured. At different times, 1-ml samples were removed (crude samples) and concentrated by speed-vacuum treatment before chemical analysis. A control was prepared using a crude E. coli BL21/pET extract.
(iii) Chemical analysis.
In order to quantify bacterial cell wall degradation, the generation of free reducing groups was monitored as previously described (35). The reducing groups liberated were characterized by the methods of Ward et al. (39) and Lemée et al. (20) with the following modifications. The concentrated crude samples were resuspended in 100 μl of distilled water, reduced with 100 μl of sodium borohydride at a concentration of 10 mg/ml in a Na2B4O7 solution (0.05 M, pH 9), and incubated for 8 h at 4°C. After reduction, the samples were hydrolyzed with 5.7 N HCl at 95°C for 16 h. The acid was removed by repeated evaporation of distilled water in a vacuum, and the samples were resuspended in 0.2 M sodium citrate buffer (pH 2.2). Glucosamine, muramic acid, and glucosaminitol contents were determined by applying the samples to a column filled with polystyrene divinyl benzene resin type Ultropac 7 (Pharmacia, Orsay, France). The column temperature was kept at 52°C. Two buffer solutions were added sequentially to the column: buffers A and B, containing 14.7 g of sodium citrate per liter, with pHs of 3 and 4.25, respectively, followed by regeneration with 0.4 M NaOH and then reequilibration with buffer A. The analysis time was 82 min. Muramicitol content was detected under the same conditions, except that buffer A consisted of 19.6 g of sodium citrate (pH 3.0) per liter. The compounds were detected at 570 nm after reaction with ninhydrin for 2.5 min at 135°C as previously described (34). Each amino acid and hexosamine was calibrated individually. Amino acid standards were from Sigma (Saint-Quentin Fallavier, France), and muramicitol and glucosaminitol standards were prepared by reducing muramic acid and glucosamine (Sigma) by the method of Hara et al. (12).
Nucleotide sequence accession number.
The nucleotide sequence of the lys gene encoding endolysin has been deposited in GenBank under the accession number AF495798.
RESULTS
Cloning of a phage endolysin-encoding gene, lys.
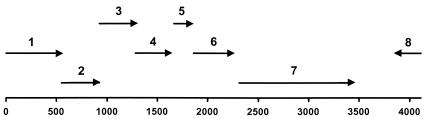
A total of 4,000 recombinant clones obtained from EcoRI digests of the φ-0303 phage DNA insert were screened for lytic activity. Clones were grown on agar plates containing autoclaved L. helveticus cells as the substrate (opaque plates). The plates were treated to chloroform vapor, which permeabilized the clones, and clones with a clearing zone were identified as positive. Only one clone had a clearing zone. This positive clone was very unstable, losing its lytic activity after two subcultures in agar medium. Lysis of the E. coli cells was not observed when the cells were grown in LB broth. The DNA insert was extracted and sequenced: its size was found to be 4.1 kb (sequence not shown). In this phage DNA fragment, seven putative ORFs were identified (Fig. 1), and all were found to be transcribed in the same direction. ORF 1, at the beginning of the cloned region, encoded a sequence of 177 amino acids but was truncated. ORFs 2, 3, 4, 5, and 6 encoded proteins of 123, 120, 112, 61, and 129 amino acids, respectively, which did not present significant homology to any known proteins in the sequence databases. ORF 7 was identified as the putative endolysin-encoding gene due to the sequence homology with several endolysins in the databases. This gene is designated lys in this work. On the other strand, transcribed in the opposite direction, one truncated ORF (ORF 8) encoding a protein of 80 amino acids was found, which had high homology with phage integrase genes of different species: Bacillus halodurans (44% in a 76-amino-acid overlap), Lactococcus lactis phages BK-5T, bIL285, and Tuc2009 (42% in a 76-amino-acid overlap). In the whole fragment cloned, no sequence corresponding to a holin-encoding gene was found.
FIG. 1.
Schematic representation of the putative ORFs found in the EcoRI-digested phage φ-0303 DNA fragment of 4.1 kb. This fragment was obtained using a library of total φ-0303 DNA in E. coli and was identified as the one encompassing the gene coding for a lytic protein.
Sequencing of the endolysin-encoding gene.
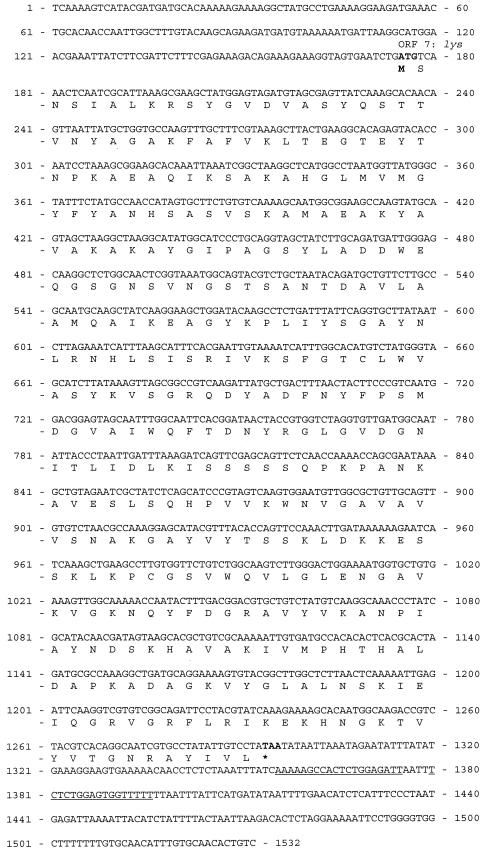
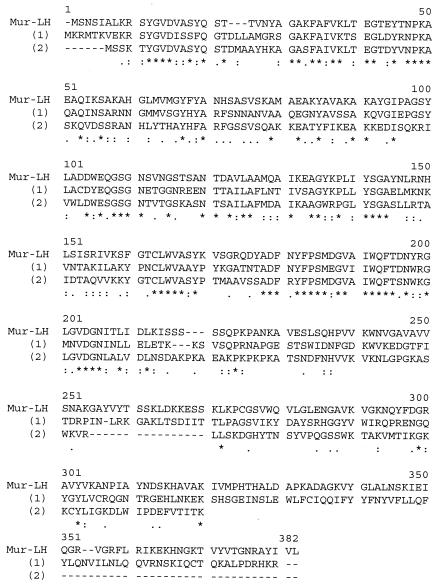
In order to ensure that no mutation had occurred during the initial cloning and manipulation of the 4.1-kb DNA fragment (suspected because of the high instability of the positive clone), the lys gene was PCR amplified, using total phage DNA as the template and two oligonucleotides encompassing this gene. The resulting 1.5-kb PCR product was then sequenced. There was no difference in the nucleotide sequence of the amplified lys gene and the previous sequence identified from the 4.1-kb DNA insert. The complete sequence of the lys gene and the surrounding regions is presented in Fig. 2. The lys gene contains 1,122 nucleotides, which encodes a protein (Mur-LH) with 373 amino acids and with a deduced molecular mass of 40,207 Da. The beginning of the gene was confirmed by determining the N-terminal sequence of the protein, which is SNSIALKRSSYGVDVA. The entire protein has a predicted theoretical isoelectric point (pI) of 9.58. The ATG codon is separated by six codons from the end of the ORF upstream. No potential ribosome binding site was found upstream of the start codon. Downstream of the lys gene, a hairpin loop structure was detected which may act as a terminator. Comparison of the Mur-LH sequence with the sequences in databases showed that it had sequence homology with numerous endolysins of other species. Percent homology values of 53 and 50 were found for the N-terminal part of Mur-LH and the endolysin of the Lactobacillus johnsonii prophage Lj965 (in a 216-amino-acid overlap) and the endolysin of the LL-H bacteriophage of L. delbrueckii subsp. lactis (in a 236-amino-acid overlap), respectively (Fig. 3). Mur-LH also had homology with the endolysins of L. gasseri bacteriophage φadh (45% in a 226-amino-acid overlap), lysA of the bacteriophage mv4 of L. delbrueckii subsp. bulgaricus (38% in a 294-amino-acid overlap), and the endolysin of the mv1 bacteriophage of L. delbrueckii subsp. bulgaricus (39% in a 181-amino-acid overlap). No significant homologies have been found in the C-terminal part of the endolysin.
FIG. 2.
Nucleotide sequence of the 1.5-kb phage DNA fragment encompassing the endolysin-encoding lys gene. The deduced amino acid sequence of the protein Mur-LH is indicated below the nucleotide sequence. The start and stop codons of the gene are shown in bold type. The underlined sequences represent the inverted repeat of the hairpin structure.
FIG. 3.
Comparison of the endolysin sequences of L. helveticus bacteriophage φ-0303 (Mur-LH), L. johnsoni bacteriophage Lj965, and L. delbrueckii bacteriophage LL-H. The endolysin sequences of L. johnsoni bacteriophage Lj965 and L. delbrueckii bacteriophage LL-H are indicated by (1) and (2), respectively, below the Mur-LH sequence. Asterisks indicate positions which have a single, fully conserved residue. Colons indicate that one of the following “strong” groups is fully conserved: STA, NEQK, NHQK, NDEQ, QHRK, MILV, MILF, HY, or FYW. Dots indicate that one of the following “weaker” groups is fully conserved: CSA, ATV, SAG, STNK, STPA, SGND, SNDEQK, NDEQHK, NEQHRK, FVLIM, or HFY. The sequences were aligned with the program Clustalw (available at the website http://www.infobiogen.fr). Gaps introduced to maximize alignment are indicated by hyphens.
Subcloning of the endolysin-encoding gene.
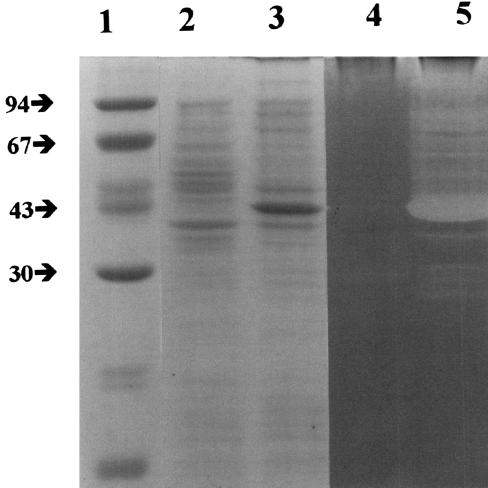
In order to determine whether the lys gene encoded a protein with lytic activity, the 1.5-kb DNA fragment previously obtained was cloned into the E. coli vector pET, and E. coli BL21 was transformed with the construct [E. coli BL21/pET(lys)]. The expression of the recombinant protein did not affect the growth of the transformed E. coli cells, and no lysis was observed. When E. coli cells harboring the recombinant plasmid were grown, the overexpression of a protein with an estimated molecular mass of 40 kDa was observed by SDS-PAGE (Fig. 4). In a zymogram of autoclaved cells of L. helveticus CNRZ 892 as the substrate, a band of activity with an estimated molecular mass of 40 kDa was detected. This band was absent from an E. coli BL21/pET extract. This result confirmed the lytic properties of the protein encoded by the lys gene.
FIG. 4.
Overexpression of the Mur-LH endolysin and demonstration of its lytic activity in a zymogram. SDS-PAGE was performed on E. coli BL21 harboring nonrecombinant plasmid pET (lane 2) and on E. coli BL21 harboring recombinant plasmid pET-(lys) expressing the endolysin of bacteriophage φ-0303 (lane 3). Lanes 4 and 5 contain the same samples in lanes 2 and 3, respectively, loaded on a zymogram with autoclaved cells of L. helveticus CNRZ 892 as a substrate. Lane 1 contains molecular mass markers. The positions of the molecular mass markers (in kilodaltons) are indicated to the left of the gel.
Lytic spectrum of the Mur-LH endolysin.
The lytic activity of Mur-LH was tested in duplicate on autoclaved cells from different species (Table 1). Thirty different cell substrates from 22 bacterial species were tested. Mur-LH hydrolyzed 16 substrates belonging to 10 of the 30 different species, showing a lytic halo around the well. When L. helveticus, L. delbrueckii, L. acidophilus, Leuconostoc lactis, or Pediococcus acidilactici was used as the substrate, strong or medium activity was detected. In contrast, no activity was detected with other lactobacilli, such as L. casei, L. fermentum, and L. brevis, or with the species Streptococcus thermophilus, Propionibacterium freudenreichii, and Clostridium sporogenes. Mur-LH exhibited a weak activity toward lactococcal species and toward L. paracasei, Brevibacterium linens, and Bacillus subtilis. No lytic activity toward gram-negative species was detected. For each strain tested as a substrate, a crude extract of E. coli BL21/pET was used as a control, and no lysis halo was observed, whatever the substrate.
TABLE 1.
Lytic spectrum of the endolysin Mur-LH of L. helveticus temperate bacteriophage φ-0303
| Strain used as bacterial cell substratea | Activityb |
|---|---|
| Lactobacillus helveticus CNRZ 892 | ++ |
| Lactobacillus helveticus CNRZ 303 | +++ |
| Lactobacillus helveticus CNRZ 32 | ++ |
| Lactobacillus helveticus CIP 103146 | +++ |
| Lactobacillus helveticus T650c | ++ |
| Lactobacillus acidophilus CNRZ 204 | ++ |
| Lactobacillus delbrueckii subsp. bulgaricus CIP 101027 | +++ |
| Lactobacillus delbrueckii subsp. lactis CNRZ 207 | +++ |
| Streptococcus thermophilus st49c | − |
| Streptococcus thermophilus st50c | − |
| Lactobacillus casei CNRZ 213 | − |
| Lactobacillus paracasei subsp. paracasei LMG 9192 | + |
| Lactobacillus fermentum CNRZ 209 | − |
| Lactobacillus brevis CNRZ 215 | − |
| Lactococcus lactis subsp. cremoris CNRZ 105 | + |
| Lactococcus lactis subsp. lactis CNRZ 144 | + |
| Brevibacterium linens ATCC 9175 | + |
| Leuconostoc lactis CIP 102422 | ++ |
| Pediococcus acidilactici CIP 103408 | +++ |
| Enterococcus faecium LMG 8149 | + |
| Enterococcus faecalis CNRZ 137 | − |
| Propionibacterium jensenii CIP 103028 | − |
| Propionibacterium freudenreichii subsp. shermanii CIP 103027 | − |
| Propionibacterium freudenreichii subsp. shermanii ITG 23c | − |
| Bacillus subtilis ATCC 6633 | + |
| Clostridium sporogenes CNRZ 692 | − |
| Listeria innocua CIP 80.11 | − |
| Escherichia coli CIP 61.11 | − |
| Pseudomonas fluorescens CIP 69.13 | − |
| Salmonella enterica serovar Abortus- ovis ATCC 31684 | − |
Strains are from the following culture collections: Centre National de Recherches Zootechniques (CNRZ) (Jouy-en-Josas, France); Collection de 1'Institut Pasteur (CIP) (Paris, France); American Type Culture Collection (ATCC) (Rockville, Md.), and Collection Laboratorium Microbiology (LMG) of Universiteit Gent (Ghent, Belgium).
Symbols: +, weak intensity; ++, middle intensity; +++, strong intensity; −, absence of lysis (no clearing zone).
Industrial strain.
Enzymatic specificity of the Mur-LH endolysin.
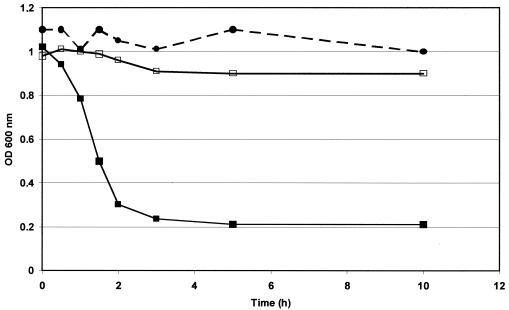
Incubating the cell walls of L. helveticus CNRZ 303 with Mur-LH resulted in a 80% reduction in the OD600 of the suspension within 3 h (Fig. 5) and a concomitant increase in the reducing power of the suspension (data not shown). This increase in reducing power indicates that glycosidic linkages of the cell walls were cleaved, demonstrating that Mur-LH is a glycosidase. In order to determine whether the glycosidase is a muramidase or transglycosidase, cell walls were incubated with Mur-LH. After 3 h of incubation, the cleaved extremities were reduced with sodium borohydride to label the free reducing groups generated. After hydrolysis with HCl, muramicitol, glucosaminitol, muramic acid, and glucosamine contents were determined. An increase of muramicitol content was detected (from 79 to 268 nmol/mg of cell walls [dry weight]), concomitant with a decrease in muramic acid (from 142 to 21 nmol/mg of cell walls [dry weight]). No change in the glucosaminitol and glucosamine contents were observed. This result demonstrated that Mur-LH is a muramidase. In the control with crude E. coli BL21/pET extract, no changes in the muramic acid, glucosamine, muramicitol, and glucosaminitol contents were observed by using the same procedure.
FIG. 5.
Lysis of cell walls of L. helveticus CNRZ 303 by the Mur-LH endolysin, measured by the decrease in absorbance at 600 nm. The cell walls were heated at 100°C for 1 h and then resuspended in a 25 mM Tris-HCl buffer (pH 8) to a final OD600 of 1 (1.9 mg [dry weight]/ml). They were incubated at 40°C with the crude endolysin extract obtained from recombinant E. coli BL21/pET(lys) (▪) or with a crude nonrecombinant E. coli BL21/pET extract (□). A control was prepared without any extract added to the cell wall (•).
DISCUSSION
In this work, cloning and sequencing of an endolysin of the temperate bacteriophage φ-0303 infecting L. helveticus was performed. The lys gene contains 1,122 nucleotides and encodes a 373 amino-acid protein, Mur-LH. Mur-LH had homologies with numerous endolysins of other bacteriophages from LAB, particularly endolysins of bacteriophages Lj965 infecting L. johnsoni (8) and LL-H (a muramidase) infecting L. delbrueckii (38). The homologies are mainly located in the N-terminal part, generally encompassing the catalytic domain of endolysins, which suggests that Mur-LH is probably a N-acetylmuramidase. This hypothesis was confirmed by biochemical analysis. The sequence of the gene also suggests that the enzyme consists of two domains, which is in agreement with the modular organization of other peptidoglycan hydrolases (5, 21, 36). A truncated gene that had homology with integrase genes was found downstream of the lys gene. This gene was translated in the direction opposite that of the lys gene, which is similar to the organization of bacteriophages of other LAB (1, 2, 37). Upstream of the lys gene, five ORFs and one truncated ORF were detected, all transcribed in the same direction. Because of the instability of the positive clone, sequence changes cannot be excluded, and accordingly, the numbers and/or sizes of putative ORFs deduced from the 4.1-kb cloned DNA fragment may differ from those existing in φ-0303 phage sequence. The nucleotide sequences of the putative ORFs did not have any homology with known sequences in the databases. In addition, none of the putative ORFs exhibited characteristics typical of holin, such as a membrane-spanning domain or β-turn (42). The absence of a holin gene downstream of the endolysin gene has been already reported for the phages bIL67 (29) and c2 (22) of Lactococcus lactis, in which the holin gene is located far (more than 12 kb) from the endolysin gene. As the holin/lysin system can be toxic for E. coli when cloned, we also cannot exclude the possibility that the positive clone detected in this work is viable only because no holin is associated with the lysin gene.
The lys gene, whose DNA sequence was obtained from φ-0303 DNA, was expressed in E. coli, yielding a protein (Mur-LH) with an apparent molecular mass of 40 kDa, in agreement with the size deduced from the sequence (40,200 Da). The endolysin exhibited lytic activity against all five strains of L. helveticus tested in this study. The efficiency of the lytic activity was strain dependent. The difference in sensitivity could be due to strain variation in the cell wall composition, but to date, the cell wall composition of lactobacilli has not been elucidated sufficiently to confirm this hypothesis. However, in lactococcal species, significant differences between the cell wall components of different strains have been detected; this is particularly true for lipoteichoic acids, which are known to be involved in endolysin activity (6). The endolysin is also active toward cells of other LAB, particularly thermophilic lactobacilli, and lactococci. Interestingly, it was also active toward nonrelated species like Bacillus subtilis, Enterococcus faecium, and Pediococcus acidilactici. Such a broad lytic spectrum appears to be rare, compared to those described in the literature. Mostly, endolysins of bacteriophages of LAB present no lytic activity toward other species (33) or present lytic activity only toward related species (25, 38). Interestingly, Mur-LH was also found to be active toward species whose peptidoglycan types are different (7). The poor homology score found in the C-terminal region of the protein could explain the broad spectrum observed for Mur-LH, as the C-terminal region is known to determine the substrate binding site and, therefore, the spectrum of the enzyme. To date, this work represents the first endolysin-encoding gene identified and biochemically characterized for L. helveticus.
Acknowledgments
We are indebted to Arilait Recherches and Rhodia-Food (France) for constructive scientific discussions.
We thank Jonathan Hanson, Anton Steen, and the staff of the MBB department for their helpful assistance and John Hannon for help in manuscript correction.
This study was supported in part by financial support from Arilait Recherches and Rhodia-Food (France) and by a grant from the “Académie d'Agriculture de France.”
REFERENCES
- 1.Arendt, E. K., C. Daly, G. F. Fitzgerald, and M. van de Guchte. 1994. Molecular characterization of lactococcal bacteriophage Tuc2009 and identification and analysis of genes encoding lysin, a putative holin, and two structural proteins. Appl. Environ. Microbiol. 60:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkeland, N.-K. 1994. Cloning, molecular characterization, and expression of the genes encoding the lytic functions of lactococcal bacteriophage φLC3: a dual lysis system of modular design. Can. J. Microbiol. 40:658-665. [DOI] [PubMed] [Google Scholar]
- 3.Boizet, B., Y. Lahbib-Mansais, L. Dupont, P. Ritzenthaler, and M. Mata. 1990. Cloning, expression and sequence analysis of an endolysin-encoding gene of Lactobacillus bulgaricus bacteriophage mv1. Gene 94:61-67. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, D. E. 1967. Ultrastructure of bacteriophages of Lactobacillus bulgaricus, L. lactis, and L. helveticus. Bacteriol. Rev. 33:230-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croux, C., C. Ronda, P. Lopez, and J. L. Garcia. 1993. Interchange of functional domains switches enzyme specificity: construction of a chimeric pneumococcal-clostridial cell wall lytic enzyme. Mol. Microbiol. 9:1019-1025. [DOI] [PubMed] [Google Scholar]
- 6.Crow, V. L., P. K. Gopal, and A. J. Wicken. 1995. Cell surfaces of lactococcal strains. Int. Dairy J. 5:45-68. [Google Scholar]
- 7.Delcour, J., T. Ferain, M. Deghorain, E. Palumbo, and P. Hols. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Leeuwenhoek 76:159-184. [PubMed] [Google Scholar]
- 8.Desiere, F., R. D. Pridmore, and H. Brüssow. 2000. Comparative genomics of the late gene cluster from Lactobacillus phages. Virology 275:294-305. [DOI] [PubMed] [Google Scholar]
- 9.Deutsch, S.-M., T. Ferain, J. Delcour, and S. Lortal. 2002. Lysis of lysogenic strains of Lactobacillus helveticus in Swiss cheeses and first evidence of concomitant Streptococcus thermophilus lysis. Int. Dairy J. 12:591-600. [Google Scholar]
- 10.Deutsch, S.-M., A. Neveu, S. Guezenec, P. Ritzenthaler, and S. Lortal. 2003. Early lysis of Lactobacillus helveticus CNRZ 303 in Swiss cheese is not prophage related. Int. J. Food Microbiol. 81:147-157. [DOI] [PubMed] [Google Scholar]
- 11.Gindreau, E., and A. Lonvaud-Funel. 1999. Molecular analysis of the region encoding the lytic system from Oenococcus oeni temperate bacteriophage φ10MC. FEMS Microbiol. Lett. 171:231-238. [DOI] [PubMed] [Google Scholar]
- 12.Hara, S., and Y. Matsushima. 1966. Crystalline muramicitol and its use in a study of the lysozyme action on the cell wall of Micrococcus lysodeikticus. Bull. Soc. Chem. Jpn. 39:1826. [Google Scholar]
- 13.Heinrich, B., B. Binishofer, and U. Bläsi. 1995. Primary structure and functional analysis of the lysis genes of Lactobacillus gasseri bacteriophage φadh. J. Bacteriol. 177:723-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hertwig, S., W. Bockelmann, and M. Teuber. 1997. Purification and characterization of the lytic activity induced by the prolate-headed bacteriophage P001 in Lactococcus lactis. J. Appl. Microbiol. 82:233-239. [DOI] [PubMed] [Google Scholar]
- 15.Horsburgh, M. J., S. J. Wharton, A. J. Cox, E. Ingham, S. Peacock, and S. J. Foster. 2002. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol. Microbiol. 44:1269-1286. [DOI] [PubMed] [Google Scholar]
- 16.Kakikawa, M., K. Yokoi, H. Kimoto, M. Nakano, K.-I. Kawasaki, A. Taketo, and K.-I. Kodaira. 2002. Molecular analysis of the lysis protein Lys encoded by Lactobacillus plantarum phage ϕgle. Gene 299:227-234. [DOI] [PubMed] [Google Scholar]
- 17.Kashige, N., Y. Nakashima, F. Miake, and K. Watanabe. 2000. Cloning, sequence analysis, and expression of Lactobacillus casei phage PL-1 lysis genes. Arch. Virol. 145:1521-1534. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Leclerc, D., and A. Asselin. 1989. Detection of bacterial cell wall hydrolysis after denaturing polyacrylamide gel electrophoresis. Can. J. Microbiol. 35:749-753. [DOI] [PubMed] [Google Scholar]
- 20.Lemée, R., S. Lortal, B. Cesselin, and J. van Heijenoort. 1994. Involvement of an N-acetylglucosaminidase in autolysis of Propionibacterium freudenreichii CNRZ 725. Appl. Environ. Microbiol. 60:4351-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopéz, G., J. L. Garcia, E. Garcia, C. Ronda, and P. Garcia. 1992. Structural analysis and biological significance of the cell wall lytic enzymes of Streptococcus pneumoniae and its bacteriophage. FEMS Microbiol. Lett. 79:439-447. [DOI] [PubMed] [Google Scholar]
- 22.Lubbers, M. W., K. Schofield, N. R. Waterfield, and K. M. Polzin. 1998. Transcription analysis of the prolate-headed lactococcal bacteriophage c2. J. Bacteriol. 180:4487-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik, A. C., G. W. Reinbold, and E. R. Vadamuthu. 1968. Evaluation of the taxonomy of Propionibacterium. Can. J. Microbiol. 14:1185-1191. [DOI] [PubMed] [Google Scholar]
- 24.Matthews, R. E. F. 1982. Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology 17:1-200. [DOI] [PubMed] [Google Scholar]
- 25.Mullan, W. M., and R. J. Crawford. 1985. Partial purification and some properties of φC2(W) lysin, a lytic enzyme produced by phage-infected cells of Streptococcus lactis C2. J. Dairy Res. 52:123-138. [DOI] [PubMed] [Google Scholar]
- 26.Sablé, S., and S. Lortal. 1995. The lysins of bacteriophages infecting lactic acid bacteria. Appl. Microbiol. Biotechnol. 43:1-6. [DOI] [PubMed] [Google Scholar]
- 27.Saboya, L. V., M.-B. Maillard, and S. Lortal. 2003. Efficient mechanical disruption of Lactobacillus helveticus, Lactococcus lactis and Propionibacterium freudenreichii by a new high-pressure homogenizer and recovery of intracellular aminotransferase activity. J. Ind. Microbiol. Biotechnol. 30:1-5. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Schouler, C., S. D. Ehrlich, and M.-C. Chopin. 1994. Sequence and organization of the lactococcal prolate-headed bIL67 phage genome. Microbiology 140:3061-3069. [DOI] [PubMed] [Google Scholar]
- 30.Séchaud, L., M. Rousseau, B. Fayard, M.-L. Callegari, P. Quénée, and J.-P. Accolas. 1992. Comparative study of 35 bacteriophages of Lactobacillus helveticus: morphology and host range. Appl. Environ. Microbiol. 58:1011-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shearman, C., K. Jury, and M. J. Gasson. 1994. Controlled expression and structural organization of a Lactococcus lactis bacteriophage lysin encoded by two overlapping genes. Appl. Environ. Microbiol. 60:3063-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shearman, C., H. Underwood, K. Jury, and M. J. Gasson. 1989. Cloning and DNA sequence analysis of a Lactococcus bacteriophage lysin gene. Mol. Gen. Genet. 218:214-221. [DOI] [PubMed] [Google Scholar]
- 33.Sheenan, M., E. Stanley, G. F. Fitzgerald, and D. van Sinderen. 1999. Identification and characterization of a lysis module present in a large proportion of bacteriophages infecting Streptococcus thermophilus. Appl. Environ. Microbiol. 65:569-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spackman, D. H., W. H. Stein, and S. Moore. 1958. Automatic recording apparatus for use in the chromatography of amino acids. Anal. Chem. 30:1190-1206. [PubMed] [Google Scholar]
- 35.Thompson, J. S., and G. D. Shockman. 1968. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal. Biochem. 22:260-268. [DOI] [PubMed] [Google Scholar]
- 36.Usobiaga, P., F. J. Medrano, M. Gasset, J. L. Garcia, J. L. Saiz, G. Rivas, J. Laynez, and M. Menéndez. 1996. Structural organization of the major autolysin from Streptococcus pneumoniae. J. Biol. Chem. 271:6832-6838. [DOI] [PubMed] [Google Scholar]
- 37.van Sinderen, D., H. Karsens, J. Kok, P. Terpstra, M. H. Ruiters, G. Venema, and A. Nauta. 1996. Sequence analysis and molecular characterization of the temperate lactococcal bacteriophage r1t. Mol. Microbiol. 19:1343-1355. [DOI] [PubMed] [Google Scholar]
- 38.Vasala, A., M. Välkkilä, J. Caldentey, and T. Alatossava. 1995. Genetic and biochemical characterization of the Lactobacillus delbrueckii subsp. lactis bacteriophage LL-H lysin. Appl. Environ. Microbiol. 61:4004-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward, J. B. 1973. The chain length of the glycans in bacterial cell walls. Biochem. J. 133:395-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward, L. J., T. P. Beresford, M. W. Lubbers, B. D. Jarvis, and A. W. Jarvis. 1993. Sequence analysis of the lysin gene region of the prolate lactococcal bacteriophage c2. Can. J. Microbiol. 39:767-774. [DOI] [PubMed] [Google Scholar]
- 41.Yoon, S. S., J. W. Kim, F. Breidt, and H. P. Fleming. 2001. Characterization of a lytic Lactobacillus plantarum bacteriophage and molecular cloning of a lysin gene in Escherichia coli. Int. J. Food Microbiol. 65:63-74. [DOI] [PubMed] [Google Scholar]
- 42.Young, R. Y. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol. Rev. 56:430-481. [DOI] [PMC free article] [PubMed] [Google Scholar]