Abstract
Folic acid deficiency during pregnancy causes birth neurocristopathic malformations resulting from aberrant development of neural crest cells. The Reduced folate carrier (RFC) is a membrane-bound receptor for facilitating transfer of reduced folate into the cells. RFC knockout mice are embryonic lethal and develop multiple malformations, including neurocristopathies. Here we show that XRFC is specifically expressed in neural crest tissues in Xenopus embryos and knockdown of XRFC by specific morpholino results in severe neurocristopathies. Inhibition of RFC blocked the expression of a series of neural crest marker genes while overexpression of RFC or injection of 5-methyltetrahydrofolate expanded the neural crest territories. In animal cap assays, knockdown of RFC dramatically reduced the mono- and trimethyl-Histone3-K4 levels and co-injection of the lysine methyltransferase hMLL1 largely rescued the XRFC morpholino phenotype. Our data revealed that the RFC mediated folate metabolic pathway likely potentiates neural crest gene expression through epigenetic modifications.
Introduction
Neural crest (NC) is a multipotent cell population which originates at the border between the neural plate and epidermis during vertebrate development. Later on, these cells migrate to various places throughout the body and give rise to various tissues, including craniofacial bones and cartilages, melanocytes, cardiac structures and peripheral nervous system [1]. Defective neural crest development leads to a broad spectrum of congenital malformations, collectively called neurocristopathies, which includes defects in pigmentation, and abnormal craniofacial and heart development. Several neurocristopathies have been documented, including frontonasal dysplasia, Waardenburg-Shah syndrome, DiGeorge syndrome, CHARGE syndrome, congenital nevi, and Hirchsprung disease [2], [3].
Induction, specification, migration, and differentiation of the NC cells are tightly regulated by a carefully orchestrated multi-step gene regulatory network (GRN) [1], [4]–[6]. Neural crest formation occurs in a series of tightly regulated steps. First, the presumptive neural crest territory is induced at the dorsal neural plate border through the interplay of different signaling pathways including BMPs, Wnts and FGFs. These signals control the broad expression of a set of transcription factors at the neural plate border region, including Pax3, Msx1 and Zic1. These neural plate border specifiers further turn on the expression of a group of genes in the emerging neural crest cells, including Snail1, Snail2, FoxD3, Sox10, Sox9, and Twist1. These neural crest genes are extensively cross-regulated and many of them have been shown to be necessary and/or sufficient for the expression of many other genes. The neural crest specifier genes further control the expression of several downstream mediators of neural crest migration. Terminal differentiation of the neural crest cells is regulated by different networks.
Folate deficiency has long been known to contribute to developmental neural defects, especially neural tube defects and neurocristopathies [7]–[10]. In humans, it has been documentated folic acid prevents the development of neural tube defects, craniofacial malformation, and heart defects [11]–[13]. In addition, in vivo and in vitro experiments suggest that altering levels of folic acid leads to aberrant cardiac NC cell migration and differentiation in chick [14], [15]. Folate is a cofactor in one-carbon metabolism and is a crucial regulator of nucleotide synthesis and methylation reactions. 5-methyltetrahydrofolate (5-MTHF) is involved in the remethylation of homocysteine to methionine, which is the precursor of S-adenosylmethionine (SAM), the primary methyl group donor for most biological methylation reactions [8]. In humans, folate metabolism and folate status has been shown to affect the global methylation of DNA [16].
As a water-soluble B class vitamin, the uptake of folate by cells is mediated by specific carriers or receptors, including folic acid receptors (FRs), proton-coupled folic acid transporter (PCFT), and reduced folic acid carrier (RFC). RFC, a 12 transmembrane protein, is believed to be the major transporter for 5-MTHF, which is the major form of folate in circulation [17]. RFC has a low affinity for folic acid and a high affinity for reduced folate and methotrexate (MTX), an antifolic acid chemotherapeutic drug [18]. RFC is widely expressed in various human tissues and mouse embryos [19], [20]. RFC1 knockout mice die shortly after implantation. Supplementation of high dose of maternal folate prolongs the survival of RFC1 null embryos until mid-gestation, but these embryos develop multiple malformations, including defects in the neural tube, craniofacial and cardiac malformations [21]. Such malformations, including the craniofacial and heart defects, coincide with neurocristopathies [3], suggesting that RFC might be required for normal neural crest development [7].
Here we show in Xenopus embryos that RFC was involved in neural crest development and knockdown of XRFC by specific morpholino led to severe neurocristopathies as observed in mice. We also provide evidence that the folate-mediated metabolism regulates neural crest gene expression is likely through epigenetic mechanisms.
Results
Xenoups RFC is expressed in the neural crest territories
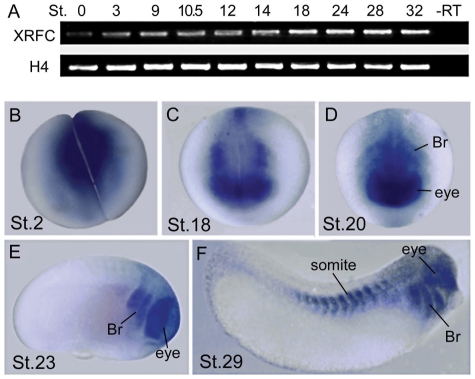
We examined the temporal and spatial expression pattern of XRFC during early Xenopus embryogenesis by RT-PCR and whole-mount in situ hybridization. XRFC was expressed in Xenopus embryos throughout the stages examined from St.0 to St.32 (Fig. 1A). Maternal XRFC transcripts were localized to the animal pole at 2-cell stage (Fig. 1B). At stage 18, XRFC was expressed at the anterior neural plate and its border (Fig. 1C). At late neurula stages (Fig. 1D, E), the expression of XRFC was detected in the prospective brain, optic vesicles, and the developing branchial arches. During the tailbud stages, XRFC expression remained high in the branchial arches, eyes, head mesenchyme and the somite regions (Fig. 1F). The expression pattern of XRFC overlaps with the neural crest territories, including the neural plate border, branchial arches, somites and the head mescenchyme. This suggests RFC may play a role in neural crest development.
Figure 1. Temporal and spatial expression pattern of XRFC.
(A) XRFC is expressed maternally and throughout the stages examined (St.0 to St.32) as detected by RT-PCR. –RT, negative control without reverse transcriptase in the RT reaction. (B–F) Expression of XRFC at the indicated stages revealed by in situ hybridization. (B) The maternal XRFC expression is detected at the animal pole at 2-cell stage. (C) At stage 18, XFRC is expressed at the anterior neural plate and its border. (D–E) At the late neurula stages, the expression of XRFC is detected in the prospective eye and forming branchial arches. (F) At tailbud stage, XRFC is strongly expressed in the branchial arches, eyes, head mesenchyme and the somites. Br, branchial arch.
XRFC is required for neural crest development
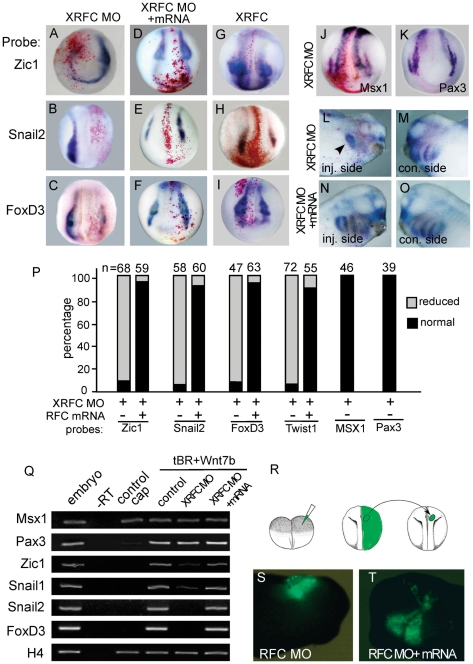
We further examined whether XRFC knockdown would affect neural crest development. XRFC-MO, together with lacZ mRNA as a lineage tracer, were injected into one blastomere of the embryos at four cell stage, such that the contralateral uninjected sides serve as an internal control. At the neurula stage, expression of neural crest maker genes Zic1, Snail2, and FoxD3 were clearly down-regulated in the injected side (Fig. 2 A–C, P) compared to the control side. This effect could be largely rescued by co-injection of a morpholino resistant XRFC mRNA (Fig. 2 D–F, P). Interestingly, over-expression of XRFC mRNA alone could promote the expression of Zic1 (92%, n = 37), Snail2 (96%, n = 47), and FoxD3 (95%, n = 41; Fig. 2 G–I). In contrast, the expression of Msx1 and Pax3 was not affected by XRFC-MO or XRFC mRNA injections (Fig. 2 J, K, P and data not shown).
Figure 2. XRFC is required for neural crest induction and migration.
(A–K) XRFC-MO and tracing lacZ mRNA were injected into one cell of four-cell stage embryos, the expression of neural crest makers were examined at stages 15–17. (A–C) Knockdown of XRFC by morphoino inhibits the expression of Zic1, Snail2 and FoxD3 in the injected sides (labeled red by staining of the tracing lacZ). (D–F) Co-injection of XRFC mRNA (800 pg) rescues the expression of Zic1, Snail2 and FoxD3. (G–I) Overexpression of XRFC expands the expression domains of Zic1, Snail2 and FoxD3. (J, K) XRFC morpholino injection does not affect the expression of Msx1 and Pax3. (L–O) Twist1 staining of neural crest cells at stage 32 of embryos injected on one side with XRFC-MO or XRFC-MO plus rescue mRNA. In XRFC-MO injected side, the neural crest cells are retained in a dorsal position (arrowhead in L). (P) Quantification of the effect of XRFC-MO on the expression of neural crest markers and the rescue by RFC mRNA. (Q) RT-PCR analysis in animal cap assay to show the effect of XRFC-MO on the expression of the indicated neural crest genes induced by co-injection of tBR and XWnt7b. (R–T) Transplantation experiment to show the effect of XRFC-MO on neural crest migration. (R) Schematic drawing showing the transplantation procedure. (S) The EGFP-labeled XRFC-MO injected neural crest tissue fails to migrate at tailbud stage after transplantation. (T) Co-injection of XRFC mRNA restores the migration ability of the neural crest cells. inj. side, injected side; con. side, control side.
We also tested whether XRFC is required for neural crest induction in animal cap assays. Neural crest cells can be induced in animal caps by coinjection of Wnts and neuralizing factors [22]. Animal caps were dissected at stage 9 from embryos injected with XWnt7b and tBR mRNA, with or without XRFC-MO. We first confirmed that XRFC was expressed in control and induced animal caps (data not shown). As in whole embryos, suppression of XRFC significantly reduced the expression of Zic1, Snail2 and FoxD3 without significantly affecting Msx1 and Pax3 expressions (Fig. 2 Q).
We checked whether RFC knockdown also affects neural crest migration using Twist1 as a marker. At the taibud stage, Twist1 labeled NC cells migrate ventrally in several streams into the hyoid and branchial arches. In the XRFC-MO injected embryos, however, Twist1 positive NC cells failed to migrate ventrally into separate streams, but were clustered and retained in a dorsal position (Fig. 2 L, M, P). This effect could be rescued by co-injection of the XRFC mRNA (Fig. 2 N, O, P). We next examined the effects of XRFC-MO on neural crest migration using a transplantation experiment ([23], Fig. 2 R). When the presumptive neural crest tissue from an embryo injected with EGFP mRNA is transplanted into similar place of a normal embryo at the neurula stage, these cells will migrate ventrally into the branchial arch regions at tailbud stage. When tissues from the XRFC-MO injected embryos were transplanted, however, these cells stayed at the transplanted position and failed to migrate ventrally (100%, n = 14, Fig. 2 S). This defect could be rescued by co-injection of RFC mRNA (100%, n = 15, Fig. 2 T). We can not rule out the possibility that the failure of neural crest migration in XRFC-MO injected embryos might be a secondary effect of the defected neural crest differentiation.
RFC knockdown leads to neurocristopathic malformations in Xenopus embryos
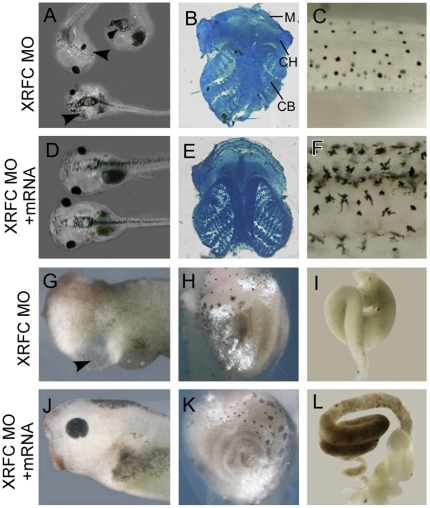
We checked whether knockdown of XRFC could induce neurocristopathic phenotypes at later stages. At tadpole stages, the XRFC-MO injected embryos develop craniofacial malformation with curled trunks (91%, n = 93; Fig. 3 A). The cranial cartilages were atrophied (Fig. 3 B). Also the embryos were hypopigmented and developed heart defects (as indicated by the enlarged pericardial cavity) and gut coiling malformations (Fig. 3 C, G, H, I). These defects could be rescued by co-injection of XRFC mRNA (94%, n = 86; Fig. 3 D, E, F, J, K, L). Thus knockdown of XRFC in Xenopus embryos reproduces the neurocristopathic phenotypes as observed in mice with deleted RFC [21], further confirming that RFC is required for neural crest development in Xenopus.
Figure 3. Knockdown of XRFC leads to multiple malformations at tadpole stages.
(A) Knockdown of XRFC caused craniofacial malformations (arrowheads) with curled trunks. (B) XRFC-MO inhibited the formation of cranial cartilage. Cartilages in late tadpoles were stained with alcian blue. Cartilage on the RFC-MO injected side was often malformed and reduced. M, meckel's cartilage; CH, ceratohyal cartilage; CB, ceratobranchial cartilage. (C) The XRFC-MO injected embryos were hypopigmented at tadpole stage. (G) The XRFC morphants showed enlarged pericardial cavity (arrowhead), indicative of heart defects. (H–I) The gut coiling in XRFC was malformed at tailbud (H) and tadpole (I) stages compared to the rescued group (K, L). (D, E, F, G, K, L) Co-injection of XRFC mRNA rescued the above mentioned malformations.
RFC is involved in the neural crest regulatory network
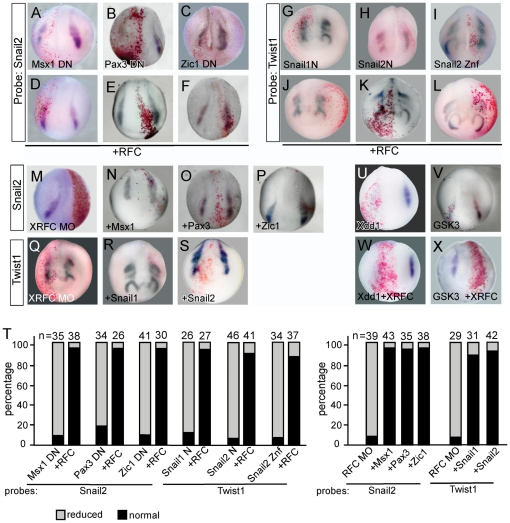
Our knockdown data suggest that RFC is required for the proper expression of a series of neural crest genes and overexpression of RFC promotes their expression. To test whether XRFC could be fit into the NC GRN linearly, we carried out a series of knockdown and rescue experiments. The neural border specifiers Pax3, Msx1 and Zic1 are at an upstream position of the NC GRN hierarchy. Interfering with their function using dominant negative constructs inhibited neural crest induction (Fig. 4 A–C, T, and Fig. S1). When XRFC was co-expressed, however, the dominant negative effects of all these three constructs were rescued (Fig. 4 D–F, T). On the other hand, Pax3, Msx1 and Zic1 could also rescue the neural crest defects in the XRFC-MO injected embryos (Fig. 4 M–P, T, and Fig. S1). Similar situation holds true for more downstream genes Snail1 and Snail2. XRFC rescued the inhibitory effect of the dominant negative Snail1 and Snail2 constructs on neural crest gene expression (Fig. 4 G–I, T and Fig. S1). Furthermore Snail1 and Snail2 also rescued the expression of the neural crest markers in the XRFC morphants (Fig. 4Q–S, T and Fig. S1). Since RFC has no direct effect on Pax3 and Msx1 expression, these data suggest that RFC might have a general role in potentiating the expression of Zic1 and downstream genes.
Figure 4. RFC is involved in the neural crest gene regulatory network.
(A–F) The inhibition of Snail2 expression by dominant negative Msx1, Pax3 and Zic1 was rescued by co-injection of XRFC. (G–L) Snail1 N, Snail2 N and Snail2 Znf inhibited the migration of neural crest (as indicated by the distribution of the Twist1 positive neural crest cells, G–I), which was rescued by co-injection of XRFC (J–L). (M–P) The inhibition of Snail2 expression by XRFC-MO was rescued by Msx1, Pax3 and Zic1 co-expression. (Q–S) The neural crest migration defects in XRFC morphants were rescued by co-injection of Snail1 or Snail2. (T) Quantification of the rescue effect of RFC on the expression of neural crest markers inhibited by dominant negative Msx1, Pax3, Msx1, Snail1, and Snail2 (left panel) and the rescue of the XRFC-MO effect by Msx1, Pax3, Zic1, Snail1, and Snail2 (right panel). (U–X) Dominant negative dishevelled (Xdd1) and GSK3β blocked the expression of Snail2 (U, V), which could not be rescued by XRFC (W, X).
In addition to its involvement in early induction stage, Wnt signaling is also required for the activation of neural crest genes by Pax3 and Zic1 [6]. Blocking of Wnt signaling by dominant negative Dishevelled (Xdd1) [24] or GSK3β abolished neural crest induction (88%, n = 44 and 85%, n = 55 respecitvely, Fig. 4 U, V). Co-injection of XRFC mRNA failed to rescue the neural crest defects (94%, n = 41 and 95%, n = 51 respectively, Fig. 4 W, X), suggesting a stringent requirement of Wnt signaling in neural crest development.
XRFC regulates neural crest development epigenetically
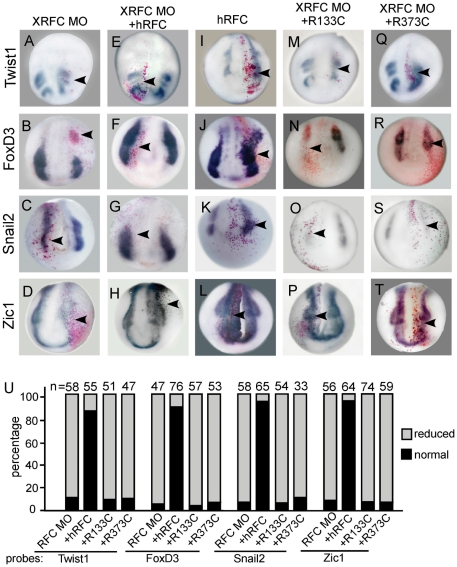
We tested whether the role of XRFC in neural crest development is associated with its function as a folate carrier. We used two loss-of-function hRFC (R133C and R373C) mutants [25], which were known to be defective in folate transportation. Co-injection of wild type hRFC rescued the effect of XRFC-MO on neural crest gene expression (Fig. 5A–H, U ) and over-expression of hRFC promoted neural crest gene expression (Fig. 5 I–L, Fig. S2). The two hRFC mutants, however, failed to rescue the XRFC knockdown phenotype (Fig. 5 M–T, U) or to promote the expression of neural crest markers (Fig. S2).
Figure 5. The effects of different hRFC constructs on neural crest gene expression.
(A–H) XRFC-MO injection blocked neural crest maker expression which was rescued by the wild type hRFC. (I–L) Overexpression of hRFC promoted of the expression of neural crest genes. (M–T) The two mutated form of hRFC (R133C, R373C) failed to rescue the neural crest gene expression in the XRFC morphants. The arrowheads indicate the injected sides. (U) Quantification of the rescue of the XRFC-MO effect by the wild type and mutated hRFC constructs.
5-methyltetrahydrofolate (5-MTHF) is the major form of folate transported by RFC. We tested whether 5-MTHF itself could rescue the XRFC knockdown phenotype. Indeed, co-injection of 5-MTHF clearly rescued XRFC-MO-induced neural crest defects (Fig. 6 A–H, Q). Injection of 5-MTHF also promoted neural crest development as indicated by the expansion of the neural crest markers (Fig. S2). On the other hand, MTX, an antagonist of folic acid, disrupted neural crest induction on the injected side, which can further be rescued by 5-MTHF (Fig. S3).
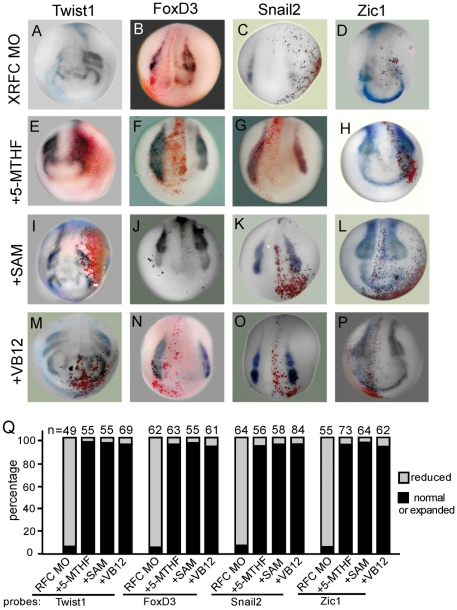
Figure 6. XRFC regulates neural crest gene expression through the one-carbon metabolism pathway.
Co-injection of 5-MTHF (20 ng/embryo, E–H), SAM (10 ng/embryo, I–L) or Vitamin B12 (50 ng/embryo, M–P) with XRFC-MO rescued the expression of the neural crest maker genes (compare with A–D). (Q) Quantification of the rescue of the XRFC-MO effect by 5-MTHF, SAM, and Vitamin B12.
We then tested whether RFC regulated neural crest development by promoting the methylation cycle. In the cell, the methyl group of 5-MTHF is transferred to homocysteine, via vitamin B12, to generate methionine, which is the substrate for the synthesis of S-adenosylmethionine (SAM). SAM is a direct donor of the methyl group in methylation of protein and DNA. Co-injection of SAM or vitamin B12 with XRFC-MO both rescued the expression of Zic1, Snail2, FoxD3, and Twist1 (Fig. 6 I–Q). Furthermore, injection of SAM or vitamin B12 also promoted the expression of the neural crest markers (Zic1, Snail2, FoxD3 and Twist1) (Fig. S2). These results suggest that RFC likely promotes neural crest development through the methylation cycle.
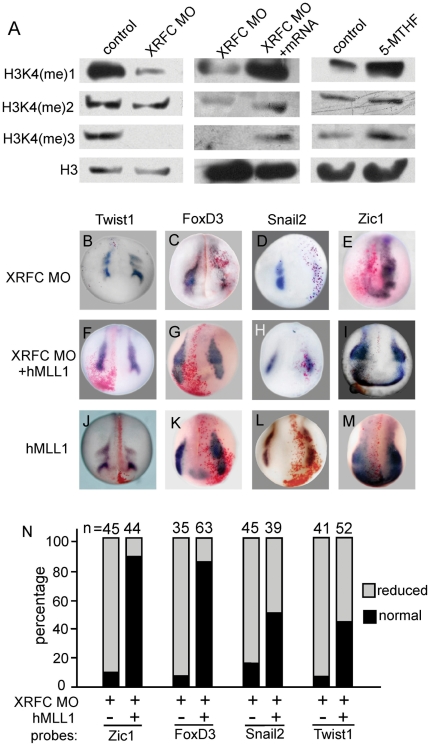
The methylation cycle may regulate gene expression via epigenetic regulations, i.e., promoting DNA and histone methylation. As DNA methylation mainly results in gene inactivation and silencing [26], we checked whether knockdown of RFC affected histone methylation levels. High levels of H3K4 trimethylation have been shown to be associated with the 5′ regions active genes [27]. We failed to detect apparent changes of H3K4 methylation levels in whole embryos injected with XRFC-MO or mRNA (data not shown), probably due to the restricted expression of endogenous XRFC and high levels of background histone methylation. We then tested the H3K4 methylation levels in the neural crest induction assay in animal caps injected with XWnt7b and tBR with or without XRFC-MO. In this system, knockdown of XRFC clearly reduced the mono- and trimethyl-H3-K4 levels (Fig. 7A) which was restored by co-injection of XRFC mRNA. Injection of 5-MTHF also increased both the mono- and trimethyl-H3-K4 levels, but not dimethyl-H3-K4 level. These data suggest that endogenous RFC is required for proper histone methylation regulation in vivo.
Figure 7. RFC regulated neural crest early development through histone modification.
(A) Levels of different methylation forms of histone 3 in animal caps injected with XWnt7b and tBR with or without XRFC-MO. Knockdown of XRFC by injection XRFC-MO decreased both the mono- and trimethyl-H3-K4 levels but not dimethyl-H3-K4 level (left panel), which was restored by co-injection of XRFC mRNA (middle panel). Injection of 5-MTHF increased the mono- and trimethyl-H3-K4 levels but not dimethyl-H3-K4 level (right panel). (B–I) hMLL1 plasmid co-injection rescued the effect of XRFC-MO on the expression of Zic1 and FoxD3, and weakly on Snail2 and Twist1. (J-M) Overexpression of hMLL1 alone promoted the expression of Zic1 and FoxD3, but had no clear effects on Snail2 and Twist1. (N) Number of embryos showed reduced expression of Zic1, FoxD3, Snail2 and Twist1 injected with XRFC-MO with or without hMLL1 plasmid (50 pg/embryo).
The expression of neural crest genes are subjected to epigenetic regulation
As a marker for gene activation, the methylated H3K4 (especially H3K4me3) is predominantly enriched surrounding transcriptional start sites [27]. We checked whether the neural crest genes are potentially regulated by histone3 methylation using published H3K4me3 chromatin immunoprecipitation data. Based on bioinformatics analyses of published genome-wide H3K4me3 pattern of Xenopus [28], zebrafish embryos, and human and mouse embryonic stem cells, H3K4me3 modification were highly enriched in the flanking and coding regions of FoxD3, Zic1, and Twist1 in all species and sporadically enriched in Snail2 (data not shown), indicating functional constraints of epigenetic regulation of these genes.
The H3K4 methylation is mediated by a group of lysine methyltransferases [29]. Among them, the MLL subfamily genes appear to play important roles in early development. Deletion of MLL1-3 genes in mouse are all lethal [30]–[32]. MLL1 knockout mice also show defects in neural crest derivatives, e.g., branchial arches[33]. We tested whether MLL1 could rescue the neural crest defects in XRFC-MO injected embryos. We used hMLL1 [34] plasmid injection in our rescue experiments. Interestingly, overexpression of hMLL1 was sufficient to rescue the expression of Zic1 and FoxD3 in XRFC-MO injected embryos (Fig. 7F–I). In contrast, the expression of Snail2 and Twist1 were only partially rescued (Fig. 7F–I, N), even using higher doses of hMLL1 (data not shown). In addition, overexpression of hMLL1 alone promoted the expression of Zic1 and FoxD3 (81%, n = 44 and 93%, n = 45 respectively, Fig. 7 K, M), but not Snail2 and Twist1 (0%, n = 39 and 0%, n = 27 respectively, Fig. 7J, L). These data suggest that the neural crest genes are differentially regulated by histone3 methylation.
Discussion
Neurocristopathies are a group of diverse disorders resulting from defective growth, differentiation, and migration of the neural crest cells. Folate deficiency has long known to be related to developmental neural defects, especially neural tube defects and neurocristopathies [7]–[9], [35], [36]. In mouse the folate receptor Folr1 is expressed at high levels in the dorsal neural tube during neural crest formation [37]. The migration of cardiac neural crest cells are affected in the Folr1 mutant embryos [38]. In chick embryos, interference with Folr1 expression also reduced the formation and migration of cardiac neural crest cells [39]. We showed here in Xenopus embryos that inhibition of RFC blocked the expression of a series of neural crest marker genes including Zic1, Snail1, Snail2, FoxD3 and Twist1, but had no clear effects on Pax3 and Msx1 (Fig. 2). At tadpole stages, the XRFC-MO injected embryos developed characteristic neurocristopathies including craniofacial malformation, hypopigmention, heart defects, and gut coiling malformations (Fig. 3).
Folate levels affect DNA synthesis, amino acid metabolism, and methylation reactions via S-adenosylmethionine (SAM) mediated one-carbon transfer reactions. Folate promotes the remethylation of homocysteine, which is cytotoxic and can induce DNA strand breakage, oxidative stress and apoptosis. Low folate induced hyperhomocysteinemia has been suggested to be involved in the developmental defects of the cardiac neural crest in chick embryos [14], [15]. In the XRFC-MO injected embryos, however, no clear change in cell apoptosis or cell proliferation were observed as compared to the control embryos (data not shown). We suggest that a basic level of folate absorption might be maintained by other folate receptors to support the proliferation of the neural crest precursors. Indeed, the classical folate receptor Folbp1 is widely expressed in the neural plate during neurula stages (data not shown). Instead, our data supported a role of RFC in the epigenetic regulation of neural crest development. First, neural crest development is sensitive to levels of folate and related molecules. Overexpression of RFC or injection of 5-MTHF, VB12 or SAM all induced expansion of the expression domains of neural crest genes, while injection of the folate antagonist MTX had an opposite effect (Figs. S2, S3). Second, the neural crest defects in XRFC morphants were largely rescued by co-injection of 5-MTHF, VB12 or SAM. And the function of RFC depended on its activity in folate transportation (Fig. 5). Third, modulating folate levels by knockdown of XRFC or addition of 5-MTHF clearly affected histone methylation levels in Xenopus animal caps (Fig. 7), supporting the idea that endogenous folate regulates neural crest gene expression via an epigenetic mechanism. Indeed, bioinformatic analyses suggested that many of the neural crest genes are subjected to epigenetic regulations. Moreover, the neural crest defects in XRFC morphants could be partially rescued by overexpression of the lysine methyltransferases hMLL1 (Fig. 7). Collectively, our data strongly suggest that RFC mediated folate metabolism regulates neural crest gene expression through epigenetic mechanisms.
Among the neural crest genes sensitive to folate levels, Zic1 is the most upstream gene in our analysis. Zic1 is also down regulated in the RFC knockout mouse as shown by microarray analysis [40]. Intriguingly, overexpression of RFC also rescued the effect of the dominant negative constructs of several downstream genes. One possibility is that the epigenetic regulation promoted by RFC is involved directly in the regulation of a panel of the neural crest GRN genes. Thus RFC might work in parallel with the canonical neural crest GRN, although we can not rule out the possibility that this phenomenon is resulted from the cross-regulation between the neural crest genes. Interestingly, overexpression of hMLL1 promoted the expression of Zic1 and FoxD3, but not Snail2 and Twist1, suggesting differential epigenetic regulations of the neural crest genes. Epigenetic mechanisms have also been implicated in folate deficiency-related neural tube defect mouse model Splotch, which carries a loss of function mutation in Pax3. In cells from caudal neural tubes of Pax3 mutant embryos, the expression of the histone demethylases KDM6B decreases and the cells exhibit increased H3K27 methylation [41]. Interestingly, these phenotypes could also be rescued by addition of exogenous folic acid [41]. Pax3 is a key regulator in neural crest development and the H3K4 level of Pax3 transcript region is also developmentally regulated [28]. However, the expression of Pax3 itself was not sensitive to folate levels in our study. These data suggest that different mechanisms are likely involved in the epigenetic regulation of the neural crest genes. There is increasing evidence that chromatin modification plays important roles in vertebrate neural crest development. For example, the CHD (chromodomain helicase DNA-binding domain) member CHD7, an ATP dependent chromatin remodeller, is shown to be essential for neural crest specification and migration [42]. Recently, the histone demethylase JmjD2A has been shown to be required for neural crest induction, which is recruited to the regulatory regions of neural crest genes and thus poises neural crest differentiation [43].
Supplementary folate has been shown to have relatively specific protective effects on neural tube and neural crest cells, suggesting that these cells might be more sensitively regulated by folate-related pathways. In addition to folate, microinjection of SAM or VB12 also affects neural crest development in Xenopus embryos (Fig. S2), possibly through promoting methylation reactions and thus epigenetic regulations. These data also manifests the role of nutrients in gene transcriptional regulation through epigenetic modification, highlighting the association of dietary intake with epigenetic modification, as well as diseases.
Materials and Methods
Ethics Statement
The care of Xenopus laevis (Nasco), in vitro fertilization procedure and embryos study were performed according to protocols approved by the Ethics Committee of Kunming Institute of Zoology, Chinese Academy of Sciences (permit number: SYDW-2006-006).
Microinjection and in situ hybridization
In vitro fertilization, embryo culture, whole mount in situ hybridization,preparation of mRNA, and microinjection were carried out as described [44]. The sequence of the antisense morpholino oligo (MO) for XRFC used was: 5′-ATTCTGTGTCATCTCGGCAGCAACT-3′, which was obtained from Gene Tools (OR). Two XRFC alleles were found in the EST database and the targeted sequence is 100% conserved between them. For in situ hybridization, the probes for Msx1, Pax3, Zic1, Snail2, FoxD3 and Twist1 were used as described [6], [45]–[47].
Plasmids construction
Full-length Xenopus laevis RFC coding region was obtained by PCR according to sequence in GenBank (accession no. BC073675) and cloned into pCS2+ vector. An EST clone (IMAGE: 3509422) containing full length hRFC was obtained from Open Biosystems and was subcloned into pCS2+ vector. To prepare the pCS-hRFC R133C and R373C mutation constructs, site-directed mutations were carried out by PCR driven overlap extension [48] using Pfu DNA polymerase (Fermentas). The GR fusion constructs for inducible expression of XSnail1, XSnail2, Snail1 N, Snail1 Znf, Snail2 N and Snail2 Znf were prepared as described [45].
Reverse transcription and polymerase chain reaction (RT-PCR) and animal cap assay
To analyze the temporal expression of XRFC during development, RT-PCR was carried out using whole embryos at different developmental stages. The primers used were: XRFC: 5′- CTGGTTCCCATAGCCATCTT-3′ and 5′- TTTGGAGGGATTTGAGGTTT-3′. H4 was used as a loading control. For animal cap assay to analyze the effect of XRFC in neural crest induction, the embryos were injected with XWnt7b and tBR mRNA with or without XRFC-MO or RFC mRNA at 2-cell stage. The animal caps were cut at stage 9 and cultured till control embryos reached stage 15–17, and processed for RT-PCR. The primers used for PCR were: Pax3: 5′-CAGCCGAATTTTGAGGAGCAAAT-3′ and 5′-GGGCAGGTCTGGTTCGGAG TC-3′; Snail2: 5′ -TCCCGCACTGAAAATGCCACGATC -3′ and 5′- CCGTCCTAAAGATGAAGGGTATCCTG -3′. The primers for Msx1, Zic1, FoxD3 and Snail1 were used as described [49]–[51].
Neural crest graft experiment and cartilage staining
The donor embryos were injected with EGFP mRNA in both blastomeres at 2-cell stage and the EGFP-labeled neural crest explants were separated at stages 14–17 and implanted into the corresponding regions of host embryos as described [23]. Cartilage staining was carried out using Alcian blue 8GX according to protocol of Dr. Richard Harland's lab (http://tropicalis.berkeley.edu/home/gene_expression/cartilage-stain/alcian.html).
Western blot analysis
Embryos were injected with XRFC-MO, XRFC-MO+mRNA, or 5-MTHF in all blasomeres at 2-cell stage and animal caps were cut at stage 9 and cultured till control embryos reached St.15–17. The injected and control animal caps were then harvested and lysed in modified TNE lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.5 mM EDTA, and 0.5% Triton X-100) containing protease inhibitor cocktail (Roche) and processed for Western blot. The antibodies used for monomethyl-, dimethyl- and trimethyl-H3-K4 were from Millipore.
Supporting Information
RFC is involved in the neural crest gene regulatory network. (A, B) The inhibition of Zic1 expression by dominant negative Msx1 can be rescued by XRFC. (C, D) Msx1 rescues the inhibition of Zic1 expression by XRFC-MO. (E–H) The inhibition of Snail2 expression by XRFC-MO can be rescued by Msx1, Pax3 and Zic1 co-expression. (I–N) The inhibition of FoxD3 expression by dominant negative Msx1, Pax3 and Zic1 can be rescued by co-injection of XRFC. (O, P) The inhibition effect on neural crest migration (as indicated by the distribution of the Twist1 positive neural crest cells) of Snail1-Znf can be rescued by co-injection of XRFC. The injected sides are indicated by the red staining of tracing lacZ. (Q) Quantification of the rescue effect of RFC on the expression of Zic1, FoxD3 or Twist1 inhibited by dominant negative Msx1, Pax3, Zic1 and Snail1 and the rescue of the XRFC-MO effect by Msx1, Pax3, and Zic1.
(TIF)
Components of the folate metabolism pathway regulate neural crest gene expression in Xenopus embryos. (A–H) Both folate transportation deficient forms of hRFC (R133C/R373C) failed to promote neural crest makers expression (Twist1, FoxD3, Snail2 and Zic1). (I–T) Injection of 5-MTHF (20ng/embryo, I–L), Vitamin B12 (50ng/embryo, M–P) or SAM (10ng/embryo, Q–T) promoted neural crest maker genes expression (Twist1, FoxD3, Snail2 and Zic1). (U) Quantification of the promoting effect on the expression of neural crest genes by hRFC, hRFC-R133C, hRFC-R373C and 5-MTHF, SAM, and Vitamin B12.
(TIF)
The folate antagonist MTX blocks neural crest induction. (A–C) Injection of MTX (10ng/embryo) inhibits the expression of Twist1, Snail2 and Zic1 in the injected sides. (D–F) Co-injection 5-MTHF (20ng/embryo) with MTX rescues the effects of MTX on neural crest gene expression. (G) Quantification of the inhibitory effect of MTX on the expression of neural crest genes and its rescue by 5-MTHF.
(TIF)
Acknowledgments
We thank Drs. A. Monsoro-Burq, R. Mayor, D. Melton, A. Zorn, S. Sokol and W. Xiao for plasmids. We thank Dr. Shuhua Zhao for helpful discussions and Mr. Philip T. T. Ly for careful reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the Key State Research Program from the Ministry of Science and Technology of China (2007CB947201), the National Natural Science Foundation of China (90919039) and the CAS Key Project (KSCX2-EW-R-05). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 2.Bolande RP. Neurocristopathy: its growth and development in 20 years. Pediatr Pathol Lab Med. 1997;17:1–25. [PubMed] [Google Scholar]
- 3.Jones MC. The neurocristopathies: reinterpretation based upon the mechanism of abnormal morphogenesis. Cleft Palate J. 1990;27:136–140. doi: 10.1597/1545-1569(1990)027<0136:tnrbut>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Gammill LS, Bronner-Fraser M. Neural crest specification: migrating into genomics. Nat Rev Neurosci. 2003;4:795–805. doi: 10.1038/nrn1219. [DOI] [PubMed] [Google Scholar]
- 5.Steventon B, Carmona-Fontaine C, Mayor R. Genetic network during neural crest induction: from cell specification to cell survival. Semin Cell Dev Biol. 2005;16:647–654. doi: 10.1016/j.semcdb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Antony AC, Hansen DK. Hypothesis: folate-responsive neural tube defects and neurocristopathies. Teratology. 2000;62:42–50. doi: 10.1002/1096-9926(200007)62:1<42::AID-TERA9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Beaudin AE, Stover PJ. Folate-mediated one-carbon metabolism and neural tube defects: balancing genome synthesis and gene expression. Birth Defects Res C Embryo Today. 2007;81:183–203. doi: 10.1002/bdrc.20100. [DOI] [PubMed] [Google Scholar]
- 9.Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitkin RM. Folate and neural tube defects. Am J Clin Nutr. 2007;85:285S–288S. doi: 10.1093/ajcn/85.1.285S. [DOI] [PubMed] [Google Scholar]
- 11.Obican SG, Finnell RH, Mills JL, Shaw GM, Scialli AR. Folic acid in early pregnancy: a public health success story. FASEB J. 2010;24:4167–4174. doi: 10.1096/fj.10-165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw GM, O'Malley CD, Wasserman CR, Tolarova MM, Lammer EJ. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. Am J Med Genet. 1995;59:536–545. doi: 10.1002/ajmg.1320590428. [DOI] [PubMed] [Google Scholar]
- 13.van Rooij IA, Ocke MC, Straatman H, Zielhuis GA, Merkus HM, et al. Periconceptional folate intake by supplement and food reduces the risk of nonsyndromic cleft lip with or without cleft palate. Prev Med. 2004;39:689–694. doi: 10.1016/j.ypmed.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Boot MJ, Steegers-Theunissen RP, Poelmann RE, van Iperen L, Gittenberger-de Groot AC. Cardiac outflow tract malformations in chick embryos exposed to homocysteine. Cardiovasc Res. 2004;64:365–373. doi: 10.1016/j.cardiores.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Boot MJ, Steegers-Theunissen RP, Poelmann RE, Van Iperen L, Lindemans J, et al. Folic acid and homocysteine affect neural crest and neuroepithelial cell outgrowth and differentiation in vitro. Dev Dyn. 2003;227:301–308. doi: 10.1002/dvdy.10303. [DOI] [PubMed] [Google Scholar]
- 16.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matherly LH. Molecular and cellular biology of the human reduced folate carrier. Prog Nucleic Acid Res Mol Biol. 2001;67:131–162. doi: 10.1016/s0079-6603(01)67027-2. [DOI] [PubMed] [Google Scholar]
- 18.Moscow JA, Gong M, He R, Sgagias MK, Dixon KH, et al. Isolation of a gene encoding a human reduced folate carrier (RFC1) and analysis of its expression in transport-deficient, methotrexate-resistant human breast cancer cells. Cancer Res. 1995;55:3790–3794. [PubMed] [Google Scholar]
- 19.Maddox DM, Manlapat A, Roon P, Prasad P, Ganapathy V, et al. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC Dev Biol. 2003;3:6. doi: 10.1186/1471-213X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whetstine JR, Flatley RM, Matherly LH. The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: identification of seven non-coding exons and characterization of a novel promoter. Biochem J. 2002;367:629–640. doi: 10.1042/BJ20020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelineau-van Waes J, Heller S, Bauer LK, Wilberding J, Maddox JR, et al. Embryonic development in the reduced folate carrier knockout mouse is modulated by maternal folate supplementation. Birth Defects Res A Clin Mol Teratol. 2008;82:494–507. doi: 10.1002/bdra.20453. [DOI] [PubMed] [Google Scholar]
- 22.Chang C, Hemmati-Brivanlou A. Neural crest induction by Xwnt7B in Xenopus. Dev Biol. 1998;194:129–134. doi: 10.1006/dbio.1997.8820. [DOI] [PubMed] [Google Scholar]
- 23.Borchers A, Epperlein HH, Wedlich D. An assay system to study migratory behavior of cranial neural crest cells in Xenopus. Dev Genes Evol. 2000;210:217–222. doi: 10.1007/s004270050307. [DOI] [PubMed] [Google Scholar]
- 24.Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- 25.Hou Z, Ye J, Haska CL, Matherly LH. Transmembrane domains 4, 5, 7, 8, and 10 of the human reduced folate carrier are important structural or functional components of the transmembrane channel for folate substrates. J Biol Chem. 2006;281:33588–33596. doi: 10.1074/jbc.M607049200. [DOI] [PubMed] [Google Scholar]
- 26.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 27.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Akkers RC, van Heeringen SJ, Jacobi UG, Janssen-Megens EM, Francoijs KJ, et al. A hierarchy of H3K4me3 and H3K27me3 acquisition in spatial gene regulation in Xenopus embryos. Dev Cell. 2009;17:425–434. doi: 10.1016/j.devcel.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Glaser S, Schaft J, Lubitz S, Vintersten K, van der Hoeven F, et al. Multiple epigenetic maintenance factors implicated by the loss of Mll2 in mouse development. Development. 2006;133:1423–1432. doi: 10.1242/dev.02302. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Lee DK, Dou Y, Lee J, Lee B, et al. Coactivator as a target gene specificity determinant for histone H3 lysine 4 methyltransferases. Proc Natl Acad Sci U S A. 2006;103:15392–15397. doi: 10.1073/pnas.0607313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagi H, Deguchi K, Aono A, Tani Y, Kishimoto T, et al. Growth disturbance in fetal liver hematopoiesis of Mll-mutant mice. Blood. 1998;92:108–117. [PubMed] [Google Scholar]
- 33.Yu BD, Hanson RD, Hess JL, Horning SE, Korsmeyer SJ. MLL, a mammalian trithorax-group gene, functions as a transcriptional maintenance factor in morphogenesis. Proc Natl Acad Sci U S A. 1998;95:10632–10636. doi: 10.1073/pnas.95.18.10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Djabali M, Selleri L, Parry P, Bower M, Young BD, et al. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet. 1992;2:113–118. doi: 10.1038/ng1092-113. [DOI] [PubMed] [Google Scholar]
- 35.Burren KA, Savery D, Massa V, Kok RM, Scott JM, et al. Gene-environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum Mol Genet. 2008;17:3675–3685. doi: 10.1093/hmg/ddn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunlevy LP, Chitty LS, Burren KA, Doudney K, Stojilkovic-Mikic T, et al. Abnormal folate metabolism in foetuses affected by neural tube defects. Brain. 2007;130:1043–1049. doi: 10.1093/brain/awm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitsu H, Ishibashi M, Nakano H, Shiota K. Spatial and temporal expression of folate-binding protein 1 (Fbp1) is closely associated with anterior neural tube closure in mice. Dev Dyn. 2003;226:112–117. doi: 10.1002/dvdy.10203. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Wlodarczyk BJ, Scott M, Yu W, Merriweather M, et al. Cardiovascular abnormalities in Folr1 knockout mice and folate rescue. Birth Defects Res A Clin Mol Teratol. 2007;79:257–268. doi: 10.1002/bdra.20347. [DOI] [PubMed] [Google Scholar]
- 39.Rosenquist TH, Chaudoin T, Finnell RH, Bennett GD. High-affinity folate receptor in cardiac neural crest migration: a gene knockdown model using siRNA. Dev Dyn. 2010;239:1136–1144. doi: 10.1002/dvdy.22270. [DOI] [PubMed] [Google Scholar]
- 40.Gelineau-van Waes J, Maddox JR, Smith LM, van Waes M, Wilberding J, et al. Microarray analysis of E9.5 reduced folate carrier (RFC1; Slc19a1) knockout embryos reveals altered expression of genes in the cubilin-megalin multiligand endocytic receptor complex. BMC Genomics. 2008;9:156. doi: 10.1186/1471-2164-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichi S, Costa FF, Bischof JM, Nakazaki H, Shen YW, et al. Folic acid remodels chromatin on Hes1 and Neurog2 promoters during caudal neural tube development. J Biol Chem. 2010;285:36922–36932. doi: 10.1074/jbc.M110.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, et al. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strobl-Mazzulla PH, Sauka-Spengler T, Bronner-Fraser M. Histone demethylase JmjD2A regulates neural crest specification. Dev Cell. 2010;19:460–468. doi: 10.1016/j.devcel.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng W, Kong Q, Li C, Mao B. Xenopus RCOR2 (REST corepressor 2) interacts with ZMYND8, which is involved in neural differentiation. Biochem Biophys Res Commun. 2010;394:1024–1029. doi: 10.1016/j.bbrc.2010.03.115. [DOI] [PubMed] [Google Scholar]
- 45.Aybar MJ, Nieto MA, Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development. 2003;130:483–494. doi: 10.1242/dev.00238. [DOI] [PubMed] [Google Scholar]
- 46.Sasai N, Mizuseki K, Sasai Y. Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development. 2001;128:2525–2536. doi: 10.1242/dev.128.13.2525. [DOI] [PubMed] [Google Scholar]
- 47.Sato T, Sasai N, Sasai Y. Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development. 2005;132:2355–2363. doi: 10.1242/dev.01823. [DOI] [PubMed] [Google Scholar]
- 48.Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2:924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- 49.Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–3124. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- 50.Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic family and its role in neural and neural crest development. Mech Dev. 1998;75:43–51. doi: 10.1016/s0925-4773(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 51.Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RFC is involved in the neural crest gene regulatory network. (A, B) The inhibition of Zic1 expression by dominant negative Msx1 can be rescued by XRFC. (C, D) Msx1 rescues the inhibition of Zic1 expression by XRFC-MO. (E–H) The inhibition of Snail2 expression by XRFC-MO can be rescued by Msx1, Pax3 and Zic1 co-expression. (I–N) The inhibition of FoxD3 expression by dominant negative Msx1, Pax3 and Zic1 can be rescued by co-injection of XRFC. (O, P) The inhibition effect on neural crest migration (as indicated by the distribution of the Twist1 positive neural crest cells) of Snail1-Znf can be rescued by co-injection of XRFC. The injected sides are indicated by the red staining of tracing lacZ. (Q) Quantification of the rescue effect of RFC on the expression of Zic1, FoxD3 or Twist1 inhibited by dominant negative Msx1, Pax3, Zic1 and Snail1 and the rescue of the XRFC-MO effect by Msx1, Pax3, and Zic1.
(TIF)
Components of the folate metabolism pathway regulate neural crest gene expression in Xenopus embryos. (A–H) Both folate transportation deficient forms of hRFC (R133C/R373C) failed to promote neural crest makers expression (Twist1, FoxD3, Snail2 and Zic1). (I–T) Injection of 5-MTHF (20ng/embryo, I–L), Vitamin B12 (50ng/embryo, M–P) or SAM (10ng/embryo, Q–T) promoted neural crest maker genes expression (Twist1, FoxD3, Snail2 and Zic1). (U) Quantification of the promoting effect on the expression of neural crest genes by hRFC, hRFC-R133C, hRFC-R373C and 5-MTHF, SAM, and Vitamin B12.
(TIF)
The folate antagonist MTX blocks neural crest induction. (A–C) Injection of MTX (10ng/embryo) inhibits the expression of Twist1, Snail2 and Zic1 in the injected sides. (D–F) Co-injection 5-MTHF (20ng/embryo) with MTX rescues the effects of MTX on neural crest gene expression. (G) Quantification of the inhibitory effect of MTX on the expression of neural crest genes and its rescue by 5-MTHF.
(TIF)