Abstract
Most probiotic lactobacilli adhere to intestinal surfaces, a phenomenon influenced by free polyunsaturated fatty acids (PUFA). The present study investigated whether free linoleic acid, γ-linolenic acid, arachidonic acid, α-linolenic acid, or docosahexaenoic acid in the growth medium alters the fatty acid composition of lactobacilli and their physical characteristics. The most abundant bacterial fatty acids identified were oleic, vaccenic, and dihydrosterculic acids. PUFA, especially conjugated linoleic acid (CLA) isomers and γ-linolenic, eicosapentaenoic, docosahexaenoic, and α-linolenic acids, also were identified in lactobacilli. When lactobacilli were cultured in MRS broth supplemented with various free PUFA, the incorporation of a given PUFA into bacterial fatty acids was clearly observed. Moreover, PUFA supplementation also resulted in PUFA-dependent changes in the proportions of other fatty acids; major interconversions were seen in octadecanoic acids (18:1), their methylenated derivatives (19:cyc), and CLA. Intermittent changes in eicosapentaenoic acid proportions also were noted. These results were paralleled by minor changes in the hydrophilic or hydrophobic characteristics of lactobacilli, suggesting that PUFA interfere with microbial adhesion to intestinal surfaces through other mechanisms. In conclusion, we have demonstrated that free PUFA in the growth medium induce changes in bacterial fatty acids in relation to the regulation of the degree of fatty acid unsaturation, cyclization, and proportions of CLA and PUFA containing 20 to 22 carbons. The potential role of lactobacilli as regulators of PUFA absorption may represent another means by which probiotics could redirect the delicate balance of inflammatory mediators derived from PUFA within the inflamed intestine.
Probiotic bacteria are defined as “live microbial food and feed ingredients beneficial to health” (19). Specific probiotics influence the microbial balance of the host and modulate host immunity. The adhesion to and subsequent temporary colonization of intestinal surfaces by probiotic bacteria is a key step in these functions (15). Thus, the ability of a probiotic strain to adhere to mucus and epithelial cell surfaces is one of the main selection criteria for candidate probiotics (13, 22). Several factors are involved in the adhesion of probiotics; one is the hydrophobicity of the bacterial cell surface. Wadström et al. (23) demonstrated that hydrophobic lactobacilli adhered better to intestinal epithelial cells than did hydrophilic strains. As bacterial cells alter their membrane fluidity under various environmental conditions, growth conditions may have a profound effect on the fatty acid compositions of their lipids and subsequently on the hydrophobicity and adhesion ability of bacterial strains. Indeed, Kankaanpää et al. recently showed that various free polyunsaturated fatty acids (PUFA) in the growth medium influence the adhesion of probiotics to mucus and epithelial cells (12).
Although most bacteria lack PUFA, it is known that the majority of bacteria can take up exogenous PUFA present in the culture medium (24). Some bacteria, mainly marine bacteria, even possess the metabolic capacity to synthesize PUFA (18). Data concerning the effects of PUFA on the physicochemical properties of lactobacilli and probiotics are scarce. In the present study, we assessed whether specific probiotic strains could incorporate exogenous free PUFA into bacterial lipids and how the resulting changes influenced the physical properties of the bacteria.
MATERIALS AND METHODS
Bacterial strains and growth conditions. Lactobacillus rhamnosus strain GG (Lactobacillus GG; ATCC 53103), Lactobacillus casei strain Shirota (Yakult Singapore Pty, Ltd.), and Lactobacillus delbrueckii subsp. bulgaricus (ATCC 11842) were selected because they are widely used probiotic (first two) or dairy starter culture strains. Bacteria were grown anaerobically in MRS broth supplemented with linoleic acid (18:2 n-6), γ-linolenic acid (18:3 n-6), arachidonic acid (20:4 n-6), α-linolenic acid (18:3 n-3), or docosahexaenoic acid (22:6 n-3) or without PUFA supplementation at 37°C for 24 h. Gentle agitation was used to facilitate mixing during the incubation period.
The fatty acid composition of nonsupplemented MRS growth medium also was analyzed (2, 26) (Table 1). Even though MRS medium is known (as well as shown) to possess unsaturated fatty acids (e.g., oleic acid, in the form of Tween 80), this medium was selected for this study because Tween 80 has been shown to be an essential growth factor for lactobacilli (16). Moreover, this particular medium is the most commonly used medium in lactobacillus research; evaluation of the relevance of the present results necessitates the use of this medium.
TABLE 1.
Fatty acid composition of MRS brotha
| Fatty acid | Relative % (wt/wt) |
|---|---|
| 12:0 + 14:0 | 0.5 |
| 16:0 | 4.7 |
| 18:0 | 1.5 |
| 19:0 | 0.4 |
| 20:0 + 22:0 | 0.4 |
| 16:1 n-7 | 1.0 |
| 18:1 n-9 | 65.9 |
| 18:1 n-7 | 2.0 |
| 20:1 n-9 | 2.1 |
| 22:1 n-9 | 0.5 |
| 18:2 n-6 | 0.1 |
| 18:2 c-9,t-11 and t-8,c-10b | 3.3 |
| 18:2 t-10,c-12 and c-11,t-13b | 3.1 |
| 20:3 n-3 | 0.2 |
| 22:6 n-3 + 24:1 | 0.6 |
| Unidentified | 13.6 |
The total fatty acid content was 0.27 mg/ml.
Overlapping peaks in the chromatograms.
Preparation of bacterial cell fatty acid extracts.
Bacteria were grown anaerobically in MRS broth supplemented with PUFA at 5 μg ml−1 or without PUFA supplementation. After cultivation, bacterial cells were harvested by centrifugation for 7 min at 1,500 × g and 4°C and washed twice with phosphate-buffered saline (pH 7.4). Each bacterial culture tube, containing approximately 100 to 500 mg of wet cell biomass, was capped and stored at 4°C prior to analysis. Bacterial fatty acid methyl esters were prepared and extracted, and bacterial cellular fatty acid gas chromatography analysis was performed by the Anaer1 method (14) of the Microbial Identification System (Microbial ID, Newark, Del.).
The saponification procedure was started by adding 1 ml of basic methanol (one portion of 3.7 M NaOH in methanol mixed with one portion of deionized distilled water) to each sample tube. The tubes were sealed tightly and vortexed for 5 to 10 s. The samples then were boiled at 100 ± 2°C for 5 min, cooled slightly, vortexed again for 5 to 10 s, and finally boiled in a water bath (100 ± 2°C) for an additional 25 min.
To methylate the fatty acids (now as sodium salts), 2 ml of methylation reagent (6.0 M HCl- methanol [13:11, vol/vol]) was added to each tube. The tubes were capped tightly, and the solutions were vortexed for 5 to 10 s and heated in an 80 ± 2°C water bath for 10 ± 1 min. The tubes then were cooled quickly to room temperature under a water tap.
Fatty acid methyl esters were transferred from the acidic aqueous phase to the organic phase with a liquid-liquid extraction procedure. A total of 1.25 ml of extraction solvent (hexane- methyl-t-butyl ether [1:1, vol/vol]) was added to each tube. The tubes were sealed tightly and mixed end-over-end for 10 min. The upper organic phase was collected.
Residual free fatty acids and residual reagents of the organic extract were removed by adding 3.0 ml of 0.3 M NaOH. The tightly capped tubes were mixed end-over-end for 5 min and then centrifuged (3 min at 1,000 × g) to clarify the interface. The upper solvent phase was removed and stored for gas chromatography analysis.
Gas chromatography analysis of bacterial extracts.
The solvent was evaporated, and the bacterial extracts were resuspended to 0.5 ml of hexane and analyzed in duplicate with a Perkin-Elmer (San Jose, Calif.) AutoSystem gas chromatograph equipped with a programmed split/splitless injector and flame ionization detector and controlled with Turbochrom Navigator 4 (Perkin-Elmer). Silica capillary column NB-351 (25 m, 0.32-mm inner diameter, 0.2-μm film thickness; HNU-Nordion Ltd., Helsinki, Finland) was used for the analysis. The injection volume was 1 μl, and a split valve was opened after 1 min. After opening of the split valve (split ratio of 1:40), the flow rate of the carrier gas (helium) was 1.7 ml/min. The temperature program was 120°C held for 2 min, increased at a rate of 3°C/min to 230°C, and 230°C held for 20 min. The injector temperature was programmed from 170 to 250°C at a rate of 20°C/min. The detector temperature was 270°C.
Peaks were identified by comparing their retention times with those of a known standard mixture (68D NuChek Prep; Elysian) or by coinjection with reference compounds; the CLA60 standard was kindly provided by K. Nurmela, Valio Ltd., Helsinki, Finland, and the 19:cyc standard containing methyl esters of dihydrosterculic acid (cis-9,10-methyleneoctadecanoate) and lactobacillic acid (cis-11,12-methyleneoctadecanoate) was purchased from Larodan Fine Chemicals AB, Malmö, Sweden. The fatty acid compositions were expressed as relative percentages (weight/weight).
Physical properties.
Microbial adhesion to solvents (MATS) was investigated by comparing bacterial cell affinities for polar and nonpolar solvents. A modification of the method described by Briandet et al. (1) was used. Briefly, the following solvent pairs were used: (i) chloroform (polar solvent) and tetradecane (nonpolar solvent) and (ii) ethyl acetate (polar solvent) and octane (nonpolar solvent). Of the polar solvents, ethyl acetate especially is a strong electron donor. The two nonpolar solvents were used to estimate the hydrophobicity properties of the lactobacilli, whereas the two polar solvents were selected for estimation of the Lewis acid/base (i.e., electron donor/electron acceptor) properties. To measure the basic characteristics of the lactobacilli, the affinities for polar acidic chloroform and for nonpolar tetradecane were compared. Similarly, the acidic characteristics of the lactobacilli were assessed by comparing the affinities for polar basic ethyl acetate and for nonpolar octane.
Bacteria were grown in standard MRS broth or in MRS broth with various free PUFA at 5 or 20 μg ml−1. Bacteria then were harvested by centrifugation for 7 min at 1,500 × g and 4°C and washed twice with and eventually resuspended in 0.15 M NaCl. The high electrolyte concentration was used to avoid charge interference (some nonpolar solvent droplets may become negatively charged in aqueous solutions and subsequently mask the cell surface charge). The turbidity of microbial suspensions at 600 nm was adjusted to 0.25 ± 0.01 (mean and standard error of the mean) (giving a CFU of 1 × 108 to 2 × 108 ml−1), and a 1-ml sample was taken (sample A0). A total of 2.4 ml of the microbial solution was vortexed for 1 min with 0.4 ml of solvent, and the mixture was allowed to stand for 15 min to completely separate the two phases. Another 1-ml sample was carefully taken from the aqueous phase (sample A). The turbidities of both samples at 400 nm were determined. The percentage of bacterial cells present in each solvent then was calculated by using the following equation: percent affinity = 100 × [1 − (A/A0)]. To facilitate evaluation of the basic and acidic characteristics of lactobacilli, ratios of specific solvent pairs, i.e., chloroform/tetradecane and ethyl acetate/octane ratios, were calculated, plotted, and statistically assessed.
Statistics.
Bacterial whole-cell fatty acid methyl esters were prepared from six independent bacterial cultures, and gas chromatography analysis of each prepared sample was performed in duplicate. Each hydrophobicity experiment was performed in duplicate with three independently prepared cultures. The results are expressed as the mean and standard error of the mean. The changes in lipid composition and hydrophobicity associated with the various growth conditions were normally distributed. Statistical differences were tested with a two-tailed paired t test. P values of less than 0.05 were considered to be significant. Statistical analysis was performed with the StatView 4.57 (Abacus Concepts Inc., Berkeley, Calif.) statistical software package.
RESULTS
Bacterial cell fatty acid composition.
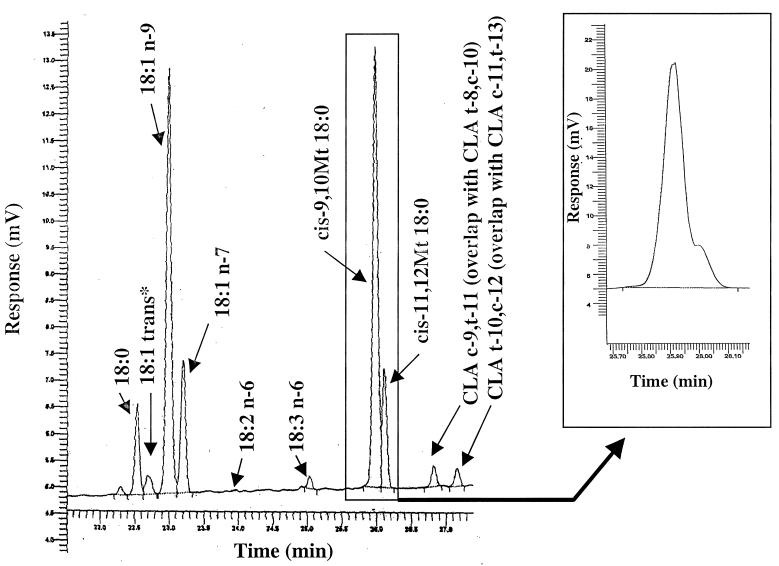
The system used in this study was able to resolve oleic and vaccenic acid methyl esters better than 95%. Pure dihydrosterculic and lactobacillic acid methyl esters also were resolved. However, when samples contained α-linolenic acid, the peaks of dihydrosterculic acid and α-linolenic acid did partially overlap (the principal component was identified as dihydrosterculic acid on the basis of coinjection with the reference compound). In this case, the peak of lactobacillic acid appeared as a shoulder with the α-linolenic acid peak (Fig. 1). Two conjugated linoleic acid (CLA) peaks also were recognized. Based on the reference material used, the first peak appeared to be a mixture of two isomers, namely, c-9,t-11 (the major isomer) and t-8,c-10, whereas the latter was a mixture of t-10,c-12 (the major isomer) and c-11,t-13 isomers.
FIG. 1.
Enlarged chromatogram of the region of the 18:0, 18:1, and CLA isomers. The chromatogram represents the cellular fatty acids of Lactobacillus GG cultured in standard, non-PUFA-supplemented MRS broth. The cyclopropenic acid region of another chromatogram (Lactobacillus GG cultured in α-linolenic acid-supplemented MRS broth) is shown in the inset. An asterisk indicates identification of a peak not based on the standard.
As shown in Tables 2, 3, and 4, all three lactobacilli grown normally (i.e., without any PUFA supplementation) possessed various PUFA. Above all, CLA isomers and γ-linolenic, eicosapentaenoic, docosahexaenoic, and α-linolenic acids were present, while linoleic and arachidonic acids were absent from all three lactobacilli tested. The most abundant bacterial fatty acids identified were oleic, vaccenic, and dihydrosterculic acids.
TABLE 2.
Effect of various free PUFA in growth medium on fatty acid compositions of total lipids of Lactobacillus GGa
| Identified fatty acid | Mean ± SEM % (wt/wt) fatty acid in Lactobacillus GG grown in MRS broth supplemented with:
|
|||||
|---|---|---|---|---|---|---|
| Nothing (control) | 18:2 n-6 | 18:3 n-6 | 20:4 n-6 | 18:3 n-3 | 22:6 n-3 | |
| SAFA | 21.5 ± 3.8 | 31.8 ± 0.6 | 31.1 ± 0.9 | 15.8 ± 0.1 | 11.9 ± 0.1** | 15.4 ± 0.1 |
| 18:1 n-9 | 21.8 ± 2.5 | 19.1 ± 1.5 | 20.6 ± 0.1 | 27.3 ± 0.2 | 28.0 ± 0.1 | 29.2 ± 0.3* |
| 18:1 n-7 | 4.3 ± 0.7 | 6.2 ± 0.4* | 5.6 ± 0.5 | 3.5 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.0 |
| 18:2 n-6 | ND | 3.6 ± 0.5* | ND | ND | ND | ND |
| 18:3 n-6 | 0.3 ± 0.1 | 0.5 ± 0.1 | 2.1 ± 0.4* | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 |
| 20:4 n-6 | ND | ND | ND | 0.5 ± 0.0* | ND | ND |
| cis-9, 10Mt 18:0 and 18:3 n-3b | 23.5 ± 1.7 | 15.8 ± 0.6* | 18.1 ± 0.8* | 31.5 ± 0.6* | 27.7 ± 0.3 | 30.2 ± 0.4* |
| cis-11, 12Mt 18:0 | 2.1 ± 1.2 | 3.7 ± 0.1 | 2.9 ± 0.6 | ND | 3.1 ± 0.0 | ND |
| 20:5 n-3 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.3 | 0.9 ± 0.0* | 0.9 ± 0.0 | 0.9 ± 0.1 |
| 22:6 n-3 + 24:1b | 1.7 ± 0.2 | 1.1 ± 0.1* | 1.1 ± 0.1** | 1.6 ± 0.2 | 1.8 ± 0.1 | 1.1 ± 0.1** |
| 18:2 c-9,t-11 and t-8,c-10b | 1.5 ± 0.2 | 0.8 ± 0.0** | 0.9 ± 0.1* | 1.9 ± 0.0 | 1.9 ± 0.1 | 2.0 ± 0.0** |
| 18:2 t-10,c-12 and c-11,t-13b | 1.4 ± 0.2 | 0.8 ± 0.0** | 0.9 ± 0.1* | 1.9 ± 0.0** | 1.9 ± 0.0 | 2.1 ± 0.0** |
The PUFA concentration was 5 μg/ml. Six independently prepared fatty acid methyl esters were analyzed in duplicate. *, P < 0.05; **, P < 0.07. SAFA, 14:0 + 16:0 + 18:0 + 20:0 + 22:0; ND, not detected.
Overlapping peaks in the chromatograms.
TABLE 3.
Effect of various free PUFA in growth medium on fatty acid compositions of total-lipids of L. delbrueckii subsp. bulgaricusa
| Identified fatty acid | Mean ± SEM % (wt/wt) fatty acid in L. bulgaricus grown in MRS broth supplemented with:
|
|||||
|---|---|---|---|---|---|---|
| Nothing (control) | 18:2 n-6 | 18:3 n-6 | 20:4 n-6 | 18:3 n-3 | 22:6 n-3 | |
| SAFA | 13.8 ± 1.4 | 13.3 ± 0.6 | 14.2 ± 1.2 | 13.6 ± 0.7 | 13.9 ± 0.2 | 17.0 ± 0.2* |
| 18:1 n-9 | 29.0 ± 3.9 | 34.3 ± 1.7 | 39.2 ± 2.4* | 37.0 ± 1.9 | 27.5 ± 0.5 | 29.7 ± 0.5 |
| 18:1 n-7 | 3.5 ± 0.1 | 3.3 ± 0.1* | 3.1 ± 0.2 | 3.4 ± 0.0 | 2.8 ± 0.0* | 3.4 ± 0.0 |
| 18:2 n-6 | ND | 3.5 ± 0.8* | ND | ND | ND | ND |
| 18:3 n-6 | 0.5 ± 0.2 | 0.8 ± 0.4 | 2.1 ± 0.6* | 0.4 ± 0.1 | 0.6 ± 0.2 | 0.5 ± 0.1 |
| 20:4 n-6 | ND | ND | ND | 0.4 ± 0.0* | ND | ND |
| cis-9,10Mt 18:0 and 18:3 n-3b | 30.3 ± 0.2 | 27.7 ± 0.3 | 21.6 ± 3.5 | 26.6 ± 1.5 | 35.6 ± 0.7* | 29.2 ± 0.7 |
| cis-11,12Mt 18:0 | ND | ND | ND | ND | ND | ND |
| 20:5 n-3 | 1.4 ± 0.6 | 1.0 ± 0.1 | 1.1 ± 0.3 | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| 22:6 n-3 + 24:1b | 1.7 ± 0.3 | 1.3 ± 0.4 | 1.1 ± 0.3 | 1.2 ± 0.4 | 1.9 ± 0.3 | 1.7 ± 0.3 |
| 18:2 c-9,t-11 and t-8, c-10b | 1.4 ± 0.2 | 1.1 ± 0.0 | 0.9 ± 0.2 | 1.1 ± 0.0 | 2.0 ± 0.0* | 1.9 ± 0.0* |
| 18:2 t-10,c-12 and c-11,t-13b | 1.3 ± 0.3 | 1.1 ± 0.0 | 0.8 ± 0.2 | 1.1 ± 0.1 | 1.9 ± 0.0** | 1.9 ± 0.0* |
The PUFA concentration was 5 μg/ml. Six independently prepared fatty acid methyl esters were analyzed in duplicate. *, P < 0.05; **, P < 0.07. SAFA, 14:0 + 16:0 + 18:0 + 20:0 + 22:0; ND, not detected.
Overlapping peaks in the chromatograms.
TABLE 4.
Effect of various free PUFA in growth medium on fatty acid compositions of total-lipids of L. casei Shirotaa
| Identified fatty acid | Mean ± SEM % (wt/wt) fatty acid in L. casei Shirota grown in MRS supplemented with:
|
|||||
|---|---|---|---|---|---|---|
| Nothing (control) | 18:2 n-6 | 18:3 n-6 | 20:4 n-6 | 18:3 n-3 | 22:6 n-3 | |
| SAFA | 13.5 ± 1.3 | 13.4 ± 0.4 | 13.9 ± 0.6 | 11.9 ± 1.0 | 11.0 ± 0.6 | 12.1 ± 1.0 |
| 18:1 n-9 | 32.4 ± 1.1 | 25.1 ± 0.9* | 28.6 ± 0.2* | 31.7 ± 1.3 | 30.5 ± 0.8 | 33.3 ± 0.8 |
| 18:1 n-7 | 3.1 ± 0.0 | 2.8 ± 0.0* | 2.8 ± 0.0* | 3.1 ± 0.0 | 2.9 ± 0.0* | 3.1 ± 0.0 |
| 18:2 n-6 | ND | 4.6 ± 0.0* | ND | ND | ND | ND |
| 18:3 n-6 | 0.4 ± 0.1 | 0.5 ± 0.0 | 3.1 ± 0.5* | 0.4 ± 0.1 | 0.6 ± 0.3 | 0.4 ± 0.1 |
| 20:4 n-6 | ND | ND | ND | 0.5 ± 0.0* | ND | ND |
| cis-9, 10Mt 18:0 and 18:3 n-3b | 35.3 ± 1.0 | 31.6 ± 1.8 | 31.1 ± 1.1* | 37.5 ± 0.2 | 40.3 ± 0.4* | 36.2 ± 0.4 |
| cis-11,12Mt 18:0 | ND | ND | ND | ND | ND | ND |
| 20:5 n-3 | 0.8 ± 0.0 | 0.9 ± 0.0* | 0.9 ± 0.0* | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 |
| 22:6 n-3 + 24:1b | 1.4 ± 0.1 | 1.9 ± 0.4 | 1.8 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.2 |
| 18:2 c-9,t-11 and t-8,c-10b | 1.7 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.6 ± 0.0 | 1.5 ± 0.0* | 1.6 ± 0.0 |
| 18:2 t-10,c-12 and c-11,t-13b | 1.7 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1** | 1.5 ± 0.0 | 1.4 ± 0.0* | 1.5 ± 0.0 |
The PUFA concentration was 5 μg/ml. Six independently prepared fatty acid methyl esters were analyzed in duplicate. *, P < 0.05; **, P < 0.07. SAFA, 14:0 + 16:0 + 18:0 + 20:0 + 22:0; ND, not detected.
Overlapping peaks in the chromatograms.
Effect of PUFA supplementation on bacterial cell fatty acid composition.
When the bacteria were cultured with various free PUFA, the incorporation of a given PUFA into cell lipids was observed (Tables 2 to 4). Culturing of Lactobacillus GG (Table 2) with linoleic acid increased the proportion of vaccenic acid (P = 0.04) but decreased the proportions of dihydrosterculic acid or α-linolenic acid (P < 0.001) and docosahexaenoic acid plus 24:1 (P = 0.03) and tended to decrease the proportions of both CLA isomers (P < 0.07 for both). γ-Linolenic acid had similar effects on bacterial fatty acids; decreases in the proportions of dihydrosterculic acid or α-linolenic acid (P = 0.02), docosahexaenoic acid plus 24:1 (P < 0.07), and both CLA isomers (P = 0.04 and P = 0.05) were observed. Arachidonic acid in the medium led to different changes in fatty acid proportions in Lactobacillus GG. The proportions of dihydrosterculic acid or α-linolenic acid (P = 0.02) and the t-10,c-12 CLA isomer (P < 0.07) were increased, while that of eicosapentaenoic acid appeared to be decreased (P = 0.04). α-Linolenic acid in the medium resulted only in decreased proportions of saturated fatty acids (SAFA) (P < 0.07), whereas the other n-3 PUFA, docosahexaenoic acid, increased the proportions of oleic acid (P = 0.04), dihydrosterculic acid or α-linolenic acid (P = 0.01), and both CLA isomers (P < 0.07 for both). Paradoxically, docosahexaenoic acid also tended to decrease its own level (P < 0.07) among the fatty acids of Lactobacillus GG. However, the chromatographic method used in the present study cannot separate the peaks of docosahexaenoic acid and 24:1; therefore, results pertaining to docosahexaenoic acid remain unclear.
The incorporation of various free PUFA into bacterial fatty acids also was observed with L. delbrueckii subsp. bulgaricus (Table 3) and L. casei Shirota (Table 4). Linoleic acid and γ-linolenic acid in the growth medium of L. delbrueckii subsp. bulgaricus increased the proportions of vaccenic acid (P = 0.03) and oleic acid (P = 0.05), respectively. The proportions of both CLA isomers were increased by α-linolenic and docosahexaenoic acids (P = 0.05 for both), the same tendency as that seen for Lactobacillus GG fatty acids. α-Linolenic acid also increased the proportions of vaccenic acid (P < 0.001) and dihydrosterculic acid or α-linolenic acid (P = 0.03). Most likely this alteration was due to an increase in the α-linolenic acid level. For L. casei Shirota (Table 4), linoleic and γ-linolenic acids decreased the proportions of oleic acid (P < 0.001 and P = 0.01, respectively) and vaccenic acid (P < 0.001 for both) and increased the proportion of eicosapentaenoic acid (P = 0.05 for both). In addition, γ-linolenic acid decreased the proportion of dihydrosterculic acid or α-linolenic acid (P = 0.02) and tended to increase the proportion of the t-10,c-12 CLA isomer (P < 0.07). Of the n-3 PUFA, docosahexaenoic acid had no effects, while α-linolenic acid decreased the proportions of vaccenic acid (P = 0.01) and both CLA isomers (P = 0.03 for both).
Effect of extracellular PUFA on physical properties of lactobacilli.
Table 5 shows the affinities for the four solvents used in the MATS method of Lactobacillus GG, L. delbrueckii subsp. bulgaricus, and L. casei Shirota cells grown in MRS broth or in MRS broth supplemented with free PUFA. Overall, the affinities of all three tested bacteria for all of the solvents tested were low. The low affinities of all three lactobacilli for nonpolar solvents (both tetradecane and octane) indicate that these bacteria exhibit a hydrophilic rather than a hydrophobic surface. When the lactobacilli were cultured with free PUFA, this modest hydrophobicity was diminished further, a phenomenon especially seen with L. casei Shirota; all tested n-6 PUFA at low concentrations reduced the affinity for octane (P < 0.05). The decrease in affinities for nonpolar solvents was less marked with Lactobacillus GG and L. delbrueckii subsp. bulgaricus, suggesting that the supplemental free PUFA altered the hydrophobicity of these two lactobacilli less than that of L.casei Shirota.
TABLE 5.
Effect of various free PUFA in growth medium on bacterial cell surface properties, measured by using the MATS method (1)a
| Bacterium | PUFA supplementation | PUFA concn (μg/ml) | Mean ± SEM % affinity for:
|
|||
|---|---|---|---|---|---|---|
| Chloroform | Tetradecane | Ethyl acetate | Octane | |||
| Lactobacillus GG | None (control) | 0 | 15 ± 1 | 8 ± 0 | 3 ± 1 | 17 ± 3 |
| 18:2 n-6 | 5 | 10 ± 4 | 13 ± 3 | 2 ± 3 | 12 ± 2 | |
| 20 | 12 ± 1 | 13 ± 4 | 0 ± 1 | 11 ± 3 | ||
| 18:3 n-6 | 5 | 10 ± 2 | 10 ± 2 | 1 ± 1 | 10 ± 0 | |
| 20 | 9 ± 6 | 7 ± 1 | 1 ± 2 | 8 ± 2b | ||
| 20:4 n-6 | 5 | 4 ± 1 | 8 ± 2 | 0 ± 1 | 11 ± 2 | |
| 20 | 8 ± 4 | 5 ± 1 | 1 ± 2 | 12 ± 2 | ||
| 18:3 n-3 | 5 | 9 ± 2 | 10 ± 4 | 2 ± 1 | 10 ± 3 | |
| 20 | 5 ± 2 | 15 ± 2 | 4 ± 2 | 14 ± 1 | ||
| 22:6 n-6 | 5 | 6 ± 1b | 7 ± 1 | 1 ± 3 | 13 ± 3 | |
| 20 | 7 ± 8 | 16 ± 6 | 0 ± 3 | 12 ± 4 | ||
| L. delbrueckii subsp. bulgaricus | None (control) | 0 | 13 ± 3 | 14 ± 2 | 3 ± 2 | 15 ± 2 |
| 18:2 n-6 | 5 | 9 ± 3 | 20 ± 6 | 1 ± 1 | 15 ± 4 | |
| 20 | 15 ± 5 | 17 ± 1 | 2 ± 1 | 11 ± 2 | ||
| 18:3 n-6 | 5 | 9 ± 2 | 22 ± 12 | 2 ± 1 | 12 ± 3 | |
| 20 | 10 ± 2 | 11 ± 2 | 1 ± 1 | 12 ± 4 | ||
| 20:4 n-6 | 5 | 8 ± 3 | 19 ± 10 | 0 ± 0 | 15 ± 1 | |
| 20 | 7 ± 0 | 10 ± 0 | 0 ± 0 | 8 ± 2 | ||
| 18:3 n-3 | 5 | 11 ± 3 | 25 ± 14 | 2 ± 2 | 14 ± 1 | |
| 20 | 13 ± 4 | 10 ± 2 | 2 ± 2 | 22 ± 4 | ||
| 22:6 n-6 | 5 | 8 ± 2 | 9 ± 3 | 1 ± 1 | 15 ± 2 | |
| 20 | 9 ± 2 | 10 ± 3 | 1 ± 1 | 24 ± 8 | ||
| L. casei Shirota | None (control) | 0 | 11 ± 1 | 12 ± 0 | 2 ± 2 | 15 ± 1 |
| 18:2 n-6 | 5 | 7 ± 0b | 14 ± 2 | 1 ± 1 | 11 ± 0b | |
| 20 | 6 ± 2 | 16 ± 2 | 2 ± 0 | 14 ± 3 | ||
| 18:3 n-6 | 5 | 5 ± 0b | 9 ± 2 | 0 ± 1 | 10 ± 1b | |
| 20 | 11 ± 1 | 12 ± 1 | 2 ± 2 | 13 ± 2 | ||
| 20:4 n-6 | 5 | 6 ± 2 | 10 ± 4 | 2 ± 2 | 8 ± 1b | |
| 20 | 10 ± 3 | 10 ± 1 | 2 ± 2 | 14 ± 3 | ||
| 18:3 n-3 | 5 | 5 ± 1 | 13 ± 1 | 0 ± 1 | 10 ± 2 | |
| 20 | 10 ± 2 | 15 ± 1 | 3 ± 1 | 12 ± 2 | ||
| 22:6 n-6 | 5 | 8 ± 2 | 13 ± 1 | 1 ± 2 | 10 ± 1b | |
| 20 | 12 ± 3 | 9 ± 1 | 0 ± 3 | 13 ± 2 | ||
The two nonpolar solvents (tetradecane and octane) were used to estimate the hydrophobicity properties of the lactobacilli, whereas the two polar solvents (chloroform and ethyl acetate) were selected for the estimation of the Lewis acid/base (i.e., electron donor/electron acceptor) character.
P < 0.05, as determined by paired t test.
Microbial adhesion to chloroform revealed an overall modest affinity for this acidic solvent and electron acceptor. Only Lactobacillus GG grown without any PUFA supplementation showed some electron donor and basic characteristics, but these characteristics were diminished when Lactobacillus GG was grown with free PUFA. The data obtained for ethyl acetate demonstrated an affinity even lower than that encountered for chloroform, indicating the nonacidic nature of the bacteria studied.
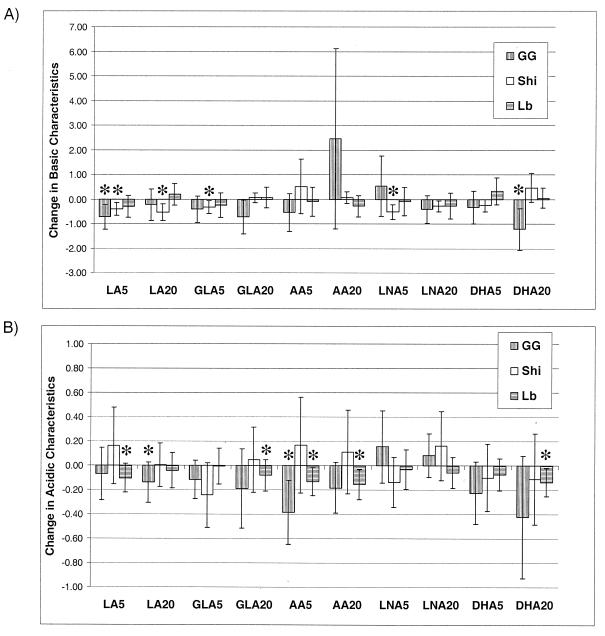
Because chloroform and tetradecane possess the same van der Waals properties, their adhesion values were compared by calculating chloroform/tetradecane ratios (Fig. 2A). Only Lactobacillus GG (control culture) showed some electron-donating nature (adhered more to the acidic chloroform than to tetradecane; ratio, 1.8); no difference in the adhesion of L. casei Shirota and L. delbrueckii subsp. bulgaricus to chloroform and tetradecane was observed (chloroform/tetradecane ratios, 0.9 and 1.0, respectively). These values were set as a baseline, and changes in basic characteristics were evaluated (Fig. 2A). All significant effects reduced the basic surface properties. Namely, low concentrations of linoleic and docosahexaenoic acids reduced the electron-donating nature of Lactobacillus GG (P < 0.05). Likewise, 20 μg of linoleic acid ml−1 and 5 μg each of γ-linolenic and α-linolenic acids ml−1 reduced the electron-donating nature of L. casei Shirota (P = 0.02, P = 0.03, and P = 0.03, respectively).
FIG. 2.
Solvent ratios. (A) The baseline represents the chloroform (CF)/tetradecane (C14) ratio for control cultures (no PUFA supplementation), and the bars represent the differences in CF/C14 ratios between test cultures (different PUFA supplementations) and control cultures; i.e., values below the baseline indicate that the CF/C14 ratio of control cultures was higher than that of test cultures (suggesting decreased basic characteristics of the bacterial surface) and vice versa. (B) The baseline represents the ethyl acetate (EA)/octane (C8) ratio for control cultures, and the bars represent the differences in EA/C8 ratios between test cultures and control cultures; i.e., values below the baseline indicate that the EA/C8 ratio of control cultures was higher than that of test cultures (suggesting increased acidic characteristics of the bacterial surface) and vice versa. GG, Lactobacillus GG; Shi, L. casei Shirota; Lb, L. delbrueckii subsp. bulgaricus. The x axes indicate culture conditions. Data are reported as the mean and standard error of the mean. Significant differences (P < 0.05) are marked by asterisks.
The weak affinity of all lactobacilli for the electron donor solvent (ethyl acetate) in comparison to the associated nonpolar solvent (octane) revealed a weak electron-accepting nature of the bacteria studied (Fig. 2B). Each of the bacteria adhered more readily to octane than to ethyl acetate, a phenomenon evidenced by low ethyl acetate/octane ratios (range, 0.15 to 0.19). Only very few changes in ethyl acetate/octane ratios under different culture conditions were seen, and all significant changes were due to lower adhesion to nonpolar octane. Interestingly, most of the changes in acidic characteristics were seen in L. delbrueckii subsp. bulgaricus; linoleic acid (5 μg ml−1), γ-linolenic acid (20 μg ml−1), arachidonic acid (5 and 20 μg ml−1), and docosahexaenoic acid (20 μg ml−1) reduced the ratio difference evaluated. Arachidonic acid (5 μg ml−1) and linoleic acid (20 μg ml−1) also resulted in lower ethyl acetate/octane ratios (P = 0.05 and P < 0.01, respectively).
DISCUSSION
The four major components in the lactobacilli analyzed, making up 65 to 75% of the cellular fatty acid pool, were SAFA, oleic acid, vaccenic acid, and dihydrosterculic acid, all of which were previously reported in lactobacilli. Most of the previous reports presented 18:1 without further identifying different isoforms, e.g., oleic and vaccenic acids. These octadecanoic acids seem to be the most predominant in lactobacilli, making up 14 to 67% of the total fatty acids (3, 5, 7, 17). The fairly substantial variation in the results most likely is due to different culture conditions (e.g., temperature, incubation time, culture medium, and strain differences). The proportions of octadecanoic acids presented here are comparable to those reported in methodologically similar studies (5, 17). The relatively high levels of oleic acid in the cellular fatty acids of all of the tested lactobacilli most likely reflect the culture medium used (MRS broth rich in oleic acid). The various proportions of vaccenic acid suggest that de novo fatty acid biosynthesis is not repressed by the high levels of oleic acid (or Tween 80) in the growth medium (25). The fourth major group of fatty acids in the present study was 19:cyc, especially dihydrosterculic acid. Previous reports regarded dihydrosterculic and lactobacillic acids, formed by methylenation of oleic and vaccenic acids, respectively, as the major fatty acids in lactobacilli (3, 7, 10, 20). Even though lactobacillic acid is the most frequently reported fatty acid in lactobacilli, we did not identify it in L. delbrueckii subsp. bulgaricus or L. casei Shirota and found it only in trace amounts in Lactobacillus GG. This finding is, however, in concordance with those of previous studies showing that a low availability of oleic acid in the culture medium (e.g., whey culture medium) would increase the cellular levels of lactobacillic acid (7), while a high availability of oleic acid (e.g., Tween 80 in MRS medium) would increase the levels of dihydrosterculic acid (10).
Linoleic acid, although fairly uncommon, has been identified in cellular fatty acids of lactobacilli, the proportions ranging from trace amounts up to 20% (3, 6, 7). In the present study, we did not identify this parent n-6 PUFA in bacterial fatty acids of three analyzed lactobacilli grown in standard MRS medium (without PUFA supplementation), although γ-linolenic acid and eicosapentaenoic acid were identified in all lactobacilli. α-Linolenic acid and docosahexaenoic acid also were recognized, although the chromatographic peaks of these two PUFA did overlap with those of other fatty acids, namely, dihydrosterculic acid and 24:1. Furthermore, in concordance with two other studies (3, 9), we also identified CLA. Coinjection with reference compounds indicated that the most predominant isomers of CLA identified in the present study were the c-9,t-11 and t-10,c-12 isomers. It must be emphasized, however, that our data cannot conclusively distinguish whether the CLA seen were naturally present in the fatty acids of lactobacilli or were assimilated from the culture medium. However, as the CLA content of the culture medium was constant and we found some differences in cellular CLA levels as well as some possible interconversion reactions, it is possible that CLA isomers indeed are part of a normal cellular fatty acid profile of lactobacilli. CLA 18:2 c-9,t-11 also was reported in the culture media of lactobacilli (4), although it was later shown to be oxidized linoleic acid (21). On the contrary, Jiang et al. (9) reported that lactobacilli do not produce extracellular CLA. Whether the cellular CLA in lactobacilli demonstrated here are excreted or not remains unknown.
The assimilation of supplemented free PUFA, especially those of the n-6 series, was clearly observed in all of the lactobacilli. In addition, PUFA-dependent differences were observed in other cellular fatty acids (including SAFA, monounsaturated fatty acids, and PUFA), suggesting that the bactericidal stress applied (12) could be balanced by fatty acid conversion reactions (7, 10). Although the PUFA-dependent changes in the proportions of bacterial fatty acids shown in the present study were rather complex and only the percentages and not the absolute contents of the fatty acids were assessed, we could identify an increase in the levels of unsaturation of fatty acids as a response to exposure to free extracellular PUFA. This results suggests that desaturase activation or hyperinduction may play an important role in the response to the stress (i.e., inhibition of growth by free exogenous PUFA) applied (12). Indeed, there is experimental evidence that anaerobic lactobacilli may possess an oxygen-consuming desaturase system to cope with environmental stress (7). However, it must be emphasized that the biosynthetic routes for fatty acids in lactobacilli still have not been studied extensively (7, 10, 20). Studies investigating the biosynthetic routes for fatty acids in lactobacilli therefore clearly are warranted.
Previous studies showed that the tested bacterial strains can adhere to different intestinal surfaces and that culturing of the bacteria with various free PUFA influences microbial adhesion to intestinal surfaces (12, 13, 22). The microbial adhesion process includes passive forces, such as hydrophobic and steric forces, as well as specific structures, such as lipoteichoic acids, lectins, and extracellular polymers (8). In the present study, all bacteria showed mediocre hydrophobic properties, and the hydrophobicity tended to decrease when the bacteria were cultivated in medium supplemented with various free PUFA. Moreover, all bacterial strains tested here had a weak electron-accepting nature, indicating their nonacidic nature. Even though hydrophobic lactobacilli may adhere better to intestinal epithelial cells than hydrophilic lactobacilli (23), the minor changes in hydrophobicity (upon culturing with PUFA) hardly explain the observed effects of free PUFA on bacterial adhesion to mucus and epithelial cells (12) but indicate that changed fatty acid compositions of probiotics may predominantly influence other factors associated with the microbial adhesion process, probably by influencing bacterial membrane fluidity and membrane-lipopeptide interactions (8).
We investigated here whether specific probiotic strains could incorporate exogenous free PUFA into cellular fatty acids and how these changes, if any, would influence the physical properties of the bacteria. The results indicate that lactobacilli do incorporate exogenous free PUFA into their cell lipids. Moreover, the present study shows that free PUFA in the growth medium of lactobacilli may induce changes in fatty acids in relation to the regulation of the degree of fatty acid unsaturation, cyclization, and proportions of PUFA containing 20 to 22 carbons and 18 carbons with conjugated double bonds. Despite these changes in cellular fatty acids, only minor alterations in the electron donor-electron acceptor properties of the lactobacilli were observed, suggesting that exogenous PUFA did not adhere to cell surfaces during their harvesting but were assimilated by the lactobacilli. As free PUFA have been shown to be antibacterial (12), the demonstrated PUFA assimilation may indeed be a detoxification mechanism used by lactobacilli (9). Consequently, the fatty acid milieu within the intestine and the delicate balance of inflammatory mediators derived from PUFA may be readjusted by members of the indigenous gut microflora. Given the demonstrated anti-inflammatory potential of probiotics (11), the present results may indicate another mechanism by which probiotics could alleviate the intestinal inflammation associated with atopy, food allergy, and inflammatory bowel disease.
Acknowledgments
This study was supported by the Technology Development Agency of Finland (TEKES) and the Academy of Finland.
We thank K. Nurmela for kindly providing us with the CLA60 standard and T. Humphreys for revision of the English language.
REFERENCES
- 1.Briandet, R., T. Meylheuc, C. Maher, and M. N. Bellon-Fontaine. 1999. Listeria monocytogenes Scott A: cell surface charge, hydrophobicity, and electron donor and acceptor characteristics under different environmental growth conditions. Appl. Environ. Microbiol. 65:5328-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christie, W. W. 1982. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 23:1072-1075. [PubMed] [Google Scholar]
- 3.Dionisi, F., P.-A. Golay, M. Elli, and L. B. Fay. 1999. Stability of cyclopropane and conjugated linoleic acids during fatty acid quantification in lactic acid bacteria. Lipids 34:1107-1115. [DOI] [PubMed] [Google Scholar]
- 4.Fairbank, J., L. Ridgway, J. Griffin, D. Wickens, A. Singer, and T. L. Dormandy. 1988. Octadeca-9,11-dienoic acid in diagnosis of cervical intraepithelial neoplasia. Lancet 2:329-330. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez Murga, M. L., G. M. Cabrera, G. Font de Valdez, A. Disalvo, and A. M. Seldes. 2000. Influence of growth temperature on cryotolerance and lipid composition of Lactobacillus acidophilus. J. Appl. Microbiol. 88:342-348. [DOI] [PubMed] [Google Scholar]
- 6.Gomez Zavaglia, A., E. A. Disalvo, and G. L. de Antoni. 2000. Fatty acid composition and freeze-thaw resistance in lactobacilli. J. Dairy Res. 67:241-247. [DOI] [PubMed] [Google Scholar]
- 7.Guerzoni, M. E., R. Lanciotti, and P. S. Cocconcelli. 2001. Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology 147:2255-2264. [DOI] [PubMed] [Google Scholar]
- 8.Gusils, C., S. Cuozzo, F. Sesma, and S. Gonzalez. 2002. Examination of adhesive determinants in three species of Lactobacillus isolated from chicken. Can. J. Microbiol. 48:34-42. [DOI] [PubMed] [Google Scholar]
- 9.Jiang, J., L. Björck, and R. Fonden. 1998. Production of conjugated linoleic acid by dairy starter cultures. J. Appl. Microbiol. 85:95-102. [DOI] [PubMed] [Google Scholar]
- 10.Johnsson, T., P. Nikkilä, L. Toivonen, H. Rosenqvist, and S. Laakso. 1995. Cellular fatty acid profiles of Lactobacillus and Lactococcus strains in relation to oleic acid content of the cultivation medium. Appl. Environ. Microbiol. 61:4497-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalliomäki, M., S. Salminen, H. Arvilommi, P. Kero, P. Koskinen, and E. Isolauri. 2001. Probiotics in primary prevention of atopic disease: a randomized placebo-controlled trial. Lancet 357:1076-1079. [DOI] [PubMed] [Google Scholar]
- 12.Kankaanpää, P. E., S. J. Salminen, E. Isolauri, and Y. K. Lee. 2001. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol. Lett. 194:149-153. [DOI] [PubMed] [Google Scholar]
- 13.Kirjavainen, P. V., A. C. Ouwehand, E. Isolauri, and S. J. Salminen. 1998. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 167:185-189. [DOI] [PubMed] [Google Scholar]
- 14.Microbial ID. 1992. Microbial identification system operational manual. Microbial ID, Newark, Del.
- 15.Ouwehand, A., P. Kirjavainen, C. Shortt, and S. Salminen. 1999. Probiotics: mechanisms and established effects. Int. Dairy, J. 9:43-52. [Google Scholar]
- 16.Partanen, L., N. Marttinen, and T. Alatossava. 2001. Fats and fatty acids as growth factors for Lactobacillus delbrueckii. Syst. Appl. Microbiol. 24:500-506. [DOI] [PubMed] [Google Scholar]
- 17.Rementzis, J., and J. Samelis. 1996. Rapid GC analysis of cellular fatty acids for characterizing Lactobacillus sake and Lact. curvatus strains of meat origin. Lett. Appl. Microbiol. 23:379-384. [DOI] [PubMed] [Google Scholar]
- 18.Russell, N. J., and D. S. Nichols. 1999. Polyunsaturated fatty acids in marine bacteria—a dogma rewritten. Microbiology 145:767-779. [DOI] [PubMed] [Google Scholar]
- 19.Salminen, S., C. Bouley, M. C. Boutron-Ruault, J. H. Cummings, A. Franck, G. R. Gibson, E. Isolauri, M. C. Moreau, M. Roberfroid, and I. Rowland. 1998. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 80:S147-S171. [DOI] [PubMed] [Google Scholar]
- 20.Suutari, M., and S. Laakso. 1992. Temperature Adaptation in Lactobacillus fermentum: interconversion of oleic acid, vaccenic acid and dihydrosterculic acid. J. Gen. Microbiol. 138:445-450. [DOI] [PubMed] [Google Scholar]
- 21.Thompson, S., and M. T. Smith. 1985. Measurement of the diene conjugated form of linoleic acid in plasma by high performance liquid chromatography: a questionable non-invasive assay of free radical activity? Chem. Biol. Interact. 55:357-366. [DOI] [PubMed] [Google Scholar]
- 22.Tuomola, E. M., and S. J. Salminen. 1998. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 41:45-51. [DOI] [PubMed] [Google Scholar]
- 23.Wadström, T., K. Andersson, M. Sydow, L. Axelsson, S. Lindgren, and B. Gullmar. 1987. Surface properties of lactobacilli isolated from the small intestine of pigs. J. Appl. Bacteriol. 62:513-520. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe, K., C. Ishikawa, H. Inoue, D. Cenhua, K. Yazawa, and K. Kondo. 1994. Incorporation of exogenous docosahexaenoic acid into various bacterial lipids. J. Am. Oil Chem. Soc. 71:325-330. [Google Scholar]
- 25.Wynn, J. P., and C. Ratledge. 2000. Evidence that the rate-limiting step for the biosynthesis of arachidonic acid in Mortierella alpina is at the level of the 18:3 to 20:3 elongase. Microbiology 146:2325-2331. [DOI] [PubMed] [Google Scholar]
- 26.Yang, B., K. Kalimo, L. Mattila, S. Kallio, J. Katajisto, O. Peltola, and H. Kallio. 1999. Effects of dietary supplementation with sea buckthorn (Hippophaë rhamnoides) seed and pulp oils on atopic dermatitis. J. Nutr. Biochem. 10:622-630. [DOI] [PubMed] [Google Scholar]