Abstract
Recently, multi-drug-resistant (MDR) Salmonella enterica subspecies enterica serovar Newport reemerged as a public and animal health problem. The antibiotic resistance of 198 isolates and the pulsed-field gel electrophoresis patterns (PFGE) of 139 isolates were determined. Serovar Newport isolates collected between 1988 and 2001 were included in the study. One hundred seventy-eight isolates were collected from the San Joaquin valley in California and came from dairy cattle clinical samples, human clinical samples, bulk tank milk samples, fecal samples from preweaned calves, and waterways. Twenty clinical isolates from humans from various regions of the United States were also included in the study. Resistance to 18 antibiotics was determined using a disk diffusion assay. PFGE patterns were determined using a single enzyme (XbaI). The PFGE and antibiogram patterns were described using cluster analysis. Although the antibiotic resistance patterns of historic (1988 to 1995) and contemporary (1999 to 2001) isolates were similar, the contemporary isolates differed from the historic isolates by being resistant to cephalosporins and florfenicol and in their general sensitivity to kanamycin and neomycin. With few exceptions, the contemporary isolates clustered together and were clearly separated from the historic isolates. One PFGE-antibiogram cluster combination was predominant for the recent isolates, which were taken from human samples from all parts of the United States, as well as in the isolates from California, indicating a rapid dissemination of this phenotypic strain. The data are consistent with the hypothesis that the reemergence of MDR serovar Newport is not simply an acquisition of further antibiotic resistance genes by the historic isolates but reflects a different genetic lineage.
In 1987, during an investigation of a human salmonella outbreak in southern California, a chloramphenicol-resistant strain of Salmonella enterica subspecies enterica serovar Newport was identified (19). The investigators traced the outbreak to contaminated hamburger and then traced the hamburger to a single slaughter plant that processed dairy-source beef. After further investigation of dairies, it was reported that the same chloramphenicol-resistant serovar Newport was found in 10.7% (8 of 75) of dairies in the San Joaquin valley (11). Pacer et al. attributed the southern California outbreak to dairy cattle and the emergence of this isolate in dairies to the use of chloramphenicol in the dairies. During the late 1980s, serovar Newport was the most common salmonella isolate from dairy cattle submitted to the California Animal Diagnostic Laboratory (data not shown). A study investigating spatial-temporal clustering of diarrhea-associated Salmonella species isolates from adult dairy cattle between 1991 and 1998 reported that serovar Newport was no longer a dominant serovar, accounting for only 1.2% of Salmonella species isolates (16).
Beginning in 1999, serovar Newport reemerged as a clinical entity in California dairy cattle (data not shown). These isolates were characterized as entero-invasive, were resistant to multiple antibiotics, and exhibited resistance specifically toward the new cephalosporin ceftiofur. The multi-drug-resistant (MDR) serovar Newport affected young and adult cattle, while at the same time humans were affected in California. More than 50 cases of serovar Newport were reported to health departments across California in 2001 to 2002. The California Department of Health Services linked many of these cases with soft cheese products and warned consumers against unpasteurized soft cheese (http://www.applications.dhs.ca.gov/pressreleases/store/PressReleases/02-11w.html). Theresistance pattern in the recent serovar Newport cases from animals and humans demonstrates resistance to several antibiotics, including ampicillin, amoxicillin-clavulanic acid, cephalothin, ceftiofur, chloramphenicol, florfenicol, streptomycin, sulfamethoxazole, and tetracycline. Cephalosporin resistance is causing concern for public health, since this category of antibiotics is used for treatment of salmonellosis. Serovar Newport was the most common serotype with reduced susceptibility to the expanded-spectrum cephalosporin ceftriaxone in the 2001 National Antimicrobial Monitoring System, a national program to study resistance trends in clinical bacterial isolates from animals and humans.
The reemergence of serovar Newport as a clinical entity has not been confined to California. Several recent reports have described outbreaks of food-associated MDR serovar Newport in humans and isolation of the organism from clinical samples from cattle (12). The National Antimicrobial Monitoring System reported that serovar Newport was the third-most-common serovar after S. enterica subspecies enterica serovar Typhimurium and S. enterica subspecies enterica serovar Enteritidis in 1999 (5). The number of cases of multi-drug-resistant serovar Newport increased significantly in 1999 compared to that for the three previous years. The surveillance data together with other recent case reports clearly indicate that a highly resistant strain of serovar Newport has quickly emerged and spread through the United States in bovines and humans. The objective of this paper is to compare the pulsed-field gel electrophoresis (PFGE) and antibiotic resistance (ABR) profiles of MDR serovar Newport isolates from the 1980s with those of the current isolates.
MATERIALS AND METHODS
Isolates of serovar Newport.
Our study included serovar Newport isolates obtained from six sources: 81 bovine and 7 equine clinical isolates from the California Animal Health and Food Safety Laboratory, 41 isolates from dairy on-farm bulk tank milk, 16 isolates from 1- to 4-week-old dairy calves without clinical disease, 8 isolates from dairy environmental samples, 16 isolates from samples of surface water used for irrigation, 10 human clinical isolates provided by local health authorities, and 20 human clinical isolates obtained from the Centers for Disease Control and Prevention (CDC) representing a variety of geographic regions within the United States. With the exception of the CDC isolates, all serovar Newport isolates were collected from the southern San Joaquin Valley, California. The majority of the isolates were collected between 1999 and 2002; some of the bovine and equine clinical isolates were collected between 1988 and 2002 (Table 1).
TABLE 1.
Source and year of isolation of serovar Newport isolates
| Source | Yr isolated
|
Total no. isolated | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | 1989 | 1993 | 1994 | 1995 | 1996 | 1998 | 2000 | 2001 | 2002 | ||
| Bovine clinical cases | 16 | 3 | 1 | 4 | 23 | 32 | 1 | 80 | |||
| Equine clinical cases | 2 | 1 | 3 | 1 | 7 | ||||||
| Human cases from CDC | 20 | 20 | |||||||||
| Local human cases | 3 | 7 | 10 | ||||||||
| Bovine bulk-milk samples | 1 | 38 | 1 | 40 | |||||||
| Bovine surveillance samples | 3 | 20 | 23 | ||||||||
| Water samples | 16 | 16 | |||||||||
| Total | 16 | 3 | 1 | 4 | 2 | 1 | 3 | 50 | 114 | 2 | 196 |
Microbiologic methods.
All putative serovar Newport isolates were reisolated and verified as serovar Newport. Briefly, the isolates were streaked for isolation on blood agar plates. Well-isolated colonies were inoculated to triple sugar iron, lysine iron, and urea agar slants and incubated at 37°C for 24 h. All salmonella isolates were serogrouped and serotyped to verify they were serovar Newport.
Antibiograms.
Antibiotic susceptibility profiles were developed for all isolates using the disk diffusion assay in accordance with NCCLS guidelines (3, 10). A panel of 18 antibiotics was used in the assay (Table 2). The zone diameters were read using a digital calibrated measuring device (Fowler Sylvac, Ultra-cal IV; Geneva Gage, Inc., Albany, Oreg.; www.1gg.com) and were recorded directly into a spreadsheet (Microsoft Excel 2000). For cluster analysis, the zone sizes were used, while for explanatory purposes the isolates are described as sensitive, intermediate resistant, or resistant to individual antibiotics in accordance with breakpoints for human isolates of Escherichia coli published by the NCCLS (10).
TABLE 2.
Antibiotics included and codes used in the disk diffusion assay and concentration of antibiotics in the disks
| Antibiotic | Code | Concentration (μg) |
|---|---|---|
| Amikacin | AMK | 30 |
| Amoxicillin-clavulanic acid | AMC | 20/10 |
| Ampicillin | AMP | 10 |
| Cephalothin | CEF | 30 |
| Ceftiofur | XNL | 30 |
| Chloramphenicol | CHL | 30 |
| Gentamicin | GEN | 10 |
| Nalidixic acid | NAL | 30 |
| Streptomycin | STR | 10 |
| Sulfisoxazole | SULF | 250 |
| Tetracycline | TET | 30 |
| Sulfamethoxazole-trimethoprim | SXT | 23.75/1.25 |
| Spectinomycin | SPT | 100 |
| Florfenicol | FFC | 30 |
| Ceftriaxone | CRO | 30 |
| Ciprofloxacin | CIP | 5 |
| Neomycin | NEO | 30 |
| Kanamycin | KAN | 30 |
PFGE.
Samples for PFGE were prepared by using a published procedure (13, 14). Briefly, the genomic DNA was prepared by embedding cells in agarose plugs and lysing the cells using lysozyme, sacrosyl, and deoxycholate. The DNA was digested in the agarose by using the restriction enzyme XbaI. The plugs were placed in a 1.2% agarose gel. The restricted fragments were separated by PFGE using 0.5× Tris-borate-EDTA buffer at 14°C and a Chef Dr III (Bio-Rad; Hercules, Calif.) gel apparatus. Electrophoresis conditions were as follows: initial switch time, 2.2 s, final switch time, 63.8 s at an angle of 120° at 6 V/cm for 20 h. Restriction fragments were visualized by using an ethidium bromide stain, and the PFGE pattern was scanned and digitized by using a Bio-Rad Fluor-S Multimager system (Quantity One, Diversity Database 2.2.0). A serovar Newport isolate obtained from the CDC (AM 01144) and a lambda ladder were used as control and size standards, respectively.
Quantitative analysis.
As shown by the antibiograms, all isolates had a profile consisting of the measured inhibition zone size for each of the 18 evaluated antibiotics. By using cluster analysis methods, serovar Newport isolates with similar inhibition zone patterns were formed into ABR clusters. The dissimilarity measure used was the squared Euclidean distance. The clusters were determined by using the average linkage algorithm also referred to as the unweighted pair-group average method. Clusters containing single isolates were excluded from the final assessment (7).
The digitized PFGE results were initially annotated using the image analysis software. From these assessments, a band set of 24 different-sized restriction fragments was defined, and it described the complete band set for our study isolates. A PFGE pattern for each isolate was defined by numbering the restriction fragments for each isolate according to the band set definitions. These patterns were exported into a spreadsheet program (Microsoft Excel 2000). Subsequently, each isolate was assigned a binary code to signify the absence or presence of a restriction fragment in the band set. Each isolate was completely defined by 24 variables. Dissimilarity between the isolates was measured by the squared Euclidean distance, and the average linkage algorithm was used to cluster the isolates. Clusters were formed with no intracluster variability. Clusters containing single isolates were excluded from the final assessments.
Cross-tabulation of the PFGE and ABR clusters was performed to assess the similarity in classification between the two descriptive methods. The recent isolates were compared to the historic isolates, and human and animal isolates were also compared.
RESULTS
ABR clusters.
Fourteen ABR clusters included 190 of the 196 serovar Newport isolates originally identified for the study. Six isolates had unique patterns that did not fit into any of the 14 clusters and are not further described in this study. The ABR patterns of 185 of 190 isolates were multiresistant (Table 3). Four equine and one bovine isolate in cluster A was sensitive to all 18 antibiotics. All remaining isolates (clusters B to N) were resistant to ampicillin, streptomycin, sulfamethoxazole, and tetracycline. Eight of the clusters (G to N) contained isolates that were resistant to all tested β-lactam antibiotics and had reduced susceptibility to ceftiofur and ceftriaxone. The isolates in these clusters were also resistant to chloramphenicol and florfenicol. These clusters exhibited the highest level of multiple-drug resistance. Seven of the clusters had isolates that were resistant to neomycin and kanamycin, and most of these clusters also contained isolates resistant to spectinomycin. All isolates were susceptible to amikacin, nalidixic acid, and ciprofloxacin.
TABLE 3.
Antibiotic susceptibility patterns of serovar Newport isolates grouped into ABR clusters A through N and number of isolates belonging to each ABR clustera
| Cluster | Antibioticb
|
No. of isolates | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AMP | CEF | XNL | CRO | CHL | FFC | AMK | GEN | KAN | NEO | STR | SULF | SXT | TET | NAL | CIP | SPT | ||
| A | 25.8 | 23 | 24.8 | 24.3 | 27.8 | 24 | 26 | 22.5 | 21.1 | 20.4 | 18.9 | 15.4 | 20 | 26 | 20 | 21.5 | 32 | 21.1 | 5 |
| B | 19 | 6 | 18.5 | 26.5 | 28 | 6 | 6 | 23.5 | 23 | 23 | 23 | 6 | 6 | 25 | 6 | 23 | 33 | 24 | 2 |
| C | 14.8 | 6 | 23 | 25.7 | 30.1 | 26 | 27 | 23 | 22.6 | 6.4 | 10.7 | 7 | 6 | 24 | 6 | 22.3 | 32 | 6 | 7 |
| D | 14.2 | 6 | 22.6 | 25.2 | 29.6 | 6 | 27 | 22.9 | 21.7 | 6 | 7.5 | 6.2 | 6 | 25 | 6 | 22.9 | 34 | 6 | 15 |
| E | 13 | 6 | 22 | 26.5 | 31 | 26 | 27 | 22 | 19.5 | 6 | 8 | 6 | 6 | 6 | 6 | 23.5 | 34 | 6 | 2 |
| F | 21 | 6 | 20 | 26 | 29.5 | 6 | 27 | 23.5 | 6 | 6 | 7.5 | 6 | 6 | 6 | 6 | 21 | 34 | 6 | 2 |
| G | 6.6 | 6 | 6 | 12.3 | 15.7 | 6 | 6 | 22.5 | 21.6 | 21.6 | 18.6 | 6 | 6 | 22 | 6.1 | 22.2 | 32 | 21.1 | 109 |
| H | 6 | 6 | 6 | 13.7 | 17.1 | 6 | 6 | 22.7 | 21.8 | 23 | 20 | 6 | 6 | 6 | 6 | 23.5 | 33 | 20.2 | 11 |
| I | 7.5 | 6 | 6 | 9.5 | 13.5 | 6 | 7.5 | 23.5 | 23.5 | 22.5 | 20 | 6 | 6 | 23 | 6 | 23 | 32 | 6 | 2 |
| J | 6.5 | 6 | 6 | 12.5 | 14.6 | 6 | 6 | 23.4 | 6.4 | 18.4 | 18.9 | 6 | 6 | 22 | 6 | 22.2 | 31 | 6.1 | 14 |
| K | 6 | 6 | 6 | 10.5 | 11 | 6 | 6 | 23 | 20 | 6 | 6 | 6 | 6 | 23 | 6 | 22 | 34 | 22 | 2 |
| L | 7.2 | 6 | 6 | 12.4 | 17.1 | 6 | 6 | 22.2 | 6.7 | 18.2 | 17.2 | 6 | 6 | 6 | 6 | 24 | 31 | 6 | 11 |
| M | 7.3 | 6 | 6 | 11 | 13.3 | 6 | 6 | 21.6 | 19.7 | 6 | 6 | 6 | 6 | 6 | 6 | 21 | 34 | 10.3 | 3 |
| N | 7.2 | 6 | 6 | 11.2 | 15.6 | 6 | 6 | 22 | 7.4 | 6 | 6.4 | 6 | 6 | 6 | 6 | 23 | 33 | 6.4 | 5 |
The susceptibility pattern of each cluster is described by the mean inhibition zone size of the isolates to the antibiotics as measured by the disk diffusion assay. The zone sizes in bold indicate that the bacteria are resistant to the antibiotic, and the underlined zone sizes indicate that the isolates exhibit intermediate resistance to the antibiotic according to the NCCLS guidelines for human E. coli. The zone sizes in regular type indicate sensitivity according to the same standard.
AMC, amoxillin-clavulanic acid; AMP, ampicillin; CEF, cephalothin; XNL, ceftiofur; CRO, ceftriaxone; CHL, chloramphenicol; FFC, florfenicol; AMK, amikacin; GEN, gentamicin; KAN, kanamycin; NEO, neomycin; STR, streptomycin; SULF, sulfisoxazole; SXT, sulfamethoxazole-trimethoprim; TET, tetracycline; NAL, nalidixic acid; CIP, ciprofloxacin; SPT, spectinomycin.
There were temporal differences that distinguished the different ABR clusters (Table 4). Ninety-eight percent (162 of 166) of the serovar Newport isolates from samples collected between 2000 and 2002 were found in clusters G to N. There was a clear trend for the isolates from 1987 to 1999 to be in clusters A to F. The distinguishing difference between these temporal clusters was the pronounced resistance to β-lactams, including reduced susceptibility to ceftiofur and ceftriaxone and resistance to florfenicol for the 2000 to 2002 isolates. Sixty-six percent (109 of 166) of the 2000 to 2002 isolates belonged to cluster G. These isolates were susceptible to kanamycin, neomycin, spectinomycin, and gentamicin. The dominant clusters for the historic isolates were C and D, which were characterized by resistance to ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, tetracycline, kanamycin, neomycin, and spectinomycin and reduced susceptibility to amoxicillin-clavulanic acid. There was no clear temporal trend in resistance to gentamicin, sulfisoxazole-trimethoprim, and spectinomycin.
TABLE 4.
Number of serovar Newport isolates belonging to each ABR cluster and their distribution among years and sources of isolation
| ABR cluster | Frequency | Yr of isolation
|
Source of isolate
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | 1989 | 1993 | 1994 | 1995 | 1996 | 1998 | 2000 | 2001 | 2002 | Bovine | Equine | Human | Water | ||
| A | 5 | 1 | 3 | 1 | 1 | 4 | |||||||||
| B | 2 | 2 | 1 | 1 | |||||||||||
| C | 7 | 4 | 2 | 1 | 7 | ||||||||||
| D | 15 | 10 | 1 | 4 | 15 | ||||||||||
| E | 2 | 2 | 2 | ||||||||||||
| F | 2 | 1 | 1 | 1 | 1 | ||||||||||
| G | 109 | 25 | 78 | 6 | 80 | 13 | 16 | ||||||||
| H | 11 | 11 | 11 | ||||||||||||
| I | 2 | 2 | 2 | ||||||||||||
| J | 14 | 12 | 2 | 12 | 2 | ||||||||||
| K | 2 | 2 | 1 | 1 | |||||||||||
| L | 11 | 3 | 8 | 11 | |||||||||||
| M | 3 | 3 | 3 | ||||||||||||
| N | 5 | 1 | 3 | 1 | 4 | 1 | |||||||||
PFGE clusters.
Fifteen PFGE clusters were developed in which all isolates had the same unique pattern for the 24 bands included in the analysis. Of the 139 isolates included in the analysis, 129 isolates were grouped into clusters 1 to 15 and 10 isolates were excluded from further analysis because their patterns were singular.
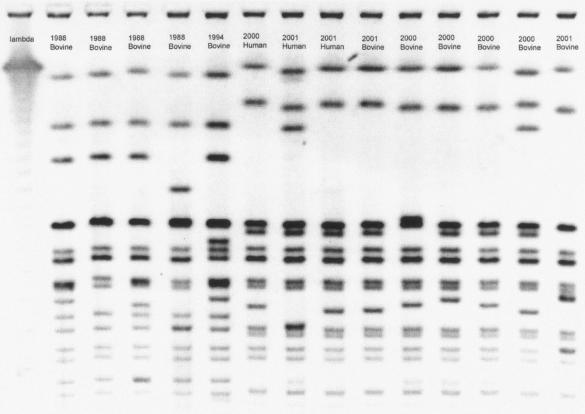
The band patterns of the most frequent clusters are shown in Fig. 1, and Table 5 gives the year of isolation, species source, and PFGE and ABR cluster group for the isolates shown in the figure. PFGE cluster 1 was the largest group, containing 40% (63 of 166) of the 2000 to 2002 isolates and no historic isolates (Table 6). The historic isolates were exclusively and evenly distributed in PFGE clusters 11 to 15.
FIG. 1.
PFGE picture of isolates selected from different PFGE and ABR clusters.
TABLE 5.
Year of isolation, species source, and PFGE and ABR cluster of isolates shown in Fig. 1
| Column | Date | Source | PFGE cluster | ABR cluster |
|---|---|---|---|---|
| 0 | Lambda | |||
| 1 | 1988 | Bovine | 11 | D |
| 2 | 1988 | Bovine | 12 | C |
| 3 | 1988 | Bovine | 14 | E |
| 4 | 1988 | Bovine | 13 | D |
| 5 | 1994 | Bovine | 15 | D |
| 6 | 2000 | Human | 1 | G |
| 7 | 2001 | Human | 4 | G |
| 8 | 2001 | Human | 2 | G |
| 9 | 2001 | Bovine | 1 | G |
| 10 | 2000 | Bovine | 6 | J |
| 11 | 2000 | Bovine | 5 | J |
| 12 | 2000 | Bovine | 9 | J |
| 13 | 2000 | Bovine | 10 | K |
| 14 | 2001 | Bovine | 3 | H |
TABLE 6.
Number of serovar Newport isolates belonging to each PFGE cluster and their distribution among years and sources of isolation
| PFGE cluster | Frequency | Yr of isolation
|
Source of isolate
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | 1989 | 1993 | 1994 | 1998 | 2000 | 2001 | 2002 | Bovine | Equine | Human | Water | ||
| 1 | 63 | 16 | 43 | 4 | 47 | 13 | 3 | ||||||
| 2 | 5 | 1 | 2 | 1 | 1 | 1 | 1 | 3 | |||||
| 3 | 15 | 2 | 13 | 15 | |||||||||
| 4 | 5 | 3 | 2 | 4 | 1 | ||||||||
| 5 | 7 | 7 | 5 | 2 | |||||||||
| 6 | 3 | 3 | 3 | ||||||||||
| 7 | 5 | 1 | 4 | 5 | |||||||||
| 8 | 2 | 2 | 2 | ||||||||||
| 9 | 2 | 1 | 1 | 1 | 1 | ||||||||
| 10 | 3 | 1 | 2 | 3 | |||||||||
| 11 | 5 | 4 | 1 | 5 | |||||||||
| 12 | 5 | 5 | 5 | ||||||||||
| 13 | 5 | 4 | 1 | 5 | |||||||||
| 14 | 2 | 1 | 1 | 2 | |||||||||
| 15 | 4 | 4 | 4 | ||||||||||
Combined data.
The PFGE clusters were cross-classified with the ABR clusters, and the results are described in Table 7. The majority of isolates within a PFGE cluster were found in a single ABR cluster. The exception to this observation was PFGE pattern 3. The isolates in this pattern were evenly divided between ABR clusters H and L and differed in their susceptibility to gentamicin and spectinomycin. In contrast, three of the ABR clusters contained 76% (100 of 131) of the cross-classified isolates, yet these isolates were scattered across 13 of 15 PFGE clusters.
TABLE 7.
Cross-classification of PFGE and ABR clusters of the serovar Newport isolates
| PFGE cluster | Frequency | ABR cluster
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | Single | ||
| 1 | 63 | 58 | 1 | 2 | 1 | 1 | ||||||||||
| 2 | 5 | 1 | 4 | |||||||||||||
| 3 | 15 | 7 | 8 | |||||||||||||
| 4 | 5 | 5 | ||||||||||||||
| 5 | 7 | 5 | 1 | 1 | ||||||||||||
| 6 | 3 | 3 | ||||||||||||||
| 7 | 5 | 4 | 1 | |||||||||||||
| 8 | 2 | 2 | ||||||||||||||
| 9 | 2 | 2 | ||||||||||||||
| 10 | 3 | 2 | 1 | |||||||||||||
| 11 | 5 | 5 | ||||||||||||||
| 12 | 5 | 4 | 1 | |||||||||||||
| 13 | 5 | 1 | 4 | |||||||||||||
| 14 | 2 | 1 | 1 | |||||||||||||
| 15 | 4 | 4 | ||||||||||||||
DISCUSSION
This study used a convenience set of serovar Newport isolates obtained from an ongoing study of Salmonella ecology in rural community, bovine, and equine diagnostic submissions to the California Animal Health and Food Safety Laboratory and from food-borne outbreaks from a variety of geographic locations in the United States. The principal aim of the study was to compare PFGE and ABR patterns in serovar Newport isolates collected from 1988 to 2002. While these data do not allow estimates of the prevalence of PFGE or ABR clusters, we may conclude that the isolates obtained prior to 1998 were distinctly different from the isolates obtained since 1998. In addition, there was a strong suggestion that the isolates obtained since 1998 were clonal and widespread in the United States.
The variability in the ABR and PFGE patterns between the recent isolates and those recovered from 1988 to 1994 is considerable. The ABR and PFGE data indicate that the recent isolates are distinct and unlikely to be related to the historic isolates. Specifically, the resistance patterns of the historic isolates differ from the recent isolates for several antibiotics. The historic isolates are susceptible to cephalosporins and florfenicol, and the majority are resistant to chloramphenicol, kanamycin, spectinomycin, and neomycin. The recent isolates exhibit resistance to cephalosporin and florfenicol (and chloramphenicol), while they are susceptible mainly to kanamycin, spectinomycin, and neomycin. This simultaneous acquisition and loss of ABR suggests that it is unlikely that the recent clones are simply a modified version of the historic strains, a suggestion that is strongly supported by the PFGE data that show clear and pronounced differences between the historic and recent isolates.
It has been hypothesized that the recent emergence of the highly resistant serovar Newport could be due to the use of antibiotics on dairies, and a focus has been placed specifically on the use of ceftiofur in the clinical treatment of animals (6). While the recent isolates exhibit resistance to more antibiotics and particularly the cephalosporins, the lack of resistance to antibiotics commonly used in animal agriculture and specifically in dairy calves (neomycin and spectinomycin) argue against that hypothesis.
Several studies have shown that ABR genes persist in environments where there are low levels of intermittent use as well as complete cessation of antibiotic use (8, 15, 17, 18). The lack of these ABR determinants in the recent isolates suggests that antibiotic use in the dairy industry cannot fully explain the emergence of these cephalosporin-resistant serovar Newport isolates.
Several of the serovar Newport isolates collected since 1998 appear to be from a clonal population that includes human, environmental, and bovine sources from a wide geographic region. The combined phenotype of ABR cluster G and PFGE cluster 1 contained a high proportion of the contemporary isolates. These isolates came from a variety of sources: 46 bovine, 2 surface water, and 10 human isolates. Of the human isolates, seven were obtained from the CDC and originated from several states. The recent strains are highly pathogenic in cattle and cause severe disease, including death. In our ongoing studies, we have found the recent serovar Newport isolates to be easily recovered from environmental samples, suggesting that they survive and persist in the environment (data not shown). Others have also reported that serovar Newport is persistent in the environment (1, 2, 4). This characteristic may play a role in its emergence and proliferation.
There are certain similarities in the dissemination of serovar Newport in the United States and the dissemination of serovar Typhimurium DT 104 in the 1990s. Both are observed in cattle and humans and are widely disseminated geographically (9), although the current multi-drug-resistant serovar Newport has yet to be reported outside of North America. In contrast to serovar Typhimurium DT 104, the recent serovar Newport strains are not as clonal and show more diversity in resistance and PFGE patterns. The greater diversity within the current serovar Newport isolates is likely explained by the observation that certain resistant traits (specifically those with cephalosporin and florfenicol resistance) are plasmid borne (12), while the multidrug resistance reported in DT 104 is chromosomally based. Further investigations of the epidemiology and biology of the emergent serovar Newport are necessary to improve our understanding of the shifts in Salmonella species populations that appear to be rapid and far reaching.
Acknowledgments
We thank the Centers for Disease Control and Prevention for the human isolates and for technical advice on PFGE methodology; A. Silva, P. Scott, and E. Potochny for technical assistance with the PFGE; and Ken Kido for antibiogram work.
This study was funded through USA/CSREES/NRI project no. 99-35212-8562.
REFERENCES
- 1.Baudart, J., J. Grabulos, J.-P. Barusseau, and P. Lebaron. 2000. Salmonella spp. and fecal coliform loads in coastal waters from a point vs. nonpoint source of pollution. J. Environ. Qual. 29:241-250. [Google Scholar]
- 2.Baudart, J., K. Lemarchand, A. Brisabois, and P. Lebaron. 2000. Diversity of Salmonella strains isolated from the aquatic environment as determined by serotyping and amplification of the ribosomal DNA spacer regions. Appl. Environ. Microbiol. 66:1544-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, A. W., W. M. Kirby, J. C. Sherris, and M. Turck. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45:493-496. [PubMed] [Google Scholar]
- 4.Burton, G. J., D. Gunnison, and G. R. Lanza. 1987. Survival of pathogenic bacteria in various freshwater sediments. Appl. Environ. Microbiol. 53:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2000. National antimicrobial resistance monitoring system. Centers for Disease Control and Prevention, Atlanta, Ga. [Online.] http://www.arru.saa.ars.usda.gov/narms/narms.htm.
- 6.Centers for Disease Control and Prevention. 2002. Outbreak of multidrug-resistant Salmonella Newport—United States, January-April 2002. Morb. Mortal. Wkly. Rep. 51:545-548. [PubMed] [Google Scholar]
- 7.Everitt, B. S., and S. Rabe-Hesketh. 1997. The analysis of proximity data. John Wiley & Sons Inc., New York, N.Y.
- 8.Gellin, G., B. E. Langlois, K. A. Dawson, and D. K. Aaron. 1989. Antibiotic resistance of gram-negative enteric bacteria from pigs in three herds with different histories of antibiotic exposure. Appl. Environ. Microbiol. 55:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock, D. D., T. E. Besser, J. Gay, D. Rice, M. A. Davis, and C. C. Gay. 2000. The global epidemiology of multiresistant Salmonella enterica serovar Typhimurium DT104, p. 217-243. In C. Brown and C. Bolin (ed.), Emerging infectious diseases of animals. ASM Press, Washington D.C.
- 10.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk susceptibility tests. M2-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Pacer, R. E., J. S. Spika, M. C. Thurmond, N. Hargrett-Bean, and M. E. Potter. 1989. Prevalence of Salmonella and multiple antimicrobial-resistant Salmonella in California dairies. J. Am. Vet. Med. Assoc. 195:59-63. [PubMed] [Google Scholar]
- 12.Rankin, S. C., H. Aceto, J. Cassidy, J. Holt, S. Young, B. Love, D. Tewari, D. S. Munro, and C. E. Benson. 2002. Molecular characterization of cephalosporin-resistant Salmonella enterica serotype Newport isolates from animals in Pennsylvania. J. Clin. Microbiol. 40:4679-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribot, E. M., R. K. Wierzba, F. J. Angulo, and T. J. Barrett. 2002. Salmonella enterica serotype Typhimurium DT104 isolated from humans, United States, 1985, 1990, and 1995. Emerg. Infect. Dis. 8:387-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salyers, A. A., and C. F. AmÂabile-Cuevas. 1997. Why are antibiotic resistance genes so resistant to elimination? Antimicrob. Agents Chemother. 41:2321-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato, K., T. E. Carpenter, J. T. Case, and R. L. Walker. 2001. Spatial and temporal clustering of Salmonella serotypes isolated from adult diarrheic dairy cattle in California. J. Vet. Diagn. Investig. 13:206-212. [DOI] [PubMed] [Google Scholar]
- 17.Schrag, S. J., V. Perrot, and B. R. Levin. 1997. Adaptation to the fitness costs of antibiotic resistance in Escherichia coli. Proc. R. Soc. Lond. B Biol. Sci. 264:1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, H. W. 1975. Persistence of tetracycline resistance in pig E. coli. Nature 258:628-630. [DOI] [PubMed] [Google Scholar]
- 19.Spika, J. S., S. H. Waterman, G. W. Hoo, M. E. St. Louis, R. E. Pacer, S. M. James, M. L. Bissett, L. W. Mayer, J. Y. Chiu, and B. Hall. 1987. Chloramphenicol-resistant Salmonella newport traced through hamburger to dairy farms. A major persisting source of human salmonellosis in California. N. Engl. J. Med. 316:565-570. [DOI] [PubMed] [Google Scholar]