Abstract
Background and Purpose
The goal of this study was to estimate the efficacy and safety of the rivastigmine transdermal patch in patients with probable Alzheimer's disease (AD) who cannot tolerate or do not respond to oral cholinesterase inhibitors (ChEIs).
Methods
A 24-week, prospective, open-label, single-arm, multicenter study was conducted from June 2009 to June 2010 in patients with probable AD. The enrolled patients had either a poor response or a decline in global function after treatment with oral ChEIs, or they were not able to tolerate treatment with oral ChEIs due to adverse events such as nausea or vomiting. A poor response was defined as a decrease of at least 2 points on the Korean version of the Mini-Mental State Examination (K-MMSE) within the previous 6 months (the decline in global function was determined by the investigator or caregiver). The efficacy of treatment was assessed using a follow-up Clinical Global Impression of Change (CGIC) assessment and K-MMSE conducted after 24 weeks, and safety was measured by the occurrence of adverse events and patient disposition.
Results
In total, 164 patients aged 74.7±7.52 years (mean±SD) and with 5.12±3.64 years of education were included. The study was completed by 70% of the patients (n=116), with 12.2% discontinuing due to adverse events. The most frequently reported adverse events (11%) were skin lesions, such as erythema or itching, followed by gastrointestinal problems (1.2%). Either an improvement or no decline in CGIC scores was reported for 82% of the patients.
Conclusions
The immediate switching of patients from an oral ChEI to the rivastigmine transdermal patch without a washout period was safe and well tolerated by the probable-AD patients in this study.
Keywords: cholinesterase inhibitors, rivastigmine transdermal patch, efficacy, safety, Alzheimer's disease
Introduction
Cholinesterase inhibitors (ChEIs) are widely used in clinical practice for the symptomatic treatment of mild-to-moderate Alzheimer's disease (AD) and Parkinson's-disease dementia. ChEIs are effective in improving the cognitive and global functioning of AD patients, and are the main pharmacological intervention in the clinical management of the disease.1-3 However, the incidence of adverse events associated with oral ChEIs, and particularly those of nausea and vomiting, increases with the administered dose, which can make it difficult to achieve and maintain high therapeutic doses in clinical practice.4-6
The recently developed rivastigmine transdermal patch represents a next generation of acetylcholinesterase treatments, and it is now available in many countries.7 By delivering the drug through the skin, directly into the bloodstream, transdermal patches avoid first-pass effects and result in reduced rates of nausea and vomiting compared with oral ChEIs.8,9 However, the different pharmacologic characteristics of the three commonly used ChEIs may influence the treatment responses of individual patients. Some AD patients do not show improvements in cognitive function and quality of life, even with prolonged intake of a maintenance dosage of ChEIs. Consistent with these observations, previous studies have shown that AD patients who have an inadequate response or intolerance of one ChEI may experience symptom improvement after switching to another ChEI.10-12 The goal of this study was to elucidate the efficacy, safety, and tolerability of switching from oral ChEI treatment to the rivastigmine transdermal patch in patients with probable AD who had experienced adverse reactions or poor responses to oral ChEI treatment.
Methods
Patients
Patients meeting the inclusion criteria for this study were women or men aged 50-85 years with a diagnosis of dementia of the Alzheimer's type, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision, and probable AD, according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association13 Eligible patients had mild-to-moderate disease, which was confirmed by a score on the Korean version of the Mini-Mental State Examination (K-MMSE) of 10-26.14 Each patient underwent a comprehensive evaluation with a neurological examination and appropriate laboratory tests.15 Patients had to have received treatment with oral ChEIs (donepezil, galantamine, or rivastigmine capsules) for a minimum of 3 months prior to baseline, and in the investigator's clinical judgment must have been responding poorly to or been deteriorating on their current treatment.
The patients were assigned to either a poor-response group or an adverse-events group. The poor-response group comprised patients with a loss of at least 2 points on the K-MMSE within the previous 6 months or a decline in the activities of daily living (ADL) or global functioning, as determined by the investigator or caregiver. All patients were required to have a caregiver in contact with them for a minimum of 3 days/week and who was available to accompany them on all visits associated with the study. The adverse-event group was classified as patients who had experienced adverse reactions such as nausea or vomiting after the administration of oral ChEIs and who could no longer take oral ChEIs.
Exclusion criteria included an advanced, severe, or unstable medical condition of any type that could interfere with the evaluation and any condition other than AD that could explain the dementia. Patients were also excluded if they had experienced any cerebrovascular accidents within 6 months prior to baseline, a current diagnosis of active, uncontrolled seizure disorder, or any psychiatric diagnosis that might interfere with the response of the patient to a clinical trial.
Prior to participation in the study, patients provided written informed consent once they were determined by the investigator to be mentally competent. If the patient was not able to provide written informed consent, this was obtained from the caregiver or an authorized representative on the patient's behalf. The study was conducted according to the ethical principles of the Declaration of Helsinki, as revised in 2000.
Study design
This was a prospective, 24-week, open-label, multicenter study conducted in South Korea from June 2009 to June 2010. After assessments of eligibility for this study, patients underwent baseline efficacy and safety assessments. They were then assigned to either the poor-response or adverse-events groups. Screening was performed at baseline (visit 1) and at visits 2, 3, and 5, which occurred during weeks 4, 12, and 24, respectively. All patients were started on the 5 cm2 rivastigmine transdermal patch, and the first patch was applied between 24 and 36 h after the last dose of oral ChEIs. If the patient had already discontinued oral ChEIs prior to the baseline visit, the time between the last dose of oral ChEI and the first application of the rivastigmine transdermal patch could not exceed 7 days. If the patient demonstrated good tolerability to the initial 5 cm2 patch, the rivastigmine patch dose was increased to 10 cm2 after 4 weeks. If the target dose was not achieved during the titration period, the investigator could resume titration during the maintenance period. Patients were maintained at their highest well-tolerated dose until the end of the study. The patch was applied by caregivers to clean, dry, and hairless skin on the patient's upper back every morning, and it was worn for 24 h. Normal activities, including bathing, were permitted. In order to minimize any skin irritation, the application site of the patch was alternated daily between the left and right sides.
Assessment
An interview for the Clinical Global Impression of Change (CGIC) scale (the primary efficacy measure) was performed at baseline, and changes from baseline were assessed at weeks 12 and 24 (or at early termination).16 The CGIC was rated on a 7-point scale ranging from 1 (very much improved) to 7 (very much worse), with 4 indicating no change. The CGIC score was used to determine the primary efficacy variable, which was the percentage of patients receiving treatment with rivastigmine (i.e., who had achieved a score of ≤4) at 24 weeks. Responders to treatment were defined as patients with a CGIC score of 4 or less and poor responders were defined as those who had a CGIC score of 5-7. According to the protocol, each patient was assessed by the same clinician who interviewed them at their baseline visit. For all ratings of change from baseline on the CGIC, the clinician relied on information obtained from the patient at the baseline visit, as well as clinical information obtained throughout the study period. The clinician did not have access to any efficacy data collected during the current study visit. For this reason, the CGIC was rated prior to all other efficacy evaluations.
The K-MMSE, the Korean Instrumental Activities of Daily Living (K-IADL), and the Clinical Dementia Rating-Sum of Box (CDR-SB) were used as secondary outcome measures to assess the effects of the treatment with the rivastigmine transdermal patch on cognitive functioning and ADL.14,17,18 These assessments were performed at baseline, and weeks 12 and 24.
Tolerability and safety evaluations were assessed at each visit during the treatment period through the collection of information concerning adverse events, including serious adverse events. Vital signs and body weight were recorded at each visit. Routine laboratory tests were performed at baseline; postbaseline laboratory tests were not performed routinely unless any abnormal clinical laboratory findings developed and induced clinical signs or symptoms that were considered clinically significant or that required therapy; these were recorded as adverse events. Skin irritation was evaluated at every visit by the investigator based on inspection of the skin at the site of application. Skin irritation also was assessed by the caregiver, who provided a summary irritation rating.
Statistical methods
All patients who took at least one dose of study medication were included in the safety analysis, which those patients who additionally provided a valid baseline and at least one postbaseline measurement were included in the intent-to-treat population. Statistical analyses of efficacy outcome measures were performed on the final group of enrolled cases of the study population. The primary analysis time point was week 24, and changes in efficacy measures from baseline were tested using a paired t-test; the level of statistical significance was set at p<0.05. The 95% confidence interval corresponds to the percentage of patients who demonstrated an improvement or no change from baseline (as assessed using CGIC scores).
Results
Study population
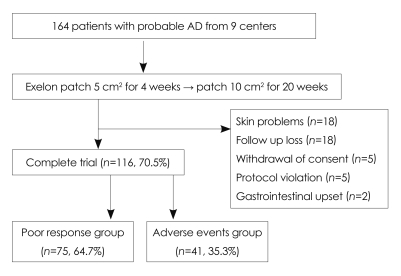
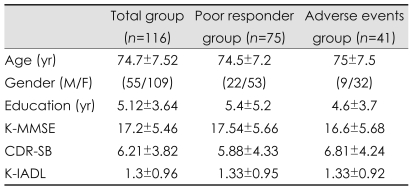
Of the 164 patients who were enrolled in this study, 116 patients (70.5%) completed all 24 weeks (Fig. 1). The patients were divided into the poor-responder group (75/116, 64.7%) and the adverse-events group (41/116, 35.3%). The patients' baseline demographic and background characteristics are summarized in Table 1. There were no significant differences between the poor-responder group and the adverse-events group at baseline.
Fig. 1.
Study flowchart for patients with probable Alzheimer's disease.
Table 1.
Demographic findings and clinical characteristics of the poor-responder and adverse-events groups in patients with probable Alzheimer's disease at baseline
K-MMSE: Korean version of Mini-Mental State Examination, CDR-SB: Clinical Dementia Rating-sum of box, K-IADL: Korean version of Instrumental Activities of Daily Living.
Efficacy
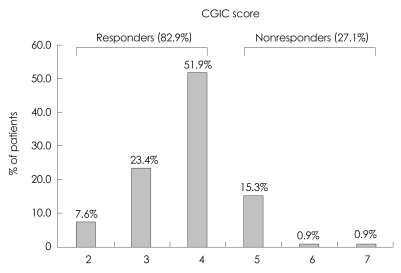
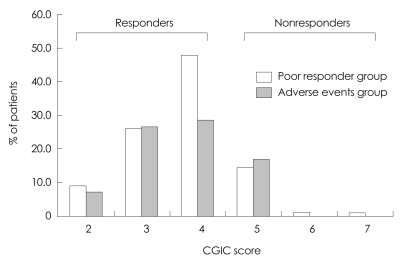
At week 24, approximately 82.8% (96/116) of the patients demonstrated either an improvement or no further deterioration in global functioning, as assessed by the CGIC rating of change from baseline (CGIC scores ≤4; Fig. 2). Similar results were observed for the two groups, with 82.7% (61/75) of the poor-responder group and 83% (34/41) of the adverse-events group demonstrating either an improvement or no further deterioration in global functioning as assessed by the CGIC rating of change from baseline (Fig. 3). Of the 116 patients with K-MMSE scores of 10-26, 64.3% showed an increase or a steady state from baseline at week 24, but those changes did not differ significantly from baseline (p>0.05). The change in K-MMSE score at week 24 relative to baseline was 0.42±0.8 (mean±SD). The K-IADL and CDR-SB scores of the entire cohort did not change significantly between baseline and week 24. There were no statistically significant differences in the subgroup analysis (poor-response and adverse-events groups) of the secondary efficacy measures. The changes from baseline for the secondary efficacy variables are given in Table 2.
Fig. 2.
Clinical Global Impression of Change (CGIC) scores at week 24 (n=116). 1: very much improved, 2: much improved, 3: slightly improved, 4: no change, 5: slightly worse, 6: much worse, 7: very much worse.
Fig. 3.
CGIC scores in the poor-responder (n=75) and adverse-events (n=41) groups at week 24. CGIC: Clinical Global Impression of Change.
Table 2.
Changes from baseline at week 24 for secondary efficacy measures among patients with probable Alzheimer's disease
K-MMSE: Korean version of the Mini-Mental State Examination, CDR-SB: Clinical Dementia Rating-Sum of Box, K-IADL: Korean version of the Instrumental Activities of Daily Living.
Safety and tolerability
Forty-eight patients (29.5%) left the study due to adverse events (n=20, 12.2%), follow-up loss (n=17, 10.5%), poor compliance (n=6, 3.7%), or protocol violation (n=5, 3%). The most common adverse event that resulted in discontinuation was skin lesions, such as erythema or an itching sensation, which occurred in 11% of the patients; there were no reported cases with serious skin problems. The next common adverse event was gastrointestinal problems, such as nausea, vomiting, or anorexia, which occurred in 1.2% of the patients. The most frequently reported adverse events are summarized in Table 3.
Table 3.
Adverse events reported during the 24-week study period in patients with probable Alzheimer's disease
Discussion
The results of this multicenter study suggest that immediate switching from oral ChEIs to the rivastigmine transdermal patch is safe and well tolerated by patients with AD. Almost 80% of this population of patients who were not responding adequately to oral ChEIs showed either an improvement or no further deterioration in global functioning, as assessed by the CGIC. The K-MMSE scores increased slightly in the overall population, but this increase was not statistically significant. The results of this study concur with those of previous clinical trials conducted in patients who were switched from donepezil to rivastigmine.12,19,20 In an open-label clinical trial, patients were switched from donepezil to rivastigmine due to a lack of efficacy (55%) or adverse events (45%), and nearly half of them demonstrated improvement in cognition while receiving rivastigmine treatment.12
A recent prospective open-label multicenter study was conducted in which 201 patients who failed previous treatment with donepezil (n=116, 57.7%) or galantamine (n=84, 41.8%) were treated with rivastigmine capsules (3-12 mg/day) for 16 weeks. Of these, 93 patients (46.3%) responded to rivastigmine as evidenced by improved (28.4%) or stabilized (17.9%) scores on the Mini-Mental State Examination scores.21 However, our study showed marked improvements on the CGIC and fewer adverse events during the 24 weeks of treatment compared to other clinical studies. We believe that this was because the oral ChEIs were switched to the rivastigmine transdermal patch rather than to capsules in our study. The rivastigmine patch was only recently developed and has been shown to provide smooth and continuous drug delivery through the skin and into the bloodstream, avoiding first-pass effects in the gut and the liver, offering potential benefits over conventional oral administration in patients with AD.9,22
The aforementioned studies and ours are limited by their open-label design and by the lack of prospective, objective, and quantitative information regarding the rate of deterioration before switching. A placebo group was not included in the study due to ethical concerns regarding not providing treatment to patients who were already poor responders to oral ChEIs. The lack of a control group and the absence of randomization limit the conclusions that can be drawn from the study. However, there are several important clinical implications of the results reported here.
The first is that it is possible to switch patients from oral ChEIs to rivastigmine without a washout period. Immediate switching to the rivastigmine patch may be beneficial to patients in that it avoids the potential loss of therapeutic effects and decline in cognitive functioning associated with a discontinuation of treatment. Our results suggest that most patients who no longer respond to oral ChEIs will still show stabilization or improvement in overall global functioning or behavior with the rivastigmine patch. This improvement or stabilization, as assessed with the CGIC, was even observed in most of the patients who had experienced a prior deterioration of 2 points or more on the K-MMSE during treatment with oral ChEIs.
The second important clinical implication is that the rivastigmine patch demonstrated good skin tolerability throughout the clinical trial phase, with any reactions usually being mild in severity. In one open-label extension study of the rivastigmine patch, skin tolerability was good in over 90% of all patients, with the most commonly reported skin irritation being erythema and pruritus (7.7% and 5.6%, respectively). A small proportion of patients (3.7%) withdrew due to application-site skin reactions.22 The results of our clinical trial demonstrated that 18 (11%) of the enrolled subjects experienced skin problems, but not serious skin reactions. The next most common adverse event was gastrointestinal side effects, such as nausea or vomiting. However, these adverse events were generally manageable and led to fewer than 2% of the patients discontinuing the treatment.
In summary, the results of this study suggest that poor responders to oral ChEIs will experience symptom improvement or stabilization when switched to the rivastigmine transdermal patch. Furthermore, an immediate switch from oral ChEIs to the rivastigmine transdermal patch was safe and generally well tolerated by most of our patients with probable AD. Given the importance of the clinical implications of this study, further randomized, controlled studies should be undertaken to determine the potential clinical benefits of switching to the rivastigmine transdermal patch for patients who are not responding to oral ChEIs.
Acknowledgements
This study was supported by a grant of the Korea healthcare technology R & D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie CW, Ames D, Clayton T, Lai R. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psychiatry. 2004;12:358–369. doi: 10.1176/appi.ajgp.12.4.358. [DOI] [PubMed] [Google Scholar]
- 3.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ. 2005;331:321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imbimbo BP. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer's disease. CNS Drugs. 2001;15:375–390. doi: 10.2165/00023210-200115050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2002:45–63. [PubMed] [Google Scholar]
- 6.Small G, Dubois B. A review of compliance to treatment in Alzheimer's disease: potential benefits of a transdermal patch. Curr Med Res Opin. 2007;23:2705–2713. doi: 10.1185/030079907x233403. [DOI] [PubMed] [Google Scholar]
- 7.Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer's disease. Expert Opin Drug Deliv. 2008;5:1377–1386. doi: 10.1517/17425240802542690. [DOI] [PubMed] [Google Scholar]
- 8.Cummings J, Lefèvre G, Small G, Appel-Dingemanse S. Pharmacokinetic rationale for the rivastigmine patch. Neurology. 2007;69:S10–S13. doi: 10.1212/01.wnl.0000281846.40390.50. [DOI] [PubMed] [Google Scholar]
- 9.Blesa R, Ballard C, Orgogozo JM, Lane R, Thomas SK. Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer disease. Neurology. 2007;69:S23–S28. doi: 10.1212/01.wnl.0000281848.25142.11. [DOI] [PubMed] [Google Scholar]
- 10.Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc. 2005;105:145–158. [PubMed] [Google Scholar]
- 11.Gauthier S, Emre M, Farlow MR, Bullock R, Grossberg GT, Potkin SG. Strategies for continued successful treatment of Alzheimer's disease: switching cholinesterase inhibitors. Curr Med Res Opin. 2003;19:707–714. doi: 10.1185/030079903125002450. [DOI] [PubMed] [Google Scholar]
- 12.Bullock R, Connolly C. Switching cholinesterase inhibitor therapy in Alzheimer's disease--donepezil to rivastigmine, is it worth it? Int J Geriatr Psychiatry. 2002;17:288–289. doi: 10.1002/gps.542. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Kang Y, Na DL, Hahn S. A validity study on the Korean mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 15.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 16.Guy W. Early Clinical Drug Evaluation Unit (ECDEU) assessment manual for psychopharmacology. Bethesda, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 17.Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH, et al. The reliability and validity of the Korean instrumental activities of daily living (K-IADL) J Korean Neurol Assoc. 2002;20:8–14. [Google Scholar]
- 18.Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. J Korean Neurol Assoc. 2001;19:585–591. [Google Scholar]
- 19.Auriacombe S, Pere JJ, Loria-Kanza Y, Vellas B. Efficacy and safety of rivastigmine in patients with Alzheimer's disease who failed to benefit from treatment with donepezil. Curr Med Res Opin. 2002;18:129–138. doi: 10.1185/030079902125000471. [DOI] [PubMed] [Google Scholar]
- 20.Sadowsky CH, Farlow MR, Atkinson L, Steadman J, Koumaras B, Chen M, et al. Switching from donepezil to rivastigmine is well tolerated: results of an open-label safety and tolerability study. Prim Care Companion J Clin Psychiatry. 2005;7:43–48. doi: 10.4088/pcc.v07n0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dantoine T, Auriacombe S, Sarazin M, Becker H, Pere JJ, Bourdeix I. Rivastigmine monotherapy and combination therapy with memantine in patients with moderately severe Alzheimer's disease who failed to benefit from previous cholinesterase inhibitor treatment. Int J Clin Pract. 2006;60:110–118. doi: 10.1111/j.1368-5031.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- 22.Grossberg G, Sadowsky C, Fröstl H, Frölich L, Nagel J, Tekin S, et al. Safety and tolerability of the rivastigmine patch: results of a 28-week open-label extension. Alzheimer Dis Assoc Disord. 2009;23:158–164. doi: 10.1097/wad.0b013e31818b1c2c. [DOI] [PubMed] [Google Scholar]